Recent Advanced Development of Acid-Resistant Thin-Film Composite Nanofiltration Membrane Preparation and Separation Performance in Acidic Environments
Abstract
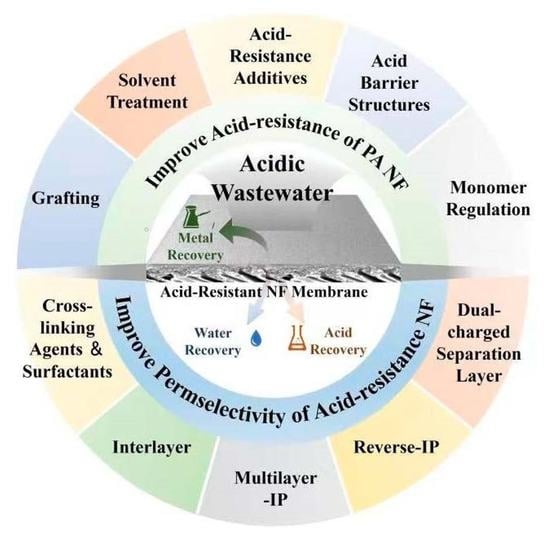
:1. Introduction
2. Challenge of NF Membranes in Acidic Environment
3. Regulation of Acid-Resistance of Polyamide NF in Conventional Acidic Environments
4. Preparation and Regulation of Acid-Resistant NF Membrane Applied in Extreme Acid Environment
5. Conclusions
6. Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reid, C.E.; Breton, E.J. Water and Ion Flow across Cellulosic Membranes. J. Appl. Polym. Sci. 1959, 1, 133–143. [Google Scholar] [CrossRef]
- Petersen, R.J. Composite Reverse Osmosis and Nanofiltration Membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar] [CrossRef]
- Feng, X.; Peng, D.; Zhu, J.; Wang, Y.; Zhang, Y. Recent Advances of Loose Nanofiltration Membranes for Dye/Salt Separation. Sep. Purif. Technol. 2022, 285, 120228. [Google Scholar] [CrossRef]
- Xu, S.; He, R.; Dong, C.; Sun, N.; Zhao, S.; He, H.; Yu, H.; Zhang, Y.-B.; He, T. Acid Stable Layer-by-Layer Nanofiltration Membranes for Phosphoric Acid Purification. J. Membr. Sci. 2022, 644, 120090. [Google Scholar] [CrossRef]
- Machado, M.T.C.; Trevisan, S.; Pimentel-Souza, J.D.R.; Pastore, G.M.; Hubinger, M.D. Clarification and Concentration of Oligosaccharides from Artichoke Extract by a Sequential Process with Microfiltration and Nanofiltration Membranes. J. Food Eng. 2016, 180, 120–128. [Google Scholar] [CrossRef]
- Suhalim, N.S.; Kasim, N.; Mahmoudi, E.; Shamsudin, I.J.; Mohammad, A.W.; Mohamed Zuki, F.; Jamari, N.L.-A. Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials 2022, 12, 437. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, K.; Liu, Y.; Xia, S. A Review on Polyester and Polyester-Amide Thin Film Composite Nanofiltration Membranes: Synthesis, Characteristics and Applications. Sci. Total Environ. 2023, 858, 159922. [Google Scholar] [CrossRef]
- Mirza, S. Reduction of Energy Consumption in Process Plants Using Nanofiltration and Reverse Osmosis. Desalination 2008, 224, 132–142. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E. Nanofiltration Membranes for Food and Pharmaceutical Industries. Emergent Mater. 2021, 5, 1329–1343. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, G.; Wan, Y.; Luo, J. Nanofiltration Membrane for Bio-separation: Process-oriented Materials Innovation. Eng. Life Sci. 2021, 21, 405–416. [Google Scholar] [CrossRef]
- Suo, Y.; Ren, Y. Research on the Mechanism of Nanofiltration Membrane Fouling in Zero Discharge Process of High Salty Wastewater from Coal Chemical Industry. Chem. Eng. Sci. 2021, 245, 116810. [Google Scholar] [CrossRef]
- Zimmermann, Y.-S.; Niewersch, C.; Lenz, M.; Kül, Z.Z.; Corvini, P.F.-X.; Schäffer, A.; Wintgens, T. Recycling of Indium From CIGS Photovoltaic Cells: Potential of Combining Acid-Resistant Nanofiltration with Liquid–Liquid Extraction. Environ. Sci. Technol. 2014, 48, 13412–13418. [Google Scholar] [CrossRef] [PubMed]
- Darvishmanesh, S.; Robberecht, T.; Luis, P.; Degrève, J.; Van der Bruggen, B. Performance of Nanofiltration Membranes for Solvent Purification in the Oil Industry. J. Am. Oil Chem. Soc. 2011, 88, 1255–1261. [Google Scholar] [CrossRef]
- Cheng, X.; Lai, C.; Zhu, X.; Shao, S.; Xu, J.; Zhang, F.; Song, J.; Wu, D.; Liang, H.; Luo, X. Tailored Ultra-Low Pressure Nanofiltration Membranes for Advanced Drinking Water Treatment. Desalination 2023, 548, 116264. [Google Scholar] [CrossRef]
- Sharma, V.; Borkute, G.; Gumfekar, S.P. Biomimetic Nanofiltration Membranes: Critical Review of Materials, Structures, and Applications to Water Purification. Chem. Eng. J. 2022, 433, 133823. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for Next-Generation Desalination and Water Purification Membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Wadekar, S.S.; Hayes, T.; Lokare, O.R.; Mittal, D.; Vidic, R.D. Laboratory and Pilot-Scale Nanofiltration Treatment of Abandoned Mine Drainage for the Recovery of Products Suitable for Industrial Reuse. Ind. Eng. Chem. Res. 2017, 56, 7355–7364. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Wang, Y. Diffusion Dialysis for Acid Recovery from Acidic Waste Solutions: Anion Exchange Membranes and Technology Integration. Membranes 2020, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Dong, F.; He, D.; Liu, S.; Chen, N.; Huo, T. Organic Acid Mediated Photoelectrochemical Reduction of U(vi) to U(Iv) in Waste Water: Electrochemical Parameters and Spectroscopy. RSC Adv. 2021, 11, 23241–23248. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A Critical Review on Remediation, Reuse, and Resource Recovery from Acid Mine Drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef]
- San Román, M.F.; Ortiz Gándara, I.; Ibañez, R.; Ortiz, I. Hybrid Membrane Process for the Recovery of Major Components (Zinc, Iron and HCl) from Spent Pickling Effluents. J. Membr. Sci. 2012, 415–416, 616–623. [Google Scholar] [CrossRef]
- Yin, C.; Yu, S.; An, B.; Lou, Z.; Huang, S.; Yuan, H.; Zhu, N. Fluorine Recovery from Etching Wastewater through CaF2-Based near-Infrared Photocatalyst Synthesis. J. Clean. Prod. 2018, 175, 267–275. [Google Scholar] [CrossRef]
- United Nations Educational, Scientific, and Cultural Organization. The United Nations World Water Development Report 2020; UNESCO: Perugia, Italy, 2020; Available online: https://unesdoc.unesco.org/ark:/48223/pf0000372876.locale=en (accessed on 21 March 2020).
- Meese, A.F.; Kim, D.J.; Wu, X.; Le, L.; Napier, C.; Hernandez, M.T.; Laroco, N.; Linden, K.G.; Cox, J.; Kurup, P.; et al. Opportunities and Challenges for Industrial Water Treatment and Reuse. ACS ES T Eng. 2021, 2, 465–488. [Google Scholar] [CrossRef]
- Yang, C.; Pan, J.; Zhu, D.; Guo, Z.; Li, X. Pyrometallurgical Recycling of Stainless Steel Pickling Sludge: A Review. J. Iron Steel Res. Int. 2019, 26, 547–557. [Google Scholar] [CrossRef]
- Das, P.; Mondal, G.C.; Singh, S.; Singh, A.K.; Prasad, B.; Singh, K.K. Effluent Treatment Technologies in the Iron and Steel Industry—A State of the Art Review. Water Environ. Res. 2018, 90, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Li, W.; Li, Y.; Wang, G.; Li, A.; Li, H. Efficient Removal of Acid Dyes Using Permanent Magnetic Resin and Its Preliminary Investigation for Advanced Treatment of Dyeing Effluents. J. Clean. Prod. 2020, 251, 119694. [Google Scholar] [CrossRef]
- Liu, H.; Xia, J.; Cui, K.; Meng, J.; Zhang, R.; Cao, B.; Li, P. Fabrication of High-Performance Pervaporation Membrane for Sulfuric Acid Recovery via Interfacial Polymerization. J. Membr. Sci. 2021, 624, 119108. [Google Scholar] [CrossRef]
- Roy, S.; Majumdar, S.; Sahoo, G.C.; Bhowmick, S.; Kundu, A.K.; Mondal, P. Removal of As(V), Cr(VI) and Cu(II) Using Novel Amine Functionalized Composite Nanofiltration Membranes Fabricated on Ceramic Tubular Substrate. J. Hazard. Mater. 2020, 399, 122841. [Google Scholar] [CrossRef]
- Foureaux, A.F.S.; Moreira, V.R.; Lebron, Y.A.R.; Santos, L.V.S.; Amaral, M.C.S. Direct Contact Membrane Distillation as an Alternative to the Conventional Methods for Value-Added Compounds Recovery from Acidic Effluents: A Review. Sep. Purif. Technol. 2020, 236, 116251. [Google Scholar] [CrossRef]
- Skousen, J.G.; Ziemkiewicz, P.F.; McDonald, L.M. Acid Mine Drainage Formation, Control and Treatment: Approaches and Strategies. Extract Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Kesieme, U.K.; Aral, H. Application of Membrane Distillation and Solvent Extraction for Water and Acid Recovery from Acidic Mining Waste and Process Solutions. J. Environ. Chem. Eng. 2015, 3, 2050–2056. [Google Scholar] [CrossRef]
- Ju, J.; Feng, Y.; Li, H.; Liu, S.; Xu, C. Separation and Recovery of V, Ti, Fe and Ca from Acidic Wastewater and Vanadium-Bearing Steel Slag Based on a Collaborative Utilization Process. Sep. Purif. Technol. 2021, 276, 119335. [Google Scholar] [CrossRef]
- Iakovleva, E.; Sillanpää, M. The Use of Low-Cost Adsorbents for Wastewater Purification in Mining Industries. Environ. Sci. Pollut. Res. 2013, 20, 7878–7899. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, S.; Zhang, H.; Deng, Y.; Baeyens, J.; Dewil, R.; Sweygers, N.; Appels, L. Adsorption of Acid Fuchsine Dye from Wastewater by Mg-Ferrite Particles. J. Environ. Manag. 2022, 317, 115427. [Google Scholar] [CrossRef] [PubMed]
- Işık, M.; Sponza, D.T. Biological Treatment of Acid Dyeing Wastewater Using a Sequential Anaerobic/Aerobic Reactor System. Enzyme Microb. Technol. 2006, 38, 887–892. [Google Scholar] [CrossRef]
- You, S.; Lu, J.; Tang, C.Y.; Wang, X. Rejection of Heavy Metals in Acidic Wastewater by a Novel Thin-Film Inorganic Forward Osmosis Membrane. Chem. Eng. J. 2017, 320, 532–538. [Google Scholar] [CrossRef]
- Cui, K.; Li, P.; Zhang, R.; Cao, B. Preparation of Pervaporation Membranes by Interfacial Polymerization for Acid Wastewater Purification. Chem. Eng. Res. Des. 2020, 156, 171–179. [Google Scholar] [CrossRef]
- Agrawal, A.; Kumari, S.; Ray, B.C.; Sahu, K.K. Extraction of Acid and Iron Values from Sulphate Waste Pickle Liquor of a Steel Industry by Solvent Extraction Route. Hydrometallurgy 2007, 88, 58–66. [Google Scholar] [CrossRef]
- Kesieme, U.; Chrysanthou, A.; Catulli, M.; Cheng, C.Y. A Review of Acid Recovery from Acidic Mining Waste Solutions Using Solvent Extraction. J. Chem. Technol. Biotechnol. 2018, 93, 3374–3385. [Google Scholar] [CrossRef] [Green Version]
- Dalvand, A.; Nabizadeh, R.; Reza Ganjali, M.; Khoobi, M.; Nazmara, S.; Hossein Mahvi, A. Modeling of Reactive Blue 19 Azo Dye Removal from Colored Textile Wastewater Using L-Arginine-Functionalized Fe3O4 Nanoparticles: Optimization, Reusability, Kinetic and Equilibrium Studies. J. Magn. Magn. Mater. 2016, 404, 179–189. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Zhang, H.; Baeyens, J. Adsorption of Congo Red Dye on FexCo3-XO4 Nanoparticles. J. Environ. Manag. 2019, 238, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Kuai, L.; De Vreese, I.; Vandevivere, P.; Verstraete, W. GAC-Amended UASB Reactor for the Stable Treatment of Toxic Textile Wastewater. Environ. Technol. 1998, 19, 1111–1117. [Google Scholar] [CrossRef]
- Bargeman, G. Recent Developments in the Preparation of Improved Nanofiltration Membranes for Extreme PH Conditions. Sep. Purif. Technol. 2021, 279, 119725. [Google Scholar] [CrossRef]
- Scott, K.; Hughes, R. Industrial Membrane Separation Technology; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Fane, A.T.; Wang, R.; Jia, Y. Membrane Technology: Past, Present and Future; Humana Press: Totowa, NJ, USA, 2010; pp. 1–45. [Google Scholar]
- López, J.; Gibert, O.; Cortina, J.L. Integration of Membrane Technologies to Enhance the Sustainability in the Treatment of Metal-Containing Acidic Liquid Wastes. An Overview. Sep. Purif. Technol. 2021, 265, 118485. [Google Scholar] [CrossRef]
- Pino, L.; Beltran, E.; Schwarz, A.; Ruiz, M.C.; Borquez, R. Optimization of Nanofiltration for Treatment of Acid Mine Drainage and Copper Recovery by Solvent Extraction. Hydrometallurgy 2020, 195, 105361. [Google Scholar] [CrossRef]
- Szymczyk, A.; Fievet, P. Ion Transport through Nanofiltration Membranes: The Steric, Electric and Dielectric Exclusion Model. Desalination 2006, 200, 122–124. [Google Scholar] [CrossRef]
- Liu, X.; Gong, W.; Liu, L. Treatment of Sulfate-Rich and Low PH Wastewater by Sulfate Reducing Bacteria with Iron Shavings in a Laboratory. Water Sci. Technol. 2013, 69, 595–600. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.; Tong, T.; Epsztein, R.; Sun, M.; Verduzco, R.; Ma, J.; Elimelech, M. Selective Removal of Divalent Cations by Polyelectrolyte Multilayer Nanofiltration Membrane: Role of Polyelectrolyte Charge, Ion Size, and Ionic Strength. J. Membr. Sci. 2018, 559, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Bowen, W.R.; Welfoot, J.S. Modelling the Performance of Membrane Nanofiltration—Critical Assessment and Model Development. Chem. Eng. Sci. 2002, 57, 1121–1137. [Google Scholar] [CrossRef]
- Ryzhkov, I.I.; Minakov, A.V. Theoretical Study of Electrolyte Transport in Nanofiltration Membranes with Constant Surface Potential/Charge Density. J. Membr. Sci. 2016, 520, 515–528. [Google Scholar] [CrossRef]
- Epsztein, R.; DuChanois, R.M.; Ritt, C.L.; Noy, A.; Elimelech, M. Towards Single-Species Selectivity of Membranes with Subnanometre Pores. Nat. Nanotechnol. 2020, 15, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Deen, W.M. Hindered Transport of Large Molecules in Liquid-Filled Pores. AIChE J. 1987, 33, 1409–1425. [Google Scholar] [CrossRef]
- Venkataraman, L.; Klare, J.E.; Nuckolls, C.; Hybertsen, M.S.; Steigerwald, M.L. Dependence of Single-Molecule Junction Conductance on Molecular Conformation. Nature 2006, 442, 904–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigelt, S.; Busse, C.; Petersen, L.; Rauls, E.; Hammer, B.; Gothelf, K.V.; Besenbacher, F.; Linderoth, T.R. Chiral Switching by Spontaneous Conformational Change in Adsorbed Organic Molecules. Nat. Mater. 2006, 5, 112–117. [Google Scholar] [CrossRef]
- Dechadilok, P.; Deen, W.M. Hindrance Factors for Diffusion and Convection in Pores. Ind. Eng. Chem. Res. 2006, 45, 6953–6959. [Google Scholar] [CrossRef]
- Ennis, J.; Zhang, H.; Stevens, G.; Perera, J.; Scales, P.; Carnie, S. Mobility of Protein through a Porous Membrane. J. Membr. Sci. 1996, 119, 47–58. [Google Scholar] [CrossRef]
- Renkin, E. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J. Gen. Physiol. 1954, 38, 225–243. [Google Scholar] [CrossRef]
- Luo, J.; Wan, Y. Effects of PH and Salt on Nanofiltration—A Critical Review. J. Membr. Sci. 2013, 438, 18–28. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Guo, S.; Luo, J.; Wan, Y. A Robust Dually Charged Membrane Prepared via Catechol-Amine Chemistry for Highly Efficient Dye/Salt Separation. J. Membr. Sci. 2021, 629, 119287. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Role of Electrostatic Interactions in the Retention of Pharmaceutically Active Contaminants by a Loose Nanofiltration Membrane. J. Membr. Sci. 2006, 286, 52–59. [Google Scholar] [CrossRef]
- Huang, B.-Q.; Tang, Y.-J.; Gao, A.-R.; Zeng, Z.-X.; Xue, S.-M.; Ji, C.-H.; Tang, C.Y.; Xu, Z.-L. Dually Charged Polyamide Nanofiltration Membranes Fabricated by Microwave-Assisted Grafting for Heavy Metals Removal. J. Membr. Sci. 2021, 640, 119834. [Google Scholar] [CrossRef]
- Guastalli, A.R.; Labanda, J.; Llorens, J. Separation of Phosphoric Acid from an Industrial Rinsing Water by Means of Nanofiltration. Desalination 2009, 243, 218–228. [Google Scholar] [CrossRef]
- Novalic, S.; Dabrowski, A.; Kulbe, K.D. Nanofiltration of Caustic and Acidic Cleaning Solutions with High COD Part 2. Recycling of HNO3. J. Food Eng. 1998, 38, 133–140. [Google Scholar] [CrossRef]
- Fernández, J.F.; Jastorff, B.; Störmann, R.; Stolte, S.; Thöming, J. Thinking in Terms of Structure-Activity-Relationships (T-SAR): A Tool to Better Understand Nanofiltration Membranes. Membranes 2011, 1, 162–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalwani, M.; Benes, N.E.; Bargeman, G.; Stamatialis, D.; Wessling, M. Effect of PH on the Performance of Polyamide/Polyacrylonitrile Based Thin Film Composite Membranes. J. Membr. Sci. 2011, 372, 228–238. [Google Scholar] [CrossRef]
- Lee, K.P.; Bargeman, G.; de Rooij, R.; Kemperman, A.J.B.; Benes, N.E. Interfacial Polymerization of Cyanuric Chloride and Monomeric Amines: PH Resistant Thin Film Composite Polyamine Nanofiltration Membranes. J. Membr. Sci. 2017, 523, 487–496. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Vecino, X.; Gibert, O.; Cortina, J.L. Comparison of Acid-Resistant Ceramic and Polymeric Nanofiltration Membranes for Acid Mine Waters Treatment. Chem. Eng. J. 2020, 382, 122786. [Google Scholar] [CrossRef]
- Lee, J.; Shin, Y.; Boo, C.; Hong, S. Performance, Limitation, and Opportunities of Acid-Resistant Nanofiltration Membranes for Industrial Wastewater Treatment. J. Membr. Sci. 2022, 666, 121142. [Google Scholar] [CrossRef]
- O’Connor, C. Acidic and Basic Amide Hydrolysis. Q. Rev., Chem. Soc. 1970, 24, 553. [Google Scholar] [CrossRef]
- Hoseinpour, H.; Peyravi, M.; Nozad, A.; Jahanshahi, M. Static and Dynamic Assessments of Polysulfonamide and Poly(Amide-Sulfonamide) Acid-Stable Membranes. J. Taiwan Inst. Chem. Eng. 2016, 67, 453–466. [Google Scholar] [CrossRef]
- Ilias, S.; Schimmel, K.A.; Assey, G.E.J.M. Effect of Viscosity on Membrane Fluxes in Cross-Flow Ultrafiltration. Sep. Sci. Technol. 1995, 30, 1669–1687. [Google Scholar] [CrossRef]
- Castricum, H.L.; Kreiter, R.; van Veen, H.M.; Blank, D.H.A.; Vente, J.F.; ten Elshof, J.E. High-Performance Hybrid Pervaporation Membranes with Superior Hydrothermal and Acid Stability. J. Membr. Sci. 2008, 324, 111–118. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Effect of Solution Chemistry on the Surface Charge of Polymeric Reverse Osmosis and Nanofiltration Membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
- Tanninen, J. Long-Term Acid Resistance and Selectivity of NF Membranes in Very Acidic Conditions. J. Membr. Sci. 2004, 240, 11–18. [Google Scholar] [CrossRef]
- Bai, L.; Wang, M.; Li, Z.; Yang, H.; Peng, Z.; Zhao, Y. Fabrication of a Novel Composite Nanofiltration Membrane with Excellent Acid Resistance and Water Flux via the Selective Bond Dissociation Method. J. Membr. Sci. 2022, 643, 120012. [Google Scholar] [CrossRef]
- Paul, M.; Jons, S.D. Chemistry and Fabrication of Polymeric Nanofiltration Membranes: A Review. Polymer 2016, 103, 417–456. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, D.; Gan, Z.; Luo, X.; Tang, X.; Cheng, X.; Bai, L.; Li, G.; Liang, H. Improving Chlorine Resistance and Separation Performance of Thin-Film Composite Nanofiltration Membranes with in-Situ Grafted Melamine. Desalination 2020, 489, 114539. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, P.; Fang, R.; Cai, J.; Zhang, L.-D.; He, Q.-Y.; Zhou, Z.-H.; Sun, S.-P.; Cao, X.-L. Constructing Positively Charged Acid-Resistant Nanofiltration Membranes via Surface Postgrafting for Efficient Removal of Metal Ions from Electroplating Rinse Wastewater. Sep. Purif. Technol. 2022, 297, 121500. [Google Scholar] [CrossRef]
- Guo, S.; Chen, X.; Wan, Y.; Feng, S.; Luo, J. Custom-Tailoring Loose Nanofiltration Membrane for Precise Biomolecule Fractionation: New Insight into Post-Treatment Mechanisms. ACS Appl. Mater. Interfaces 2020, 12, 13327–13337. [Google Scholar] [CrossRef]
- Wei, X.; Xu, X.; Wu, J.; Li, C.; Chen, J.; Lv, B.; Zhu, B.; Xiang, H. SiO2 -Modified Nanocomposite Nanofiltration Membranes with High Flux and Acid Resistance. J. Appl. Polym. Sci. 2019, 136, 47436. [Google Scholar] [CrossRef]
- Puthai, W.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. SiO2-ZrO2 Nanofiltration Membranes of Different Si/Zr Molar Ratios: Stability in Hot Water and Acid/Alkaline Solutions. J. Membr. Sci. 2017, 524, 700–711. [Google Scholar] [CrossRef] [Green Version]
- Chae, H.-R.; Lee, J.; Lee, C.-H.; Kim, I.-C.; Park, P.-K. Graphene Oxide-Embedded Thin-Film Composite Reverse Osmosis Membrane with High Flux, Anti-Biofouling, and Chlorine Resistance. J. Membr. Sci. 2015, 483, 128–135. [Google Scholar] [CrossRef]
- Chae, H.-R.; Kim, I.-C.; Kwon, Y.-N. Acid-Resistance Enhancement of Thin-Film Composite Membrane Using Barrier Effect of Graphene Oxide Nanosheets. Materials 2021, 14, 3151. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.G.; Kwon, S.J.; Park, H.; Park, Y.-I.; Lee, J.-H. High-Performance and Acid-Resistant Nanofiltration Membranes Prepared by Solvent Activation on Polyamide Reverse Osmosis Membranes. J. Membr. Sci. 2020, 595, 117590. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of Membrane Chemistry and Coating Layer on Physiochemical Properties of Thin Film Composite Polyamide RO and NF Membranes. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, L.; Zhang, L.; Yu, J.Q. An Acid Resistant Nanofiltration Membrane Prepared from a Precursor of Poly(s-Triazine-Amine) by Interfacial Polymerization. J. Membr. Sci. 2018, 546, 225–233. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, J.; Chen, C.; Wan, Y. Highly Permeable Acid-Resistant Nanofiltration Membrane Based on a Novel Sulfonamide Aqueous Monomer for Efficient Acidic Wastewater Treatment. Chem. Eng. J. 2021, 425, 131791. [Google Scholar] [CrossRef]
- Park, H.M.; Takaba, H.; Lee, Y.T. Preparation and Characterization of TFC NF Membrane with Improved Acid Resistance Behavior. J. Membr. Sci. 2020, 616, 118620. [Google Scholar] [CrossRef]
- Park, H.M.; Ismael, M.; Takaba, H.; Lee, Y.T. Acid-Resistant Thin-Film Composite Nanofiltration Membrane Prepared from Polyamide-Polyurea and the Behavior of Density Functional Theory Study. J. Membr. Sci. 2022, 645, 120175. [Google Scholar] [CrossRef]
- Han, R.; Zhang, S.; Hu, L.; Guan, S.; Jian, X. Preparation and Characterization of Thermally Stable Poly(Piperazine Amide)/PPBES Composite Nanofiltration Membrane. J. Membr. Sci. 2011, 370, 91–96. [Google Scholar] [CrossRef]
- Mikhailenko, S.D.; Wang, K.; Kaliaguine, S.; Xing, P.; Robertson, G.P.; Guiver, M.D. Proton Conducting Membranes Based on Cross-Linked Sulfonated Poly(Ether Ether Ketone) (SPEEK). J. Membr. Sci. 2004, 233, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wan, Y.; Chen, C.; Luo, J. Preparation of Acid-Resistant Nanofiltration Membrane with Dually Charged Separation Layer for Enhanced Salts Removal. Sep. Purif. Technol. 2022, 292, 120974. [Google Scholar] [CrossRef]
- Shen, K.; Cheng, C.; Zhang, T.; Wang, X. High Performance Polyamide Composite Nanofiltration Membranes via Reverse Interfacial Polymerization with the Synergistic Interaction of Gelatin Interlayer and Trimesoyl Chloride. J. Membr. Sci. 2019, 588, 117192. [Google Scholar] [CrossRef]
- Wang, X.; Yeh, T.-M.; Wang, Z.; Yang, R.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. Nanofiltration Membranes Prepared by Interfacial Polymerization on Thin-Film Nanofibrous Composite Scaffold. Polymer 2014, 55, 1358–1366. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Z.; Wang, H.; Jiang, H.; Li, Y.; Wu, C. An Acid-Stable Positively Charged Polysulfonamide Nanofiltration Membrane Prepared by Interfacial Polymerization of Polyallylamine and 1,3-Benzenedisulfonyl Chloride for Water Treatment. RSC Adv. 2019, 9, 2042–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Zhou, Q.; Shuai, S.; Yao, G.; Ma, M.; Gao, C. Thin-Film Composite Nanofiltration Membranes with Improved Acid Stability Prepared from Naphthalene-1,3,6-Trisulfonylchloride (NTSC) and Trimesoyl Chloride (TMC). Desalination 2013, 315, 164–172. [Google Scholar] [CrossRef]
- Bali Eslami, A.; Peyravi, M.; Jahanshahi, M.; Hosseinpour, H. Polysulfonamide Coating Layer Polymerized By1,3-Disulfonyl Chloride and Polyethylenimine to Achieve Acid Resistant TFC Membranes. Chem. Eng. Res. Des. 2020, 155, 172–179. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Dong, Y.; Zhao, X.; Jiang, Z.; Zhang, R.; Zhao, J. Separation Performance of Thin-Film Composite Nanofiltration Membrane through Interfacial Polymerization Using Different Amine Monomers. Desalination 2014, 333, 59–65. [Google Scholar] [CrossRef]
- Liu, M.; Yao, G.; Cheng, Q.; Ma, M.; Yu, S.; Gao, C. Acid Stable Thin-Film Composite Membrane for Nanofiltration Prepared from Naphthalene-1,3,6-Trisulfonylchloride (NTSC) and Piperazine (PIP). J. Membr. Sci. 2012, 415–416, 122–131. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, Y.; Liu, C.; Lee, K.-R.; Hung, W.-S.; Wang, Z.; Li, Y.; Elimelech, M.; Jin, J.; Lin, S. Polyamide Nanofiltration Membrane with Highly Uniform Sub-Nanometre Pores for Sub-1 Å Precision Separation. Nat. Commun. 2020, 11, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S. Effect of Diamine Composition on the Gas Transport Properties in 6FDA-Durene/3,3′-Diaminodiphenyl Sulfone Copolyimides. J. Membr. Sci. 2002, 202, 165–176. [Google Scholar] [CrossRef]
- Lasisi, K.H.; Ajibade, T.F.; Zhang, K. 3, 3′-Diaminodiphenyl Sulfone Engagement in Polysulfonamide-Based Acid-Resistant Nanofiltration Membrane Fabrication for Efficient Separation Performance and Heavy Metal Ions Removal from Wastewater. J. Membr. Sci. 2022, 661, 120909. [Google Scholar] [CrossRef]
- Lai, W.; Shan, L.; Bai, J.; Xiao, L.; Liu, L.; Wang, S.; Gong, L.; Jiao, Y.; Xie, W.; Liu, H.; et al. Highly Permeable and Acid-Resistant Nanofiltration Membrane Fabricated by in-Situ Interlaced Stacking of COF and Polysulfonamide Films. Chem. Eng. J. 2022, 450, 137965. [Google Scholar] [CrossRef]
- Zhu, Y.; Dou, P.; He, H.; Lan, H.; Xu, S.; Zhang, Y.; He, T.; Niu, J. Improvement of Permeability and Rejection of an Acid Resistant Polysulfonamide Thin-Film Composite Nanofiltration Membrane by a Sulfonated Poly(Ether Ether Ketone) Interlayer. Sep. Purif. Technol. 2020, 239, 116528. [Google Scholar] [CrossRef]
- Ali, M.E.A.; Wang, L.; Wang, X.; Feng, X. Thin Film Composite Membranes Embedded with Graphene Oxide for Water Desalination. Desalination 2016, 386, 67–76. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Delnavaz, M.; Vatanpour, V. Investigation of Raw and Oxidized Multiwalled Carbon Nanotubes in Fabrication of Reverse Osmosis Polyamide Membranes for Improvement in Desalination and Antifouling Properties. Desalination 2017, 410, 1–9. [Google Scholar] [CrossRef]
- Khorshidi, B.; Biswas, I.; Ghosh, T.; Thundat, T.; Sadrzadeh, M. Robust Fabrication of Thin Film Polyamide-TiO2 Nanocomposite Membranes with Enhanced Thermal Stability and Anti-Biofouling Propensity. Sci. Rep. 2018, 8, 784. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, H.; Jiang, H.; Sheng, A.; Wei, Z.; Li, Y.; Wu, C.; Li, H. Positively Charged Polysulfonamide Nanocomposite Membranes Incorporating Hydrophilic Triazine-Structured COFs for Highly Efficient Nanofiltration. ACS Appl. Nano Mater. 2020, 3, 9329–9339. [Google Scholar] [CrossRef]
- Paltrinieri, L.; Remmen, K.; Müller, B.; Chu, L.; Köser, J.; Wintgens, T.; Wessling, M.; de Smet, L.C.P.M.; Sudhölter, E.J.R. Improved Phosphoric Acid Recovery from Sewage Sludge Ash Using Layer-by-Layer Modified Membranes. J. Membr. Sci. 2019, 587, 117162. [Google Scholar] [CrossRef]
- Yuan, T.; Hu, Y.; He, M.; Zhao, S.; Lan, H.; Li, P.; Jason Niu, Q. Spinning-assist Layer-by-layer Assembled Polysulfonamide Membrane for Reverse Osmosis from Naphthalene-1,3,6-trisulfonylchloride and Piperazine. J. Appl. Polym. Sci. 2018, 136, 47138. [Google Scholar] [CrossRef]
- He, M.; Yuan, T.; Dong, W.; Li, P.; Jason Niu, Q.; Meng, J. High-Performance Acid-Stable Polysulfonamide Thin-Film Composite Membrane Prepared via Spinning-Assist Multilayer Interfacial Polymerization. J. Mater. Sci. 2018, 54, 886–900. [Google Scholar] [CrossRef]
- Lee, K.P.; Zheng, J.; Bargeman, G.; Kemperman, A.J.B.; Benes, N.E. PH Stable Thin Film Composite Polyamine Nanofiltration Membranes by Interfacial Polymerisation. J. Membr. Sci. 2015, 478, 75–84. [Google Scholar] [CrossRef]
- Just, G.; Pokorny, I.; Pritzkow, W. Kinetic studies on the Reaction of Cyanuric Chloride with Amines. J. Prakt. Chem. 1995, 337, 133–135. [Google Scholar] [CrossRef]
- Fierz-David, H.E.; Matter, M. Azo and Anthraquinonoid Dyes Containing the Cyanuric Ring. J. Soc. Dyers Colour. 1937, 53, 424–436. [Google Scholar] [CrossRef]
- Liu, S.; Wu, C.; Hung, W.-S.; Lu, X.; Lee, K.-R. One-Step Constructed Ultrathin Janus Polyamide Nanofilms with Opposite Charges for Highly Efficient Nanofiltration. J. Mater. Chem. A 2017, 5, 22988–22996. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Xu, L.; Liu, Q.; Borjigin, B.; Hou, D.; Xiang, J.; Wang, J. One Step Prepared Janus Acid-Resistant Nanofiltration Membranes with Opposite Surface Charges for Acidic Wastewater Treatment. Sep. Purif. Technol. 2020, 250, 117245. [Google Scholar] [CrossRef]
- He, B.; Peng, H.; Chen, Y.; Zhao, Q. High Performance Polyamide Nanofiltration Membranes Enabled by Surface Modification of Imidazolium Ionic Liquid. J. Membr. Sci. 2020, 608, 118202. [Google Scholar] [CrossRef]
- Xiao, H.-F.; Chu, C.-H.; Xu, W.-T.; Chen, B.-Z.; Ju, X.-H.; Xing, W.; Sun, S.-P. Amphibian-Inspired Amino Acid Ionic Liquid Functionalized Nanofiltration Membranes with High Water Permeability and Ion Selectivity for Pigment Wastewater Treatment. J. Membr. Sci. 2019, 586, 44–52. [Google Scholar] [CrossRef]
- Bai, J.; Lai, W.; Gong, L.; Xiao, L.; Wang, G.; Shan, L.; Luo, S. Ionic Liquid Regulated Interfacial Polymerization Process to Improve Acid-Resistant Nanofiltration Membrane Permeance. J. Membr. Sci. 2022, 641, 119882. [Google Scholar] [CrossRef]
- Yu, L.; Li, K.; Zhang, Y.; Wang, J.; Zhang, G. Improved Permeability of Tight Acid Resistant Nanofiltration Membrane via Citric Acid Post-Treatment. J. Membr. Sci. 2022, 648, 120381. [Google Scholar] [CrossRef]
- Jun, B.-M.; Lee, H.K.; Kwon, Y.-N. Acid-Catalyzed Hydrolysis of Semi-Aromatic Polyamide NF Membrane and Its Application to Water Softening and Antibiotics Enrichment. Chem. Eng. J. 2018, 332, 419–430. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, J.; Feng, S.; Chen, X.; Luo, J.; Wan, Y. Tuning Pore Size and Surface Charge of Poly(Piperazinamide) Nanofiltration Membrane by Enhanced Chemical Cleaning Treatment. J. Membr. Sci. 2022, 643, 120054. [Google Scholar] [CrossRef]
- Şakar-Deliormanlı, A. Interaction of Sodium Dodecyl Sulfate with Poly(Ethyleneimine) in Bulk Solution and at the Air-Solution Interface. J. Dispers. Sci. Technol. 2009, 31, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Wang, M.; Yang, H.; Peng, Z.; Zhao, Y.; Li, Z. A Nanofiltration Membrane Fabricated on a Surfactant Activated Substrate with Improved Separation Performance and Acid Resistance. New J. Chem. 2021, 45, 14381–14391. [Google Scholar] [CrossRef]
- Mansourpanah, Y.; Alizadeh, K.; Madaeni, S.S.; Rahimpour, A.; Soltani Afarani, H. Using Different Surfactants for Changing the Properties of Poly(Piperazineamide) TFC Nanofiltration Membranes. Desalination 2011, 271, 169–177. [Google Scholar] [CrossRef]
- Lasisi, K.H.; Yao, W.; Xue, Q.; Liu, Q.; Zhang, K. High Performance Polyamine-Based Acid-Resistant Nanofiltration Membranes Catalyzed with 1,4-Benzenecarboxylic Acid in Interfacial Cross-Linking Polymerization Process. J. Membr. Sci. 2021, 640, 119833. [Google Scholar] [CrossRef]
- Lasisi, K.H.; Zhang, K. Polyamine-Based Thin-Film Composite Nanofiltration Membrane Embedded with Catalytic Chemical Additive for Enhanced Separation Performance and Acid Stability. J. Membr. Sci. 2022, 644, 120155. [Google Scholar] [CrossRef]
- Sendijarevic, V.; Sendijarevic, A.; Sendijarevic, I.; Bailey, R.E.; Pemberton, D.; Reimann, K.A. Hydrolytic Stability of Toluene Diisocyanate and Polymeric Methylenediphenyl Diisocyanate Based Polyureas under Environmental Conditions. Environ. Sci. Technol. 2004, 38, 1066–1072. [Google Scholar] [CrossRef]
- Iqbal, N.; Tripathi, M.; Parthasarathy, S.; Kumar, D.; Roy, P.K. Polyurea Coatings for Enhanced Blast-Mitigation: A Review. RSC Adv. 2016, 6, 109706–109717. [Google Scholar] [CrossRef]
- Cadotte, J.E. Reverse Osmosis Membrane. US. Patent 4 (39) (1977) 440, 2 August 1977. [Google Scholar]
- Zhang, Y.; Wan, Y.; Li, Y.; Pan, G.; Yu, H.; Du, W.; Shi, H.; Wu, C.; Liu, Y. Thin-Film Composite Nanofiltration Membrane Based on Polyurea for Extreme PH Condition. J. Membr. Sci. 2021, 635, 119472. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadrzadeh, M.; Sudhölter, E.J.R.; de Smet, L.C.P.M. Surface Modification Methods of Organic Solvent Nanofiltration Membranes. Chem. Eng. J. 2016, 289, 562–582. [Google Scholar] [CrossRef]
- Liu, P.; Hou, J.; Zhang, Y.; Li, L.; Lu, X.; Tang, Z. Two-Dimensional Material Membranes for Critical Separations. Inorg. Chem. Front. 2020, 7, 2560–2581. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhang, X.; Huang, X.; Liao, W.; Zhao, Y. MoS2-Based Membranes in Water Treatment and Purification. Chem. Eng. J. 2021, 422, 130082. [Google Scholar] [CrossRef]
- Feng, Y.; Meng, X.; Wu, Z.; Chen, J.; Sun, C.; Huo, S. A Novel Polyurea Nanofiltration Membrane Constructed by PEI/TA-MoS2 for Efficient Removal of Heavy Metal Ions. Sep. Purif. Technol. 2022, 300, 121785. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Based Membranes for Water and Wastewater Treatment. Colloids Surf. A 2019, 578, 123513. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M.V. A Review of Water Treatment Membrane Nanotechnologies. Energy Environ. Sci. 2011, 4, 1946. [Google Scholar] [CrossRef] [Green Version]
- Árki, P.; Hecker, C.; Tomandl, G.; Joseph, Y. Streaming Potential Properties of Ceramic Nanofiltration Membranes–Importance of Surface Charge on the Ion Rejection. Sep. Purif. Technol. 2019, 212, 660–669. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration Membranes Review: Recent Advances and Future Prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, S.; Wu, X.; Qi, H. Fabrication and Characterization of TiO2/ZrO2 Ceramic Membranes for Nanofiltration. Microporous Mesoporous Mater. 2018, 260, 125–131. [Google Scholar] [CrossRef]
- Benfer, S.; Árki, P.; Tomandl, G. Ceramic Membranes for Filtration Applications—Preparation and Characterization. Adv. Eng. Mater. 2004, 6, 495–500. [Google Scholar] [CrossRef]
- Chen, P.; Ma, X.; Zhong, Z.; Zhang, F.; Xing, W.; Fan, Y. Performance of Ceramic Nanofiltration Membrane for Desalination of Dye Solutions Containing NaCl and Na2SO4. Desalination 2017, 404, 102–111. [Google Scholar] [CrossRef]
- Weber, R.; Chmiel, H.; Mavrov, V. Characteristics and Application of Ceramic Nanofiltration Membranes. Ann. N. Y. Acad. Sci. 2003, 984, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Nghiem, L.D. Nanofiltration of Trace Organic Chemicals: A Comparison between Ceramic and Polymeric Membranes. Sep. Purif. Technol. 2014, 136, 258–264. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Comparison of Polypropylene and Ceramic Microfiltration Membranes Applied for Separation of 1,3-PD Fermentation Broths and Saccharomyces Cerevisiae Yeast Suspensions. Membranes 2021, 11, 44. [Google Scholar] [CrossRef]
- Saw, E.T.; Ang, K.L.; He, W.; Dong, X.; Ramakrishna, S. Molecular Sieve Ceramic Pervaporation Membranes in Solvent Recovery: A Comprehensive Review. J. Environ. Chem. Eng. 2019, 7, 103367. [Google Scholar] [CrossRef]
- Liu, T.; Qin, Z.; Liu, Q.; Li, X.; Liu, Y.; An, Q.-F.; Guo, H. In Situ Growth of a Tubular MoS2 Membrane on a Ceramic Tube with Improved Organic Solvent Nanofiltration Performance. Mater. Chem. Front. 2021, 5, 3184–3191. [Google Scholar] [CrossRef]
- Guo, F.; Su, D.; Liu, Y.; Wang, J.; Yan, X.; Chen, J.; Chen, S. High Acid Resistant SiOC Ceramic Membranes for Wastewater Treatment. Ceram. Int. 2018, 44, 13444–13448. [Google Scholar] [CrossRef]
- Issaoui, M.; Limousy, L. Low-Cost Ceramic Membranes: Synthesis, Classifications, and Applications. C. R. Chim. 2019, 22, 175–187. [Google Scholar] [CrossRef]
- Voigt, I.; Richter, H.; Stahn, M.; Weyd, M.; Puhlfürß, P.; Prehn, V.; Günther, C. Scale-up of Ceramic Nanofiltration Membranes to Meet Large Scale Applications. Sep. Purif. Technol. 2019, 215, 329–334. [Google Scholar] [CrossRef]






| Regulation Method | Membrane Type and IP Monomer | Flux (L·m−2·h−1·bar−1) | Rejection % | Acid Stability |
|---|---|---|---|---|
| Surface grafting | PA PEI-TMC-ANPI | 7.33 | 97.3% a | Soaking in 10 wt% H2SO4 for 21 days or soaking in 10 wt% H3PO4 for 15 days [81] |
| Adding acid-resistant additives | PA HGPN-SiO2/PIP-TMC | 13.75 | 97% b | Soaking in (pH > 2) H2SO4 for 216 h [83] |
| PA GONs/PIP-TMC | 3.3 | 95% c | Soaking in 50% H2SO4 for 7 days [86] | |
| Solvent treatment | PA MPD-TMC-DMSO solvent-activated | 14.5 | 99.1% a 99.9% b 99.9% c | Soaking in 15 wt% H2SO4 for 28 days [88] |
| Novel monomer | triazine PA TPT-TMC | 8.68 | 98.6% b | Soaking in 0.05 M H2SO4 for 30 days [90] |
| PASA ABSA-TMC-multilayer IP | 12.4 | 95% b | Soaking in 20 wt% H2SO4 for 30 days [91] | |
| Mixed monomers | PASA PIP-TMC/BDSC | 2.2 3.8 e | 68% b 41% e | Soaking in 10 wt% H2SO4 at 55 °C for 24 h [73] |
| PASA MPD-TMC/BDSC | 1.4 7.4 e | 71% b 34% e | Soaking in 10 wt% H2SO4 at 55 °C for 24 h [73] | |
| PA-PSA-PE PIP/SA/SMF-TMC | 7 | 96% c | Soaking in 15 wt% H2SO4 for 30 days [92] | |
| PA-PU EDA-TMC/TDI | 5.1 | 98% c | Soaking in 15 wt% H2SO4 for 83 days [93] | |
| PA/PU-PASA/PU ABSA-TMC/PPDI-PEI r-IP | 2.6 | 93% a 97.7% b 97.7% c | Soaking in 10 wt% H2SO4 for 400 h [96] |
| IP Monomer | Regulation Method | Flux L·m−2·h−1·bar−1 | Rejection % | Acid Stability |
|---|---|---|---|---|
| PEI-BDSC | None | 3.2 | 90% a | Soaking in 20 wt% H2SO4 at 70 °C for 24 h [101] |
| Introducing small molecule amine monomer: PIP | 4.4 | 88% a | ||
| Introducing small molecule amine monomer: MPD | 4 | 89% a | ||
| PAH-BDSC | Introducing amine-rich polymer | 7.8 | 88.3% b | Soaking in 20.0% (wt/v) H2SO4 [99] |
| PIP-NTSC | Introducing multifunctional monomer: NTSC Introducing surfactants: SDS | 5.8 | 86.5% c | Soaking in 20% (wt/v) H2SO4 for 60 days or running 4.9% (wt/v) H2SO4 for 60 days [103] |
| BPEI-BDSC | Introducing cross-linking agents: DDS | 13.4 | 97.3% b | Soaking in 15 wt% H2SO4 for 720 h [106] |
| PEI-BDSC | Manufacture interlayer: COF TpPa | 43.3 | 49.5% b 92.7% RE3+ | Soaking in acidic (pH = 1) for 90 days [107] |
| DETA-NTSC | None | 0.73 | 91.8% c | Soaking in 8 wt% H2SO4 for 24 h [108] |
| Manufacture interlayer: SPEEK | 1.74 | 99.7% c | ||
| PAH-BDSC | None | 6 | 88.7% b | Soaking in 20% (wt/v) H2SO4 for 30 days [112] |
| Introducing COF into aqueous phase: NENP-1 | 15.1 | 93.3% b | ||
| PIP-TCSP | None | 1.5 | 92.9% d | Soaking in 20 wt% H2SO4 at 90 °C for 24 h [115] |
| Spinning-assist multilayer IP | 3.7 | 99.4% d |
| IP Monomer | Regulation Method | Flux L·m−2·h−1·bar−1 | Rejection % | Acid Stability |
|---|---|---|---|---|
| DETA-CC | None | 1.5 | 85.2% a | Soaking in acidic 0.1 M HNO3 for 30 days [69] |
| PEI-CC | None | 2.2 | 66% a | Soaking in acidic 0.1 M HNO3 for 30 days [69] |
| PEI-CC | None | 2.6 | 57.7% b | Soaking in 3 wt% HCl for 1800 h [120] |
| Low CC concentration and LC-CT method | 5.2 | 92.3% b 71.7% c | ||
| PEI-CC | None | 58.2 | 97.4% d | Soaking in (pH = 1) HCl for 30 days [123] |
| ILs regulated: AEMIC | 79.1 | 97.5% d | ||
| ILs regulated: AEMIT | 68.6 | 99.1% d | ||
| PEI-CC | None | 0.28 | 92.7% b | Soaking in 3 wt% HCl at 50 °C for 72 h [124] |
| LC-CT method and CA post-treatment | 2.3 | 97.6% b | ||
| PEI-CC | Introducing surfactants: SDS | 13.7 | 92.2% b | Soaking in 0.1 M HNO3 for 30 days [128] |
| BPEI-CC | Introducing IP catalytic: TPA | 12.8 | 94.7% b | Soaking in 25 wt% H2SO4 for 720 h [130] |
| BPEI-CC | None | 8.7 | 88.9% b | Soaking in 25 wt% H2SO4 for 720 h [131] |
| Introducing IP catalytic: TPA | 14.1 | 96.7% b |
| IP Monomer | Regulation Method | Flux L·m−2·h−1·bar−1 | Rejection % | Acid Stability |
|---|---|---|---|---|
| PEI-PDI | Introducing small molecule amine monomer: PIP | 0.65 2.63 b 4.22 c | 97.1% a 91.9% a,b 86.9% a,c | Soaking in 20% (wt/v) HCl or H2SO4 for 1 year [135] |
| PEI-TDI | Introducing small molecule amine monomer: PIP | 1 5.4 d | 97.1% a 42% a,d | Soaking in 20% (wt/v) HCl for 180 days, unstable [135] |
| PEI-TDI | None | 2 | 94% a | NO TEST [139] |
| Introducing nanosheets into aqueous phase: TA-MoS2 | 5.75 | 93.2% a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, W.; Zhang, X.; Guo, H.; Liang, Y. Recent Advanced Development of Acid-Resistant Thin-Film Composite Nanofiltration Membrane Preparation and Separation Performance in Acidic Environments. Separations 2023, 10, 20. https://doi.org/10.3390/separations10010020
Li M, Zhang W, Zhang X, Guo H, Liang Y. Recent Advanced Development of Acid-Resistant Thin-Film Composite Nanofiltration Membrane Preparation and Separation Performance in Acidic Environments. Separations. 2023; 10(1):20. https://doi.org/10.3390/separations10010020
Chicago/Turabian StyleLi, Mowen, Wenhai Zhang, Xuehong Zhang, Hongxia Guo, and Yucang Liang. 2023. "Recent Advanced Development of Acid-Resistant Thin-Film Composite Nanofiltration Membrane Preparation and Separation Performance in Acidic Environments" Separations 10, no. 1: 20. https://doi.org/10.3390/separations10010020