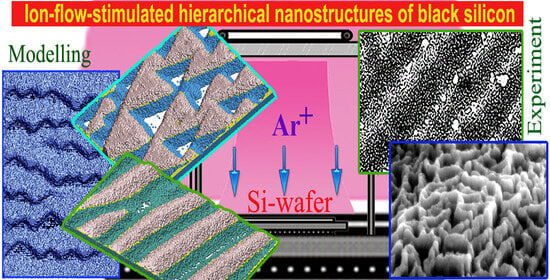
Hierarchical Structuring of Black Silicon Wafers by Ion-Flow-Stimulated Roughening Transition: Fundamentals and Applications for Photovoltaics
Abstract
:1. Introduction
2. Numerical Model
3. The Numerical Experiment Results and Their Analysis
4. Experimental Texturing of Silicon Wafers for Photovoltaics
5. Discussion of the Results
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisquert, J. The Physics of Solar Cells: Perovskites, Organics, and Photovoltaic Fundamentals; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1-351-39558-0. [Google Scholar]
- Chai, J.Y.-H.; Wong, B.T.; Juodkazis, S. Black-Silicon-Assisted Photovoltaic Cells for Better Conversion Efficiencies: A Review on Recent Research and Development Efforts. Mater. Today Energy 2020, 18, 100539. [Google Scholar] [CrossRef]
- Scheul, T.E.; Khorani, E.; Rahman, T.; Charlton, M.D.B.; Boden, S.A. Light Scattering from Black Silicon Surfaces and Its Benefits for Encapsulated Solar Cells. Sol. Energy Mater. Sol. Cells 2022, 235, 111448. [Google Scholar] [CrossRef]
- Liu, X.; Coxon, P.R.; Peters, M.; Hoex, B.; Cole, J.M.; Fray, D.J. Black Silicon: Fabrication Methods, Properties and Solar Energy Applications. Energy Environ. Sci. 2014, 7, 3223–3263. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, T.; Zhang, P.; Zhao, Y.; Li, S. Review Application of Nanostructured Black Silicon. Nanoscale Res. Lett. 2018, 13, 110. [Google Scholar] [CrossRef]
- Tan, Q.; Lu, F.; Xue, C.; Zhang, W.; Lin, L.; Xiong, J. Nano-Fabrication Methods and Novel Applications of Black Silicon. Sens. Actuators A Phys. 2019, 295, 560–573. [Google Scholar] [CrossRef]
- Rajab, F.H.; Whitehead, D.; Liu, Z.; Li, L. Characteristics of Hierarchical Micro/Nano Surface Structure Formation Generated by Picosecond Laser Processing in Water and Air. Appl. Phys. B 2017, 123, 282. [Google Scholar] [CrossRef]
- Chartier, C.; Bastide, S.; Lévy-Clément, C. Metal-Assisted Chemical Etching of Silicon in HF–H2O2. Electrochim. Acta 2008, 53, 5509–5516. [Google Scholar] [CrossRef]
- Romano, L.; Kagias, M.; Jefimovs, K.; Stampanoni, M. Self-Assembly Nanostructured Gold for High Aspect Ratio Silicon Microstructures by Metal Assisted Chemical Etching. RSC Adv. 2016, 6, 16025–16029. [Google Scholar] [CrossRef]
- Chen, K.; Pasanen, T.P.; Vahanissi, V.; Savin, H. Effect of MACE Parameters on Electrical and Optical Properties of ALD Passivated Black Silicon. IEEE J. Photovolt. 2019, 9, 974–979. [Google Scholar] [CrossRef]
- Bhushan, B. Biomimetics: Bioinspired Hierarchical-Structured Surfaces for Green Science and Technology; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 3-319-28284-0. [Google Scholar]
- Zhou, H.; Xiao, C.; Yang, Z.; Du, Y. 3D Structured Materials and Devices for Artificial Photosynthesis. Nanotechnology 2020, 31, 282001. [Google Scholar] [CrossRef] [PubMed]
- Cuenat, A.; George, H.B.; Chang, K.-C.; Blakely, J.M.; Aziz, M.J. Lateral Templating for Guided Self-Organization of Sputter Morphologies. Adv. Mater. 2005, 17, 2845–2849. [Google Scholar] [CrossRef]
- Frost, F.; Ziberi, B.; Höche, T.; Rauschenbach, B. The Shape and Ordering of Self-Organized Nanostructures by Ion Sputtering. Nucl. Instrum. Meth. B 2004, 216, 9–19. [Google Scholar] [CrossRef]
- Motta, F.C.; Shipman, P.D.; Bradley, R.M. Highly Ordered Nanoscale Surface Ripples Produced by Ion Bombardment of Binary Compounds. J. Phys. D Appl. Phys. 2012, 45, 122001. [Google Scholar] [CrossRef]
- Som, T.; Kanjilal, D. Nanofabrication by Ion-Beam Sputtering: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2012; ISBN 981-4303-75-5. [Google Scholar]
- Vorobyev, A.Y.; Guo, C. Direct Femtosecond Laser Surface Nano/Microstructuring and Its Applications: Direct Femtosecond Laser Surface Nano/Microstructuring and Its Applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Jo, S.; Jun, J.; Lee, E.; Yoon, S.M.; Seo, J.; Muñoz-García, J.; Cuerno, R.; Kim, J.-S. Order Improvement of Surface Nanopatterns via Substrate Rocking under Ion Bombardment: Experiments and Nonlinear Models. Phys. Rev. B 2020, 102, 045421. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.; Salahuddin, U.; Gao, P.-X. Hierarchical and Scalable Integration of Nanostructures for Energy and Environmental Applications: A Review of Processing, Devices, and Economic Analyses. Nano Futures 2020, 4, 012002. [Google Scholar] [CrossRef]
- Gabovich, A.M.; Semeniuk, V.F.; Semeniuk, N.I. Effect of Trampoline Sputtering on Surface Morphology and Coatings Properties. J. Phys. D Appl. Phys. 2021, 54, 255301. [Google Scholar] [CrossRef]
- Marks, M.R.; Cheong, K.Y.; Hassan, Z. A Review of Laser Ablation and Dicing of Si Wafers. Precis. Eng. 2022, 73, 377–408. [Google Scholar] [CrossRef]
- Brandao, E.; Nakhoul, A.; Duffner, S.; Emonet, R.; Garrelie, F.; Habrard, A.; Jacquenet, F.; Pigeon, F.; Sebban, M.; Colombier, J.-P. Learning Complexity to Guide Light-Induced Self-Organized Nanopatterns. Phys. Rev. Lett. 2023, 130, 226201. [Google Scholar] [CrossRef]
- Bradley, R.M.; Pearson, D.A. Nanoscale Pattern Formation Produced by Ion Bombardment of a Rotating Target: The Decisive Role of the Ion Energy. Phys. Rev. E 2023, 107, 014801. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.; Min, S.W.; Hammerick, K.E.; Vyakarnam, M.; Greco, R.S.; Prinz, F.B.; Fasching, R.J. The Construction of Three-Dimensional Micro-Fluidic Scaffolds of Biodegradable Polymers by Solvent Vapor Based Bonding of Micro-Molded Layers. Biomaterials 2007, 28, 1174–1184. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Chen, C.S. Tissue Engineering at the Micro-Scale. Biomed. Microdevices 1999, 2, 131–144. [Google Scholar] [CrossRef]
- Ferrie, S.; Darwish, N.; Gooding, J.J.; Ciampi, S. Harnessing Silicon Facet-Dependent Conductivity to Enhance the Direct-Current Produced by a Sliding Schottky Diode Triboelectric Nanogenerator. Nano Energy 2020, 78, 105210. [Google Scholar] [CrossRef]
- Borsoni, G.; Le Roux, V.; Laffitte, R.; Kerdilès, S.; Béchu, N.; Vallier, L.; Korwin-Pawlowski, M.L.; Vannuffel, C.; Bertin, F.; Vergnaud, C.; et al. Ultrathin SiO2 Layers Formation by Ultraslow Single- and Multicharged Ions. Solid-State Electron. 2002, 46, 1855–1862. [Google Scholar] [CrossRef]
- Ostrikov, K. Colloquium: Reactive Plasmas as a Versatile Nanofabrication Tool. Rev. Mod. Phys. 2005, 77, 489–511. [Google Scholar] [CrossRef]
- Chuang, A.T.H.; Boskovic, B.O.; Robertson, J. Freestanding Carbon Nanowalls by Microwave Plasma-Enhanced Chemical Vapour Deposition. Diam. Relat. Mater. 2006, 15, 1103–1106. [Google Scholar] [CrossRef]
- Anders, A. Metal Plasmas for the Fabrication of Nanostructures. J. Phys. D Appl. Phys. 2007, 40, 2272. [Google Scholar] [CrossRef]
- De Temmerman, G.; Bystrov, K.; Zielinski, J.J.; Balden, M.; Matern, G.; Arnas, C.; Marot, L. Nanostructuring of Molybdenum and Tungsten Surfaces by Low-Energy Helium Ions. J. Vac. Sci. Technol. A 2012, 30, 041306. [Google Scholar] [CrossRef]
- Randel, E.; Bradley, R.M.; Menoni, C.S. Deposition of Conformal Thin Film Coatings on Sawtooth Substrates Using Ion Bombardment. J. Appl. Phys. 2021, 130, 125303. [Google Scholar] [CrossRef]
- Li, J.; Yang, G.; Bradley, R.M.; Liu, Y.; Frost, F.; Hong, Y. Enhancing the Quality of Self-Organized Nanoripples by Ar-Ion Bombardment of a Bilayer System. Nanotechnology 2021, 32, 385301. [Google Scholar] [CrossRef]
- Gentile, P.; Cuoco, M.; Volkov, O.M.; Ying, Z.-J.; Vera-Marun, I.J.; Makarov, D.; Ortix, C. Electronic Materials with Nanoscale Curved Geometries. Nat. Electron. 2022, 5, 551–563. [Google Scholar] [CrossRef]
- Zheng, H.; Zhai, T.; Yu, M.; Xie, S.; Liang, C.; Zhao, W.; Wang, S.C.I.; Zhang, Z.; Lu, X. TiO2@C Core–Shell Nanowires for High-Performance and Flexible Solid-State Supercapacitors. J. Mater. Chem. C 2013, 1, 225–229. [Google Scholar] [CrossRef]
- Jo, J.; Choi, J.; Lee, S.; Song, C.; Noh, H.; Jang, J. Mass Fabrication of 3D Silicon Nano-/Microstructures by Fab-Free Process Using Tip-Based Lithography. Small 2021, 17, 2005036. [Google Scholar] [CrossRef]
- Duarte, M.; Lasagni, A.; Giovanelli, R.; Narciso, J.; Louis, E.; Mücklich, F. Increasing Lubricant Film Lifetime by Grooving Periodical Patterns Using Laser Interference Metallurgy. Adv. Eng. Mater. 2008, 10, 554–558. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Lee, Y.K.; Ge, Y.; Ren, S.; Roth, J.E.; Kamins, T.I.; Miller, D.A.B.; Harris, J.S. Strong Quantum-Confined Stark Effect in Germanium Quantum-Well Structures on Silicon. Nature 2005, 437, 1334–1336. [Google Scholar] [CrossRef] [PubMed]
- Ostrikov, K.; Neyts, E.C.; Meyyappan, M. Plasma Nanoscience: From Nano-Solids in Plasmas to Nano-Plasmas in Solids. Adv. Phys. 2013, 62, 113–224. [Google Scholar] [CrossRef]
- Pai, Y.-Y.; Tylan-Tyler, A.; Irvin, P.; Levy, J. Physics of SrTiO3 -Based Heterostructures and Nanostructures: A Review. Rep. Prog. Phys. 2018, 81, 036503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, P.; Qi, G.; Zhang, Z.M.; Cheng, P. An Experimental Study of a Nearly Perfect Absorber Made from a Natural Hyperbolic Material for Harvesting Solar Energy. J. Appl. Phys. 2020, 127, 233102. [Google Scholar] [CrossRef]
- Yin, H.; Xing, K.; Zhang, Y.; Dissanayake, D.M.A.S.; Lu, Z.; Zhao, H.; Zeng, Z.; Yun, J.-H.; Qi, D.-C.; Yin, Z. Periodic Nanostructures: Preparation, Properties and Applications. Chem. Soc. Rev. 2021, 50, 6423–6482. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, N.; Schroers, J. Nanofabrication through Molding. Prog. Mater. Sci. 2022, 125, 100891. [Google Scholar] [CrossRef]
- Hussain, I.; Lamiel, C.; Sahoo, S.; Ahmad, M.; Chen, X.; Javed, M.S.; Qin, N.; Gu, S.; Li, Y.; Nawaz, T.; et al. Factors Affecting the Growth Formation of Nanostructures and Their Impact on Electrode Materials: A Systematic Review. Mater. Today Phys. 2022, 27, 100844. [Google Scholar] [CrossRef]
- Keilmann, F. Laser-Driven Corrugation Instability of Liquid Metal Surfaces. Phys. Rev. Lett. 1983, 51, 2097–2100. [Google Scholar] [CrossRef]
- Young, J.F.; Sipe, J.E.; Van Driel, H.M. Laser-Induced Periodic Surface Structure. III. Fluence Regimes, the Role of Feedback, and Details of the Induced Topography in Germanium. Phys. Rev. B 1984, 30, 2001–2015. [Google Scholar] [CrossRef]
- Tsibidis, G.D.; Skoulas, E.; Papadopoulos, A.; Stratakis, E. Convection Roll-Driven Generation of Supra-Wavelength Periodic Surface Structures on Dielectrics upon Irradiation with Femtosecond Pulsed Lasers. Phys. Rev. B 2016, 94, 081305. [Google Scholar] [CrossRef]
- Rudenko, A.; Abou-Saleh, A.; Pigeon, F.; Mauclair, C.; Garrelie, F.; Stoian, R.; Colombier, J.P. High-Frequency Periodic Patterns Driven by Non-Radiative Fields Coupled with Marangoni Convection Instabilities on Laser-Excited Metal Surfaces. Acta Mater. 2020, 194, 93–105. [Google Scholar] [CrossRef]
- Ivanov, D.S.; Kuznetsov, A.I.; Lipp, V.P.; Rethfeld, B.; Chichkov, B.N.; Garcia, M.E.; Schulz, W. Short Laser Pulse Nanostructuring of Metals: Direct Comparison of Molecular Dynamics Modeling and Experiment. Appl. Phys. A 2013, 111, 675–687. [Google Scholar] [CrossRef]
- Ubbelohde, A.R. Melting and Crystal Structure. Q. Rev. Chem. Soc. 1950, 4, 356. [Google Scholar] [CrossRef]
- Frenkel, J. A General Theory of Heterophase Fluctuations and Pretransition Phenomena. J. Chem. Phys. 1939, 7, 538–547. [Google Scholar] [CrossRef]
- Frenkel, J. Kinetic Theory of Liquids; Peter Smith Publisher, Incorporated: Gloucester, MA, USA, 1984; ISBN 978-0-8446-2094-7. [Google Scholar]
- Rauber, M.; Muench, F.; Toimil-Molares, M.E.; Ensinger, W. Thermal Stability of Electrodeposited Platinum Nanowires and Morphological Transformations at Elevated Temperatures. Nanotechnology 2012, 23, 475710. [Google Scholar] [CrossRef]
- Li, H.; Biser, J.M.; Perkins, J.T.; Dutta, S.; Vinci, R.P.; Chan, H.M. Thermal Stability of Cu Nanowires on a Sapphire Substrate. J. Appl. Phys. 2008, 103, 024315. [Google Scholar] [CrossRef]
- Granberg, F.; Parviainen, S.; Djurabekova, F.; Nordlund, K. Investigation of the Thermal Stability of Cu Nanowires Using Atomistic Simulations. J. Appl. Phys. 2014, 115, 213518. [Google Scholar] [CrossRef]
- Naik, J.P.; Das, K.; Prewett, P.D.; Raychaudhuri, A.K.; Chen, Y. Liquid-like Instabilities in Gold Nanowires Fabricated by Focused Ion Beam Lithography. Appl. Phys. Lett. 2012, 101, 163108. [Google Scholar] [CrossRef]
- Volk, A.; Knez, D.; Thaler, P.; Hauser, A.W.; Grogger, W.; Hofer, F.; Ernst, W.E. Thermal Instabilities and Rayleigh Breakup of Ultrathin Silver Nanowires Grown in Helium Nanodroplets. Phys. Chem. Chem. Phys. 2015, 17, 24570–24575. [Google Scholar] [CrossRef] [PubMed]
- Plateau, J. Experimental and Theoretical Statics of Liquids Subject to Molecular Forces Only; GAUTHIER-VILLARS, TRÜBNER and Co: London, UK, 1873; Volume 1, ISBN 978-0-428-50082-5. [Google Scholar]
- Rayleigh, F.R.S.L. On the Instability of Jets. Proc. Lond. Math. Soc. 1878, s1-10, 4–13. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Kassubek, F.; Stafford, C.A. Surface Fluctuations and the Stability of Metal Nanowires. Phys. Rev. B 2003, 68, 165414. [Google Scholar] [CrossRef]
- Nichols, F.A.; Mullins, W.W. Surface-(interface-) and volume-diffusion contributions to morphological changes driven by capillarity. Trans. Metall. Soc. AIME 1965, 233, 1840–1848. [Google Scholar]
- Gorshkov, V.N.; Tereshchuk, V.V.; Sareh, P. Diversity of Anisotropy Effects in the Breakup of Metallic FCC Nanowires into Ordered Nanodroplet Chains. CrystEngComm 2020, 22, 2601–2611. [Google Scholar] [CrossRef]
- Gorshkov, V.N.; Sareh, P.; Tereshchuk, V.V.; Soleiman-Fallah, A. Dynamics of Anisotropic Break-Up in Nanowires of FCC Lattice Structure. Adv. Theory Simul. 2019, 2, 1900118. [Google Scholar] [CrossRef]
- Karim, S.; Toimil-Molares, M.E.; Balogh, A.G.; Ensinger, W.; Cornelius, T.W.; Khan, E.U.; Neumann, R. Morphological Evolution of Au Nanowires Controlled by Rayleigh Instability. Nanotechnology 2006, 17, 5954. [Google Scholar] [CrossRef]
- Karim, S.; Toimil-Molares, M.E.; Ensinger, W.; Balogh, A.G.; Cornelius, T.W.; Khan, E.U.; Neumann, R. Influence of Crystallinity on the Rayleigh Instability of Gold Nanowires. J. Phys. D Appl. Phys. 2007, 40, 3767–3770. [Google Scholar] [CrossRef]
- Vigonski, S.; Jansson, V.; Vlassov, S.; Polyakov, B.; Baibuz, E.; Oras, S.; Aabloo, A.; Djurabekova, F.; Zadin, V. Au Nanowire Junction Breakup through Surface Atom Diffusion. Nanotechnology 2017, 29, 015704. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Xu, M.; Zhao, Y.; Wang, J.; Jiang, X.; Yu, L.; Wang, J.; Xu, J.; Shi, Y.; Chen, K.; et al. Engineering Island-Chain Silicon Nanowires via a Droplet Mediated Plateau-Rayleigh Transformation. Nat. Commun. 2016, 7, 12836. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, V.N.; Tereshchuk, V.V.; Sareh, P. Restructuring and Breakup of Nanowires with the Diamond Cubic Crystal Structure into Nanoparticles. Mater. Today Commun. 2020, 22, 100727. [Google Scholar] [CrossRef]
- Claudin, P.; Durán, O.; Andreotti, B. Dissolution Instability and Roughening Transition. J. Fluid Mech. 2017, 832, R2. [Google Scholar] [CrossRef]
- Maxson, J.B.; Savage, D.E.; Liu, F.; Tromp, R.M.; Reuter, M.C.; Lagally, M.G. Thermal Roughening of a Thin Film: A New Type of Roughening Transition. Phys. Rev. Lett. 2000, 85, 2152–2155. [Google Scholar] [CrossRef]
- Andersen, M.; Ghoniem, N.M. Surface Roughening Mechanisms for Tungsten Exposed to Laser, Ion, and X-Ray Pulses. Fusion Sci. Technol. 2007, 52, 579–583. [Google Scholar] [CrossRef]
- Scott, J.F.; Hayward, S.A.; Miyake, M. High Temperature Phase Transitions in Barium Sodium Niobate: The Wall Roughening 1q-2q Incommensurate Transition and Mean Field Tricritical Behaviour in a Disordered Exclusion Model. J. Phys. Condens. Matter 2005, 17, 5911–5926. [Google Scholar] [CrossRef]
- Heyraud, J.C.; Métois, J.J.; Bermond, J.M. The Roughening Transition of the Si113 and Si110 Surfaces—An in Situ, Real Time Observation. Surf. Sci. 1999, 425, 48–56. [Google Scholar] [CrossRef]
- Suzuki, T.; Minoda, H.; Tanishiro, Y.; Yagi, K. REM Studies of the Roughening Transitions of Si High Index Surfaces. Thin Solid Films 1999, 343–344, 423–426. [Google Scholar] [CrossRef]
- Dashti-Naserabadi, H.; Saberi, A.A.; Rouhani, S. Roughening Transition and Universality of Single Step Growth Models in (2+1)-Dimensions. New J. Phys. 2017, 19, 063035. [Google Scholar] [CrossRef]
- Xu, S.; Li, P.; Lu, Y. In Situ Atomic-Scale Analysis of Rayleigh Instability in Ultrathin Gold Nanowires. Nano Res. 2018, 11, 625–632. [Google Scholar] [CrossRef]
- Dai, H.; Yu, X.; Zhao, Z.; Shi, D.; Shi, X.; Zhao, J.; Dong, X.; Zhang, D. Low Temperature RF-Plasma Initiated Rapid and Highly Ordered Fracture on Ag Nanowires. Appl. Sci. 2020, 10, 1338. [Google Scholar] [CrossRef]
- Gorshkov, V.N.; Tereshchuk, V.V.; Sareh, P. Roughening transition as a driving factor in the formation of self-ordered one-dimensional nanostructures. CrystEngComm 2021, 23, 1836–1848. [Google Scholar] [CrossRef]
- Gorshkov, V.N.; Tereshchuk, V.V.; Bereznykov, O.V.; Boiger, G.K.; Fallah, A.S. Dynamics of Quasi-One-Dimensional Structures under Roughening Transition Stimulated by External Irradiation. Nanomaterials 2022, 12, 1411. [Google Scholar] [CrossRef]
- Misbah, C.; Pierre-Louis, O.; Saito, Y. Crystal Surfaces in and out of Equilibrium: A Modern View. Rev. Mod. Phys. 2010, 82, 981–1040. [Google Scholar] [CrossRef]
- Sigmund, P. Particle Penetration and Radiation Effects: General Aspects and Stopping of Swift Point Charges; Spinger Series in Solid-State Science; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2008; ISBN 978-3-540-72622-7. [Google Scholar]
- Mott, N.F. The Cohesive Forces in Metals and Alloys. Rep. Prog. Phys. 1962, 25, 218–243. [Google Scholar] [CrossRef]
- Inglesfield, J.E. Surface Electronic Structure. Rep. Prog. Phys. 1982, 45, 223–284. [Google Scholar] [CrossRef]
- Srivastava, G.P.; Weaire, D. The Theory of the Cohesive Energies of Solids. Adv. Phys. 1987, 36, 463–517. [Google Scholar] [CrossRef]
- Sigmund, P. Theory of Sputtering. I. Sputtering Yield of Amorphous and Polycrystalline Targets. Phys. Rev. 1969, 184, 383–416. [Google Scholar] [CrossRef]
- Chan, W.L.; Chason, E. Making Waves: Kinetic Processes Controlling Surface Evolution during Low Energy Ion Sputtering. J. Appl. Phys. 2007, 101, 121301. [Google Scholar] [CrossRef]
- Muñoz-García, J.; Vázquez, L.; Castro, M.; Gago, R.; Redondo-Cubero, A.; Moreno-Barrado, A.; Cuerno, R. Self-Organized Nanopatterning of Silicon Surfaces by Ion Beam Sputtering. Mater. Sci. Eng. R Rep. 2014, 86, 1–44. [Google Scholar] [CrossRef]
- Frost, F.; Schindler, A.; Bigl, F. Roughness Evolution of Ion Sputtered Rotating InP Surfaces: Pattern Formation and Scaling Laws. Phys. Rev. Lett. 2000, 85, 4116–4119. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.R.; Sinha, O.P.; Krok, F.; Zembok, T.; Pedrys, R.; Szymonski, M. Temperature-Dependent Surface Modification of InSb(001) Crystal by Low-Energy Ion Bombardment. Nucl. Instrum. Meth. B 2009, 267, 2752–2756. [Google Scholar] [CrossRef]
- Gabovich, A.M.; Semeniuk, V.F.; Semeniuk, N.I. New Collective Trampoline Mechanism of Accelerated Ion-Plasma Sputtering. J. Phys. D Appl. Phys. 2019, 52, 185201. [Google Scholar] [CrossRef]
- Gorshkov, V.; Tereshchuk, V.; Sareh, P. Heterogeneous and Homogeneous Nucleation in the Synthesis of Quasi-One-Dimensional Periodic Core–Shell Nanostructures. Cryst. Growth Des. 2021, 21, 1604–1616. [Google Scholar] [CrossRef]
- Day, R.W.; Mankin, M.N.; Gao, R.; No, Y.-S.; Kim, S.-K.; Bell, D.C.; Park, H.-G.; Lieber, C.M. Plateau–Rayleigh Crystal Growth of Periodic Shells on One-Dimensional Substrates. Nat. Nanotechnol. 2015, 10, 345–352. [Google Scholar] [CrossRef]
- Day, R.W.; Mankin, M.N.; Lieber, C.M. Plateau–Rayleigh Crystal Growth of Nanowire Heterostructures: Strain-Modified Surface Chemistry and Morphological Control in One, Two, and Three Dimensions. Nano Lett. 2016, 16, 2830–2836. [Google Scholar] [CrossRef]
- Gorshkov, V.; Privman, V. Kinetic Monte Carlo Model of Breakup of Nanowires into Chains of Nanoparticles. J. Appl. Phys. 2017, 122, 204301. [Google Scholar] [CrossRef]
- Privman, V.; Gorshkov, V.; Zavalov, O. Formation of Nanoclusters and Nanopillars in Nonequilibrium Surface Growth for Catalysis Applications: Growth by Diffusional Transport of Matter in Solution Synthesis. Heat Mass Transf. 2014, 50, 383–392. [Google Scholar] [CrossRef]
- Gorshkov, V.; Zavalov, O.; Atanassov, P.B.; Privman, V. Morphology of Nanoclusters and Nanopillars Formed in Nonequilibrium Surface Growth for Catalysis Applications. Langmuir 2011, 27, 8554–8561. [Google Scholar] [CrossRef]
- Gorshkov, V.; Zavalov, A.; Privman, V. Shape Selection in Diffusive Growth of Colloids and Nanoparticles. Langmuir 2009, 25, 7940–7953. [Google Scholar] [CrossRef]
- Gorshkov, V.; Privman, V. Models of Synthesis of Uniform Colloids and Nanocrystals. Phys. E Low Dimens. Syst. Nanostruct. 2010, 43, 1–12. [Google Scholar] [CrossRef]
- Privman, V.; Gorshkov, V.; Yaish, Y.E. Kinetics Modeling of Nanoparticle Growth on and Evaporation off Nanotubes. J. Appl. Phys. 2017, 121, 014301. [Google Scholar] [CrossRef]
- Gorshkov, V.; Kuzmenko, V.; Privman, V. Nonequilibrium Kinetic Modeling of Sintering of a Layer of Dispersed Nanocrystals. CrystEngComm 2014, 16, 10395–10409. [Google Scholar] [CrossRef]
- Gorshkov, V.; Kuzmenko, V.; Privman, V. Modeling of Growth Morphology of Core–Shell Nanoparticles. J. Phys. Chem. C 2014, 118, 24959–24966. [Google Scholar] [CrossRef]
- Manning, J.R. Diffusion Kinetics for Atoms in Crystals; Van Nostrand: Princeton, NJ, USA, 1968; ISBN 978-0-608-10295-5. [Google Scholar]
- Tran, R.; Xu, Z.; Radhakrishnan, B.; Winston, D.; Sun, W.; Persson, K.A.; Ong, S.P. Surface Energies of Elemental Crystals. Sci. Data 2016, 3, 160080. [Google Scholar] [CrossRef] [PubMed]
- Eaglesham, D.J.; White, A.E.; Feldman, L.C.; Moriya, N.; Jacobson, D.C. Equilibrium Shape of Si. Phys. Rev. Lett. 1993, 70, 1643–1646. [Google Scholar] [CrossRef]
- Kuang, Y.; Vece, M.D.; Rath, J.K.; Dijk, L.V.; Schropp, R.E.I. Elongated Nanostructures for Radial Junction Solar Cells. Rep. Prog. Phys. 2013, 76, 106502. [Google Scholar] [CrossRef]
- Voorhees, P.W. The Theory of Ostwald Ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef]
- Shikida, M.; Tokoro, K.; Uchikawa, D.; Sato, K. Surface Morphology of Anisotropically Etched Single-Crystal Silicon. J. Micromech. Microeng. 2000, 10, 522. [Google Scholar] [CrossRef]
- Lee, S.; Liang, C.-W.; Martin, L.W. Synthesis, Control, and Characterization of Surface Properties of Cu2O Nanostructures. ACS Nano 2011, 5, 3736–3743. [Google Scholar] [CrossRef]
- Nishimori, H.; Ouchi, N. Formation of Ripple Patterns and Dunes by Wind-Blown Sand. Phys. Rev. Lett. 1993, 71, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Pye, K.; Tsoar, H. Aeolian Sand and Sand Dunes; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-85909-3. [Google Scholar]
- Singh, P.; Srivastava, S.K.; Prajapati, V.; Sivaiah, B.; Rauthan, C.M.S.; Singh, P.K. Low Reflecting Hierarchically Textured Silicon by Silver Assisted Chemical Etching for Potential Solar Cell Application. Mater. Today Proc. 2018, 5, 23258–23267. [Google Scholar] [CrossRef]
- Macdonald, D.H.; Cuevas, A.; Kerr, M.J.; Samundsett, C.; Ruby, D.; Winderbaum, S.; Leo, A. Texturing Industrial Multicrystalline Silicon Solar Cells. Sol. Energy 2004, 76, 277–283. [Google Scholar] [CrossRef]
- Schrödinger, E. What Is Life? The Physical Aspect of the Living Cell; with, Mind and Matter; & Autobiographical Sketches; Canto Classics; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1992; ISBN 978-1-107-60466-7. [Google Scholar]
- Schiffmann, Y. The Non-Equilibrium Basis of Turing Instability and Localised Biological Work. Prog. Biophys. Mol. Biol. 2017, 127, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Brillouin, L. Maxwell’s Demon Cannot Operate: Information and Entropy. I. J. Appl. Phys. 1951, 22, 334–337. [Google Scholar] [CrossRef]
- Kondepudi, D.; Prigogine, I. Modern Thermodynamics: From Heat Engines to Dissipative Structures, 2nd ed.; John Wiley & Sons Inc.: Chichester, UK, 2015; ISBN 978-1-118-69870-9. [Google Scholar]
- Wilson, J.A. Entropy, Not Negentropy. Nature 1968, 219, 535–536. [Google Scholar] [CrossRef]
- Olby, R. Schrödinger’s Problem: What Is Life? J. Hist. Biol. 1971, 4, 119–148. [Google Scholar] [CrossRef]
- Ulanowicz, R.E.; Hannon, B.M. Life and the Production of Entropy. Proc. R. Soc. Lond. B. 1987, 232, 181–192. [Google Scholar] [CrossRef]
- Stephens, G.L.; O’Brien, D.M. Entropy and Climate. I: ERBE Observations of the Entropy Production of the Earth. Q. J. R. Meteorol. Soc. 1993, 119, 121–152. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Y. Radiation Entropy Flux and Entropy Production of the Earth System. Rev. Geophys. 2010, 48, RG2003. [Google Scholar] [CrossRef]
- Brunsell, N.A.; Schymanski, S.J.; Kleidon, A. Quantifying the Thermodynamic Entropy Budget of the Land Surface: Is This Useful? Earth Syst. Dynam. 2011, 2, 87–103. [Google Scholar] [CrossRef]
- Ozawa, H.; Ohmura, A.; Lorenz, R.D.; Pujol, T. The Second Law of Thermodynamics and the Global Climate System: A Review of the Maximum Entropy Production Principle: Thermodynamics of climate. Rev. Geophys. 2003, 41, 4. [Google Scholar] [CrossRef]
- Lorenz, R. Full Steam Ahead--Probably. Science 2003, 299, 837–838. [Google Scholar] [CrossRef]
- Dewar, R.C. 4 Maximum Entropy Production and Non-Equilibrium Statistical Mechanics. In Non-Equilibrium Thermodynamics and the Production of Entropy: Life, Earth, and Beyond; Kleidon, A., Lorenz, R.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 41–55. ISBN 978-3-540-32359-4. [Google Scholar]
- Martyushev, L.M.; Seleznev, V.D. Maximum Entropy Production Principle in Physics, Chemistry and Biology. Phys. Rep. 2006, 426, 1–45. [Google Scholar] [CrossRef]
- Priolo, F.; Gregorkiewicz, T.; Galli, M.; Krauss, T.F. Silicon Nanostructures for Photonics and Photovoltaics. Nat. Nanotechnol. 2014, 9, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, P.T.; Baruch, P. The Thermodynamics of the Conversion of Radiation Energy for Photovoltaics. J. Phys. A Math. Gen. 1989, 22, 1911–1926. [Google Scholar] [CrossRef]
- Gnilitskyi, I.; Derrien, T.J.-Y.; Levy, Y.; Bulgakova, N.M.; Mocek, T.; Orazi, L. High-Speed Manufacturing of Highly Regular Femtosecond Laser-Induced Periodic Surface Structures: Physical Origin of Regularity. Sci. Rep. 2017, 7, 8485. [Google Scholar] [CrossRef]
- Florian, C.; Kirner, S.V.; Krüger, J.; Bonse, J. Surface Functionalization by Laser-Induced Periodic Surface Structures. J. Laser Appl. 2020, 32, 022063. [Google Scholar] [CrossRef]
- Bonse, J.; Krüger, J.; Höhm, S.; Rosenfeld, A. Femtosecond Laser-Induced Periodic Surface Structures. J. Laser Appl. 2012, 24, 042006. [Google Scholar] [CrossRef]
- He, S.; Nivas, J.J.; Anoop, K.K.; Vecchione, A.; Hu, M.; Bruzzese, R.; Amoruso, S. Surface Structures Induced by Ultrashort Laser Pulses: Formation Mechanisms of Ripples and Grooves. Appl. Surf. Sci. 2015, 353, 1214–1222. [Google Scholar] [CrossRef]
- He, S.; Nivas, J.J.; Vecchione, A.; Hu, M.; Amoruso, S. On the Generation of Grooves on Crystalline Silicon Irradiated by Femtosecond Laser Pulses. Opt. Express 2016, 24, 3238. [Google Scholar] [CrossRef] [PubMed]
- Vorobyev, A.Y.; Guo, C. Femtosecond Laser-Induced Periodic Surface Structure Formation on Tungsten. J. Appl. Phys. 2008, 104, 063523. [Google Scholar] [CrossRef]
- Sipe, J.E.; Young, J.F.; Preston, J.S.; Van Driel, H.M. Laser-Induced Periodic Surface Structure. I. Theory. Phys. Rev. B 1983, 27, 1141–1154. [Google Scholar] [CrossRef]
- Bonse, J.; Gräf, S. Ten Open Questions about Laser-Induced Periodic Surface Structures. Nanomaterials 2021, 11, 3326. [Google Scholar] [CrossRef]
- Kafle, B.; Schön, J.; Fleischmann, C.; Werner, S.; Wolf, A.; Clochard, L.; Duffy, E.; Hofmann, M.; Rentsch, J. On the Emitter Formation in Nanotextured Silicon Solar Cells to Achieve Improved Electrical Performances. Sol. Energy Mater. Sol. Cells 2016, 152, 94–102. [Google Scholar] [CrossRef]
- Huo, C.; Wang, J.; Fu, H.; Li, X.; Yang, Y.; Wang, H.; Mateen, A.; Farid, G.; Peng, K. Metal-Assisted Chemical Etching of Silicon in Oxidizing HF Solutions: Origin, Mechanism, Development, and Black Silicon Solar Cell Application. Adv. Funct. Mater. 2020, 30, 2005744. [Google Scholar] [CrossRef]
- Fan, Z.; Cui, D.; Zhang, Z.; Zhao, Z.; Chen, H.; Fan, Y.; Li, P.; Zhang, Z.; Xue, C.; Yan, S. Recent Progress of Black Silicon: From Fabrications to Applications. Nanomaterials 2020, 11, 41. [Google Scholar] [CrossRef]
- Ghosh, D.K.; Bose, S.; Das, G.; Acharyya, S.; Nandi, A.; Mukhopadhyay, S.; Sengupta, A. Fundamentals, Present Status and Future Perspective of TOPCon Solar Cells: A Comprehensive Review. Surf. Interf. 2022, 30, 101917. [Google Scholar] [CrossRef]
- Jansen, H.V.; De Boer, M.J.; Unnikrishnan, S.; Louwerse, M.C.; Elwenspoek, M.C. Black Silicon Method: X. A Review on High Speed and Selective Plasma Etching of Silicon with Profile Control: An in-Depth Comparison between Bosch and Cryostat DRIE Processes as a Roadmap to next Generation Equipment. J. Micromech. Microeng. 2009, 19, 033001. [Google Scholar] [CrossRef]
- Gabovich, A.M.; Gudymenko, O.Y.; Kladko, V.P.; Lytvyn, P.M.; Nasieka, I.M.; Romaniuk, B.M.; Semeniuk, V.F.; Semeniuk, N.I.; Strelchuk, V.V.; Styopkin, V.I.; et al. Nanosized structure formation by trampoline ion-plasma sputtering. Nanosist. Nanomater. Nanotehnol. 2020, 18, 357–372. [Google Scholar] [CrossRef]
- Nenow, D. Surface Premelting. Prog. Cryst. Growth Charact. 1984, 9, 185–225. [Google Scholar] [CrossRef]
- Li, Y.; Somorjai, G.A. Surface Premelting of Ice. J. Phys. Chem. C 2007, 111, 9631–9637. [Google Scholar] [CrossRef]
- Slater, B.; Michaelides, A. Surface Premelting of Water Ice. Nat. Rev. Chem. 2019, 3, 172–188. [Google Scholar] [CrossRef]
- Llombart, P.; Noya, E.G.; Sibley, D.N.; Archer, A.J.; MacDowell, L.G. Rounded Layering Transitions on the Surface of Ice. Phys. Rev. Lett. 2020, 124, 065702. [Google Scholar] [CrossRef]
- Cui, S.; Chen, H. Transition Dynamics and Metastable States during Premelting and Freezing of Ice Surfaces. Phys. Rev. B 2023, 108, 045413. [Google Scholar] [CrossRef]
- Sun, C.Q.; Huang, Y.; Zhang, X.; Ma, Z.; Wang, B. The Physics behind Water Irregularity. Phys. Rep. 2023, 998, 1–68. [Google Scholar] [CrossRef]
- Faraday, M.I. Note on Regelation. Proc. R. Soc. Lond. 1860, 10, 440–450. [Google Scholar] [CrossRef]
- Sun, Z.; Pan, D.; Xu, L.; Wang, E. Role of Proton Ordering in Adsorption Preference of Polar Molecule on Ice Surface. Proc. Natl. Acad. Sci. USA 2012, 109, 13177–13181. [Google Scholar] [CrossRef]
- Shultz, M.J. Ice Surfaces. Annu. Rev. Phys. Chem. 2017, 68, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Pickering, I.; Paleico, M.; Sirkin, Y.A.P.; Scherlis, D.A.; Factorovich, M.H. Grand Canonical Investigation of the Quasi Liquid Layer of Ice: Is It Liquid? J. Phys. Chem. B 2018, 122, 4880–4890. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, H.; Landman, U. Superheating, Melting, and Annealing of Copper Surfaces. Phys. Rev. Lett. 1993, 71, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Landa, A.; Wynblatt, P.; Häkkinen, H.; Barnett, R.N.; Landman, U. Equilibrium Interphase Interfaces and Premelting of the Pb(110) Surface. Phys. Rev. B 1995, 51, 10972–10980. [Google Scholar] [CrossRef]
- Chen, E.T.; Barnett, R.N.; Landman, U. Surface Melting of Ni(110). Phys. Rev. B 1990, 41, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Kochev, V.D.; Seidov, S.S.; Grigoriev, P.D. On the Size of Superconducting Islands on the Density-Wave Background in Organic Metals. Magnetochemistry 2023, 9, 173. [Google Scholar] [CrossRef]
- Kagan, M.Y.; Aksenov, S.V.; Turlapov, A.V.; Ikhsanov, R.S.; Kugel, K.I.; Mazur, E.A.; Kuznetsov, E.A.; Silkin, V.M.; Burovski, E.A. Formation of Droplets of the Order Parameter and Superconductivity in Inhomogeneous Fermi–Bose Mixtures (Brief Review). JETP Lett. 2023, 117, 755–764. [Google Scholar] [CrossRef]
- Gabovich, A.M.; Voitenko, A.I. Influence of Order-Parameter Nonhomogeneities on Low-Temperature Properties of Superconductors. Phys. Rev. B 1999, 60, 7465–7472. [Google Scholar] [CrossRef]
- Phillips, J.C.; Saxena, A.; Bishop, A.R. Pseudogaps, Dopants, and Strong Disorder in Cuprate High-Temperature Superconductors. Rep. Prog. Phys. 2003, 66, 2111–2182. [Google Scholar] [CrossRef]
- Chen, Q.; Pugno, N.M. Bio-Mimetic Mechanisms of Natural Hierarchical Materials: A Review. J. Mech. Behav. Biomed. Mater. 2013, 19, 3–33. [Google Scholar] [CrossRef]
- Toor, F.; Branz, H.M.; Page, M.R.; Jones, K.M.; Yuan, H.-C. Multi-scale surface texture to improve blue response of nanoporous black silicon solar cells. Appl. Phys. Lett. 2011, 99, 103501. [Google Scholar] [CrossRef]
- Nguyen, K.N.; Abi-Saab, D.; Basset, P.; Richalot, E.; Malak, M.; Pavy, N.; Flourens, F.; Marty, F.; Angelescu, D.; Leprince-Wang, Y.; et al. Study of black silicon obtained by cryogenic plasma etching: Approach to achieve the hot spot of a thermoelectric energy harvester. Microsyst. Technol. 2012, 18, 1807–1814. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorshkov, V.N.; Stretovych, M.O.; Semeniuk, V.F.; Kruglenko, M.P.; Semeniuk, N.I.; Styopkin, V.I.; Gabovich, A.M.; Boiger, G.K. Hierarchical Structuring of Black Silicon Wafers by Ion-Flow-Stimulated Roughening Transition: Fundamentals and Applications for Photovoltaics. Nanomaterials 2023, 13, 2715. https://doi.org/10.3390/nano13192715
Gorshkov VN, Stretovych MO, Semeniuk VF, Kruglenko MP, Semeniuk NI, Styopkin VI, Gabovich AM, Boiger GK. Hierarchical Structuring of Black Silicon Wafers by Ion-Flow-Stimulated Roughening Transition: Fundamentals and Applications for Photovoltaics. Nanomaterials. 2023; 13(19):2715. https://doi.org/10.3390/nano13192715
Chicago/Turabian StyleGorshkov, Vyacheslav N., Mykola O. Stretovych, Valerii F. Semeniuk, Mikhail P. Kruglenko, Nadiia I. Semeniuk, Victor I. Styopkin, Alexander M. Gabovich, and Gernot K. Boiger. 2023. "Hierarchical Structuring of Black Silicon Wafers by Ion-Flow-Stimulated Roughening Transition: Fundamentals and Applications for Photovoltaics" Nanomaterials 13, no. 19: 2715. https://doi.org/10.3390/nano13192715