Porous Hexagonal Boron Nitride as Solid-Phase Microextraction Coating Material for Extraction and Preconcentration of Polycyclic Aromatic Hydrocarbons from Soil Sample
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Instruments
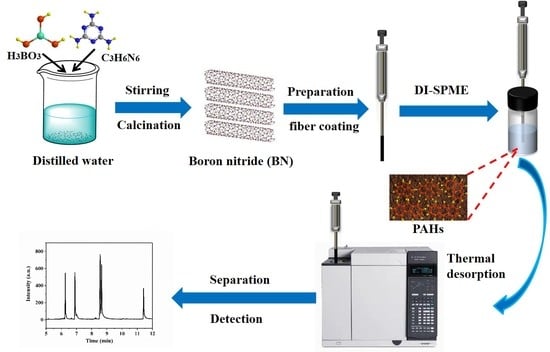
2.3. Synthesis of h-BN Nanorods
2.4. Preparation of Sol-SiO2
2.5. Preparation of h-BN Coated Fiber and Stability Test
2.6. Standard Solution and Real Sample Preparation
2.7. SPME Procedures
2.8. Protocols for Method Validation
3. Results and Discussion
3.1. Characterization of h-BN
3.2. Optimization of SPME Experimental Conditions
3.2.1. Extraction Temperature
3.2.2. Extraction Time
3.2.3. Desorption Temperature
3.2.4. Desorption Time
3.2.5. Agitation Speed
3.2.6. Ionic Strength
3.3. Method Evaluation
3.4. Enrichment Factor and Extraction Mechanism
3.5. Analysis of Real Soil Samples
3.6. Comparison with Other Fiber Coating Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, C.; Qu, L.; Wu, L.; Wu, X.; Sun, R.; Li, Y. Advances in analysis of nitrated polycyclic aromatic hydrocarbons in various matrices. TrAC-Trend. Anal. Chem. 2020, 127, 115878. [Google Scholar] [CrossRef]
- Kariyawasam, T.; Doran, G.S.; Howitt, J.A.; Prenzler, P.D. Polycyclic aromatic hydrocarbon contamination in soils and sediments: Sustainable approaches for extraction and remediation. Chemosphere 2021, 291, 132981. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, J.; Zhang, X.; Han, L.; Qin, P.; Tian, S.; Zhou, Q.; Zhang, X.; Lu, M. Preparation of Al-doped mesoporous crystalline material-41 as fiber coating material for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons from human urine. J. Chromatogr. A 2020, 1626, 461354. [Google Scholar] [CrossRef] [PubMed]
- Ncube, S.; Madikizela, L.; Cukrowska, E.; Chimuka, L. Recent advances in the adsorbents for isolation of polycyclic aromatic hydrocarbons (PAHs) from environmental sample solutions. TrAC-Trend. Anal. Chem. 2018, 99, 101–116. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Ihsanullah, I. Novel materials for dispersive (micro) solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples: A review. Anal. Chim. Acta 2021, 1141, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Kou, H.; Wang, X.; Zhou, Z.; Du, X.; Lu, X. Porous cage-like hollow magnetic carbon-doped CoO nanocomposite as an advanced sorbent for magnetic solid-phase extraction of nine polycyclic aromatic hydrocarbons. Talanta 2021, 227, 122149. [Google Scholar] [CrossRef]
- Feng, J.; Feng, J.; Loussala, H.M.; Han, S.; Ji, X.; Li, C.; Sun, H.; Sun, M. Dendritic mesoporous silica nanospheres@ porous carbon for in-tube solid-phase microextraction to detect polycyclic aromatic hydrocarbons in tea beverages. Food Chem. 2021, 364, 130379. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Yang, J.; Zhou, Z.; Du, X.; Lu, X. Rapid synthesis of graphite phase carbon nitride/zeolitic imidazolate framework-8 with hierarchical structure and its superior adsorption of polycyclic aromatic hydrocarbons from aqueous solution. J. Chromatogr. A 2021, 1659, 462639. [Google Scholar] [CrossRef]
- Reddy, A.V.B.; Moniruzzaman, M.; Aminabhavi, T.M. Polychlorinated biphenyls (PCBs) in the environment: Recent updates on sampling, pretreatment, cleanup technologies and their analysis. Chem. Eng. J. 2019, 358, 1186–1207. [Google Scholar] [CrossRef]
- Dimpe, K.M.; Nomngongo, P.N. Current sample preparation methodologies for analysis of emerging pollutants in different environmental matrices. TrAC-Trend. Anal. Chem. 2016, 82, 199–207. [Google Scholar] [CrossRef]
- Olasupo, A.; Suah, F.B.M. Trends in hollow fiber liquid phase microextraction for the preconcentration of pharmaceutically active compounds in aqueous solution: A case for polymer inclusion membrane. J. Hazard. Mater. 2022, 431, 128573. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, R.; Li, Y.; Sun, C. Sample preparation and analytical methods for polycyclic aromatic hydrocarbons in sediment. Trends Environ. Anal. Chem. 2019, 24, e00074. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Kim, K.H.; Shamsipur, M.; Deep, A.; Hong, J. Recent advances in liquid-phase microextraction techniques for the analysis of environmental pollutants. TrAC-Trend. Anal. Chem. 2017, 97, 83–95. [Google Scholar] [CrossRef]
- Gao, M.; Wang, D.; Deng, L.; Liu, S.; Zhang, K.; Quan, T.; Gao, D. High-Crystallinity Covalent Organic Framework Synthesized in Deep Eutectic Solvent: Potentially Effective Adsorbents Alternative to Macroporous Resin for Flavonoids. Chem. Mater. 2021, 33, 8036–8051. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, X.; Qin, P.; Yang, Y.; Tian, S.; Yang, H.; Lu, M. Simultaneous determination of melatonin, l-tryptophan, and two l-tryptophan-derived esters in food by HPLC with graphene oxide/SiO2 nanocomposite as the adsorbent. Food Anal. Methods 2018, 11, 2438–2446. [Google Scholar] [CrossRef]
- Han, L.; Liu, X.; Zhang, X.; Li, M.; Li, D.; Qin, P.; Tian, S.; Lu, M.; Cai, Z. Preparation of multivariate zirconia metal-organic frameworks for highly efficient adsorption of endocrine disrupting compounds. J. Hazard. Mater. 2022, 424, 127559. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Qin, P.; Han, L.; Zhu, W.; Duan, S.; Lu, M.; Cai, Z. Facile preparation of nano-g-C3N4/UiO-66-NH2 composite as sorbent for high-efficient extraction and preconcentration of food colorants prior to HPLC analysis. Chin. Chem. Lett. 2022, 33, 903–906. [Google Scholar] [CrossRef]
- Mashile, G.P.; Mpupa, A.; Nomngongo, P.N. Magnetic mesoporous carbon/β-cyclodextrin–chitosan nanocomposite for extraction and preconcentration of multi-class emerging contaminant residues in environmental samples. Nanomaterials 2021, 11, 540. [Google Scholar] [CrossRef]
- Qiao, L.Z.; Yu, C.M.; Sun, R.T. Preparation of Amino-functionalized Guanidinium Ionic Liquid-Modified Magnetic Materials and Application in Solid-Phase Extraction of Pollutants in Water. J. Anal. Test. 2021, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Li, W.; Yang, Y.; Qin, P.; Zhang, X.; Lu, M. Magnetic graphene oxide nanocomposites as the adsorbent for extraction and pre-concentration of azo dyes in different food samples followed by high-performance liquid chromatography analysis. Food Addit. Contam. A 2018, 35, 2099–2110. [Google Scholar] [CrossRef]
- Liu, X.; Song, R.; Wei, R. Rapid Determination of Vitamin D3 in Aquatic Products by Polypyrrole-Coated Magnetic Nanoparticles Extraction Coupled with High-Performance Liquid Chromatography Detection. Nanomaterials 2022, 12, 1226. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Han, L.; Zhang, X.; Li, M.; Li, D.; Lu, M.; Cai, Z. MIL-101 (Fe)-derived magnetic porous carbon as sorbent for stir bar sorptive-dispersive microextraction of sulfonamides. Microchim. Acta 2021, 188, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiao, Z.; Wang, W.; Zhang, S.; Wu, Q.; Wang, C.; Wang, Z. Rational integration of porous organic polymer and multiwall carbon nanotube for the microextraction of polycyclic aromatic hydrocarbons. Microchim. Acta 2020, 187, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qin, P.; Han, L.; Zhang, X.; Li, D.; Li, M.; Wang, Y.; Zhang, X.; Lu, M.; Cai, Z. Gas-cycle-assisted headspace solid-phase microextraction coupled with gas chromatography for rapid analysis of organic pollutants. Chem. Commun. 2021, 57, 8810–8813. [Google Scholar] [CrossRef]
- Xu, S.; Liu, H.; Long, A.; Li, H.; Chen, C.; Feng, S.; Fan, J. Carbon Dot-Decorated Graphite Carbon Nitride Composites for Enhanced Solid-Phase Microextraction of Chlorobenzenes from Water. Nanomaterials 2022, 12, 335. [Google Scholar] [CrossRef]
- Zhang, N.; Lei, X.; Huang, T.; Su, L.; Zhang, L.; Xie, Z.; Wu, X. Guanidyl-functionalized polyhedral oligomeric silsesquioxane porous hybrid polymer coating for specific solid phase microextraction of phthalate esters in foodstuff. Chem. Eng. J. 2020, 386, 124003. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Chen, L.; Wang, S.; Xu, J.; Zhu, F.; Cui, S.; Zheng, J.; Ouyang, G. MOF-74/polystyrene-derived Ni-doped hierarchical porous carbon for structure-oriented extraction of polycyclic aromatic hydrocarbons and their metabolites from human biofluids. J. Hazard. Mater. 2022, 424, 127465. [Google Scholar] [CrossRef]
- Rickert, D.A.; Singh, V.; Thirukumaran, M.; Grandy, J.J.; Belinato, J.R.; Lashgari, M.; Pawliszyn, J. Comprehensive analysis of multiresidue pesticides from process water obtained from wastewater treatment facilities using solid-phase microextraction. Environ. Sci. Technol. 2022, 54, 15789–15799. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Zhu, W.; Qin, P.; Lu, M.; Zhang, X.; Miao, Y.; Cai, Z. Mesoporous graphitic carbon nitride@ NiCo2O4 nanocomposite as a solid phase microextraction coating for sensitive determination of environmental pollutants in human serum samples. Chem. Commun. 2019, 55, 10019–10022. [Google Scholar] [CrossRef]
- Grandy, J.J.; Galpin, V.; Singh, V.; Pawliszyn, J. Development of a drone-based thin-film solid-phase microextraction water sampler to facilitate on-site screening of environmental pollutants. Anal. Chem. 2020, 92, 12917–12924. [Google Scholar] [CrossRef]
- Sun, M.; Feng, J.; Han, S.; Ji, X.; Li, C.; Feng, J.; Sun, H.; Fan, J. Poly (ionic liquid)-hybridized silica aerogel for solid-phase microextraction of polycyclic aromatic hydrocarbons prior to gas chromatography-flame ionization detection. Microchim. Acta 2021, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, D.; Li, M.; Qin, P.; Zhu, S.; Gao, Y.; Mu, M.; Zhang, N.; Wang, Y.; Lu, M. Flower-like Co3O4/C3N5 composite as solid-phase microextraction coating for high-efficiency adsorption and preconcentration of polycyclic aromatic hydrocarbons and polychlorinated biphenyls in water. Chem. Eng. J. 2022, 443, 136293. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, P.; Zhang, J.; Li, W.; Zhu, J.; Lu, M.; Cai, Z. Fabrication of nanoscale graphitic carbon nitride/copper oxide hybrid composites coated solid-phase microextraction fibers coupled with gas chromatography for determination of polycyclic aromatic hydrocarbons. J. Chromatogr. A 2018, 1570, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Huang, Y.; Ouyang, S.; Huang, J.; Shi, Y.; Tong, Y.J.; Zhao, X.; Li, E.; Zheng, J.; Zheng, J.; et al. Efficient solid phase microextraction of organic pollutants based on graphene oxide/chitosan aerogel. Anal. Chim. Acta 2022, 1195, 339462. [Google Scholar] [CrossRef]
- Feng, Z.; Huang, C.; Guo, Y.; Liu, W.; Zhang, L. Graphitic carbon nitride derivative with large mesopores as sorbent for solid-phase microextraction of polycyclic aromatic hydrocarbons. Talanta 2020, 209, 120541. [Google Scholar] [CrossRef]
- Kong, J.; Zhu, F.; Huang, W.; He, H.; Hu, J.; Sun, C.; Xian, Q.; Yang, S. Sol–gel based metal-organic framework zeolite imidazolate framework-8 fibers for solid-phase microextraction of nitro polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbons in water samples. J. Chromatogr. A 2019, 1603, 92–101. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, Y.; Zhang, W.; Ma, W.; Wang, J.; Chen, H.; Ding, Q.; Zhang, L. Rapidly covalent immobilization of β-ketoenamine-linked covalent organic framework on fibers for efficient solid-phase microextraction of phthalic acid esters. Talanta 2022, 243, 123380. [Google Scholar] [CrossRef]
- Wang, S.; Xin, Y.; Yuan, J.; Wang, L.; Zhang, W. Direct conversion of methane to methanol on boron nitride-supported copper single atoms. Nanoscale 2022, 14, 5547. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, Q.; Li, Y.; Pang, J. Cu-Doped Boron Nitride Nanosheets for Solid-Phase Extraction and Determination of Rhodamine B in Foods Matrix. Nanomaterials 2022, 12, 318. [Google Scholar] [CrossRef]
- Cao, X.M.; Zhou, H.; Zhao, L.; Chen, X.; Hu, P. Screening performance of methane activation over atomically dispersed metal catalysts on defective boron nitride monolayers: A density functional theory study. Chin. Chem. Lett. 2021, 32, 1972–1976. [Google Scholar] [CrossRef]
- García Doménech, N.; Purcell-Milton, F.; Sanz Arjona, A.; Casasín García, M.L.; Ward, M.; Cabré, M.B.; Rafferty, A.; Mckelvey, K.; Dunne, P.; Gun’ko, Y.K. High-Performance Boron Nitride-Based Membranes for Water Purification. Nanomaterials 2022, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Sathish, C.I.; Kothandam, G.; Selvarajan, P.; Lei, Z.; Lee, J.; Qu, J.; Al-Muhtaseb, A.; Yu, X.; Breese, M.; Zheng, R.; et al. Ordered Mesoporous Boron Carbon Nitrides with Tunable Mesopore Nanoarchitectonics for Energy Storage and CO2 Adsorption Properties. Adv. Sci. 2022, 2105603. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yu, Z.; Chou, K.C. Facile synthesis of hexagonal boron nitride fibers with uniform morphology. Ceram. Int. 2013, 39, 6427–6431. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, P.; Zhang, X.; Niu, J.; Tian, S.; Lu, M.; Zhu, J.; Cai, Z. Layer-by-layer fabrication of g-C3N4 coating for headspace solid-phase microextraction of food additives followed by gas chromatography-flame ionization detection. Anal. Methods 2018, 10, 322–329. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J.; Lin, J.; Wang, Q.; Yu, C.; Fang, Y.; Huang, Y. Synthesis of Nanostructured Boron Nitride Aerogels by Rapid Pyrolysis of Melamine Diborate Aerogels via Induction Heating: From Composition Adjustment to Property Studies. ACS Appl. Nano Mater. 2021, 4, 13788–13797. [Google Scholar] [CrossRef]
- Lin, J.; Yuan, X.; Li, G.; Huang, Y.; Wang, W.; He, X.; Tang, C. Self-assembly of porous boron nitride microfibers into ultralight multifunctional foams of large sizes. ACS Appl. Mater. Interfaces 2017, 9, 44732–44739. [Google Scholar] [CrossRef]
- Lin, L.X.; Zheng, Y.; Zheng, Y.; Wei, K.M. Facile synthesis of hexagonal boron nitride fibers and flowers. Mater. Lett. 2007, 61, 1735–1737. [Google Scholar] [CrossRef]
- Chen, Y.; Gerald, J.F.; Williams, J.S.; Bulcock, S. Synthesis of boron nitride nanotubes at low temperatures using reactive ball milling. Chem. Phys. Lett. 1999, 299, 260–264. [Google Scholar] [CrossRef]
- Nazarov, A.S.; Demin, V.N.; Grayfer, E.D.; Bulavchenko, A.I.; Arymbaeva, A.T.; Shin, H.J.; Choi, J.Y.; Fedorov, V.E. Functionalization and Dispersion of Hexagonal Boron Nitride (h-BN) Nanosheets Treated with Inorganic Reagents. Chem. Asian J. 2012, 7, 554–560. [Google Scholar] [CrossRef]
- Sachdev, H.; Müller, F.; Hüfner, S. BN analogues of graphene: On the formation mechanism of boronitrene layers—Solids with extreme structural anisotropy. Diam. Rela. Mater. 2010, 19, 1027–1033. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Liu, X.; Jiang, S. A novel multiwalled carbon nanotubes bonded fused-silica fiber for solid phase microextraction–gas chromatographic analysis of phenols in water samples. Talanta 2009, 78, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Abolghasemi, M.M.; Fattahpour, P. A hexagonally ordered nanoporous silica-based fiber coating for SPME of polycyclic aromatic hydrocarbons from water followed by GC–MS. Chromatographia 2011, 74, 807–815. [Google Scholar] [CrossRef]
- Fagan, S.B.; Souza Filho, A.G.; Lima, J.O.G.; Filho, J.M.; Ferreira, O.P.; Mazali, I.O.; Dresselhaus, M.S. 1,2-Dichlorobenzene interacting with carbon nanotubes. Nano Lett. 2004, 4, 1285–1288. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Carbon nanotubes as superior sorbent for dioxin removal. J. Am. Chem. Soc. 2001, 123, 2058–2059. [Google Scholar] [CrossRef] [PubMed]
- Doong, R.A.; Chang, S.M. Determination of distribution coefficients of priority polycyclic aromatic hydrocarbons using solid-phase microextraction. Anal. Chem. 2000, 72, 3647–3652. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.B.; Fan, Y.H.; Mou, X.X.; Li, X.S.; Qi, S.H. Preparation of phenyl-modified magnetic silica as a selective magnetic solid-phase extraction adsorbent for polycyclic aromatic hydrocarbons in soils. J. Chromatogr. A 2018, 1568, 29–37. [Google Scholar] [CrossRef]
- Xu, S.; Li, H.; Wu, H.; Xiao, L.; Dong, P.; Feng, S.; Fan, J. A facile cooling-assisted solid-phase microextraction device for solvent-free sampling of polycyclic aromatic hydrocarbons from soil based on matrix solid-phase dispersion technique. Anal. Chim. Acta 2020, 1115, 7–15. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, M.; Zhang, G.; Zang, X.; Li, H.; Zhang, S.; Wang, Z. Carbon nanospheres as solid-phase microextraction coating for the extraction of polycyclic aromatic hydrocarbons from water and soil samples. J. Sep. Sci. 2020, 43, 2594–2601. [Google Scholar] [CrossRef]
- Li, H.; Hou, B.; Wang, L.; Zang, X.; Wang, C.; Wang, Z. Boron nitride modified reduced graphene oxide as solid-phase microextraction coating material for the extraction of seven polycyclic aromatic hydrocarbons from water and soil samples. J. Sep. Sci. 2021, 44, 1521–1528. [Google Scholar] [CrossRef]
- Ma, X.; Huang, P.; Dang, X.; Ai, Y.; Zheng, D.; Chen, H. MWCNTs/MnO2 nanocomposite-based polythiophene coating for solid-phase microextraction and determination of polycyclic aromatic hydrocarbons in soil. Microchem. J. 2019, 146, 1026–1032. [Google Scholar] [CrossRef]





| Analyte | Linear Range (ng mL−1) | R2 | LOQs (ng mL−1/ng g−1) a | LODs (ng mL−1/ng g−1) | RSD% (n = 3) | ||
|---|---|---|---|---|---|---|---|
| Intraday | Interday | Fiber-to-Fiber | |||||
| ACE | 0.1–50 | 0.9911 | 0.10/2.00 | 0.033/0.66 | 5.94 | 10.40 | 6.14 |
| FLU | 0.02–50 | 0.9910 | 0.02/0.40 | 0.004/0.08 | 3.36 | 10.07 | 7.46 |
| PHE | 0.02–50 | 0.9938 | 0.02/0.40 | 0.004/0.08 | 6.39 | 11.81 | 8.53 |
| ANC | 0.02–50 | 0.9925 | 0.02/0.40 | 0.004/0.08 | 3.44 | 4.58 | 5.63 |
| PYR | 0.05–50 | 0.9921 | 0.05/1.00 | 0.014/0.28 | 6.24 | 7.06 | 8.82 |
| Analyte | Molecular Formula | Structure | logKow | EFs |
|---|---|---|---|---|
| ACE | C12H10 |  | 3.92 | 3000 |
| FLU | C13H10 |  | 4.18 | 3278 |
| PHE | C14H10 |  | 4.46 | 4327 |
| ANC | C14H10 |  | 4.45 | 4398 |
| PYR | C16H10 |  | 4.88 | 1526 |
| Analyte | Campus Soil | Zhengkai Avenue Soil | ||||
|---|---|---|---|---|---|---|
| Found (ng g−1) | Added (ng g−1) | Recovery, % (RSD, %) | Found (ng g−1) | Added (ng g−1) | Recovery, % (RSD, %) | |
| ACE | N.D. | 10 | 120.0 (2.6) | N.D. | 10 | 116.0 (5.9) |
| 100 | 86.0 (2.5) | 100 | 80.0 (3.0) | |||
| 400 | 81.2 (0.4) | 400 | 92.0 (2.0) | |||
| FLU | 16.2 | 10 | 80.0 (5.5) | 15.2 | 10 | 80.0 (4.0) |
| 100 | 80.0 (3.4) | 100 | 89.8 (5.6) | |||
| 400 | 113.4 (3.1) | 400 | 97.6 (9.8) | |||
| PHE | 12.0 | 10 | 83.7 (4.7) | 12.2 | 10 | 80.0 (2.5) |
| 100 | 80.0 (3.5) | 100 | 80.2 (1.7) | |||
| 400 | 92.0 (6.5) | 400 | 89.4 (7.9) | |||
| ANC | 6.4 | 10 | 81.4 (2.4) | 5.6 | 10 | 82.8 (4.0) |
| 100 | 103.0 (5.1) | 100 | 80.0 (0.6) | |||
| 400 | 84.9 (1.9) | 400 | 78.0 (4.0) | |||
| PYR | 255.6 | 10 | 80.1 (5.8) | 251.0 | 10 | 79.9 (0.5) |
| 100 | 86.8 (5.8) | 100 | 94.3 (1.9) | |||
| 400 | 89.0 (0.1) | 400 | 82.7 (2.8) | |||
| Sorbents | Extraction Methods | Detection Techniques | Linearity Range (ng g−1) | LODs (ng g−1) | Thermal Stability (°C) | Refs. |
|---|---|---|---|---|---|---|
| Fe3O4@mSiO2-Ph-PTSA a | MSPE | GC-MS | 5–500 | 0.07–0.41 | N.P. b | [56] |
| PDMS | SPME | GC-MS | 40–4000 | 4.2–8.5 | N.P. | [57] |
| Carbon nanospheres | SPME | GC-FID | 6.0–2700 | 1.53–2.70 | 350 | [58] |
| BN@rGO | SPME | GC-FID | 1.0–400 | 0.3–0.5 | 400 | [59] |
| MWCNTs/MnO2/PEDOT c | SPME | GC-FID | 0.5–250 | 0.1–0.8 | 300 | [60] |
| h-BN | SPME | GC-FID | 0.4–1000 | 0.08–0.66 | 800 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, M.; Zhu, S.; Gao, Y.; Mu, M.; Zhang, N.; Wang, Y.; Lu, M. Porous Hexagonal Boron Nitride as Solid-Phase Microextraction Coating Material for Extraction and Preconcentration of Polycyclic Aromatic Hydrocarbons from Soil Sample. Nanomaterials 2022, 12, 1860. https://doi.org/10.3390/nano12111860
Li D, Li M, Zhu S, Gao Y, Mu M, Zhang N, Wang Y, Lu M. Porous Hexagonal Boron Nitride as Solid-Phase Microextraction Coating Material for Extraction and Preconcentration of Polycyclic Aromatic Hydrocarbons from Soil Sample. Nanomaterials. 2022; 12(11):1860. https://doi.org/10.3390/nano12111860
Chicago/Turabian StyleLi, Dan, Mengyuan Li, Shiping Zhu, Yanmei Gao, Mengyao Mu, Ning Zhang, Youmei Wang, and Minghua Lu. 2022. "Porous Hexagonal Boron Nitride as Solid-Phase Microextraction Coating Material for Extraction and Preconcentration of Polycyclic Aromatic Hydrocarbons from Soil Sample" Nanomaterials 12, no. 11: 1860. https://doi.org/10.3390/nano12111860