Core-Shell Structured Carbon@Al2O3 Membrane with Enhanced Acid Resistance for Acid Solution Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
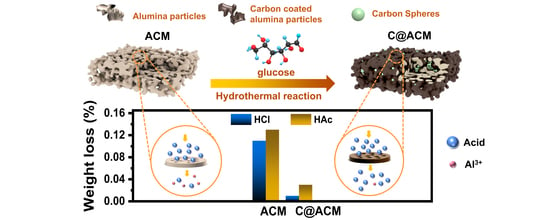
2.2. Fabrication of C@ACM
2.3. Characterization
2.4. Acid Resistance Test
2.5. Adsorption during the Filtration Process and Model Fitting
2.5.1. Adsorption Kinetics
2.5.2. Adsorption Isotherm
2.6. Membrane Performance
2.6.1. Penetration of Acid Components
2.6.2. Performance of the Acid Extract Solution
2.7. Coating Stability Evaluation and Recyclability Verification
3. Results
3.1. Characterization of ACM and C@ACM
3.2. Acid Resistance Properties
3.3. Adsorption Kinetics and Thermodynamics Fitting
3.4. Membrane Performance
3.4.1. Penetration of Acid Components
3.4.2. Performance of Acidic Extract Solutions
3.5. Durability Test and Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ojalvo, C.; Fuentes, M.J.; Zhang, W.J.; Guiberteau, F.; Candelario, V.M.; Ortiz, A.L. Fabrication of B4C ultrafiltration membranes on SiC supports. J. Eur. Ceram. Soc. 2022, 42, 3118–3126. [Google Scholar] [CrossRef]
- Alftessi, S.A.; Othman, M.H.D.; Adam, M.R.; Farag, T.M.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Habib, M.A.; Raji, Y.O.; Hubadillah, S.K. Novel silica sand hollow fiber ceramic membrane for oily wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 104975. [Google Scholar] [CrossRef]
- Mao, X.; Zhao, L.; Zhang, K.; Wang, Y.Y.; Ding, B. Highly flexible ceramic nanofibrous membranes for superior thermal insulation and fire retardancy. Nano Res. 2022, 15, 2592–2598. [Google Scholar] [CrossRef]
- Liu, F.; Yao, H.; Sun, S.B.; Tao, W.J.; Wei, T.; Sun, P.Z. Photo-fenton activation mechanism and antifouling performance of an FeOCl-coated ceramic membrane. Chem. Eng. J. 2020, 402, 125477. [Google Scholar] [CrossRef]
- Gu, Q.L.; Albert Ng, T.C.; Zhang, L.; Lyn, Z.Y.; Zhang, Z.X.; Yong, H.; Wang, J. Interfacial diffusion assisted chemical deposition (ID-CD) for confined surface modification of alumina microfiltration membranes toward high flux and anti-fouling. Sep. Purif. Technol. 2020, 325, 116177. [Google Scholar] [CrossRef]
- Liu, W.; Yang, G.; Huang, M.P.; Liang, J.W.; Zeng, B.B.; Fu, C.; Wu, H.D. Ultrarobust and biomimetic hierarchically macroporous ceramic membrane for oil-water separation templated by emulsion-assisted self-assembly method. ACS Appl. Mater. Inter. 2020, 12, 35555–35562. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z.H. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Zhong, W.W.; Zheng, D.Y.; Zou, H.H.; Zhuo, Z.W.; Guo, L.W. A novel approach for evaluating the concentrations of Indicative components in liquid and solid in the pharmaceutical process of TCM manufacturing using membrane-based clarification--example given in the water extracts of eucommiae folium. Chin. Tradit. Herb. Drugs. 2021, 52, 3234–3238. [Google Scholar] [CrossRef]
- Li, C.; Lu, Z.D.; Ao, X.W.; Sun, W.J.; Huang, X. Degradation kinetics and removal efficiencies of pharmaceuticals by photocatalytic ceramic membranes using ultraviolet light-emitting diodes. Chem. Eng. J. 2022, 427, 130828. [Google Scholar] [CrossRef]
- Aloulou, W.; Aloulou, H.; Attia, A.; Chakraborty, S.; Amar, R.B. Treatment of tuna cooking juice via ceramic ultrafiltration membrane: Optimization using response surface methodology. Membranes 2022, 12, 813. [Google Scholar] [CrossRef]
- Mo, J.H.; Li, X.H.; Yang, Z.F. Dissecting the structure-property relationship of ceramic membrane with asymmetric multilayer structures for maximizing permselectivity. Water Res. 2022, 220, 118658. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Chen, X.F.; Drioli, E.; Qiu, M.H.; Fan, Y.Q. Facile mixing process to fabricate fly ash-enhanced alumina-based membrane supports for industrial microfiltration applications. Ind. Eng. Chem. Res. 2019, 58, 8712–8723. [Google Scholar] [CrossRef]
- Hang, Y.T.; Liu, G.P.; Huang, K.; Jin, W.Q. Mechanical properties and interfacial adhesion of composite membranes probed by in-situ nano-indentation/scratch technique. J. Mem. Sci. 2015, 494, 205–215. [Google Scholar] [CrossRef]
- Lu, M.; Hu, M.Z. Novel porous ceramic tube-supported polymer layer membranes for acetic acid/water separation by pervaporation dewatering. Sep. Purif. Technol. 2020, 236, 116312. [Google Scholar] [CrossRef]
- Bukhari, S.Z.A.; Ha, J.H.; Lee, J.M.; Song, I.H. Fabrication and optimization of a clay-bonded SiC flat tubular membrane support for microfiltration applications. Ceram. Int. 2017, 43, 7736–7742. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Azarova, T.A.; Agabekov, V.E.; Azarov, S.M.; Batsukg, C.; Batsuren, D.; Prozorovich, V.G.; Pat’to, A.A. Effect of phase composition of natural quartz raw material on characterization of microfiltration ceramic membranes. Ceram. Int. 2016, 42, 16571–16578. [Google Scholar] [CrossRef]
- Hoffman, R.; Pippardt, U.; Kriegel, R. Impact of sintering temperature on permeation and long-term development of support structure and stability for asymmetric oxygen transporting BSCF membranes. J. Mem. Sci. 2019, 581, 270–282. [Google Scholar] [CrossRef]
- Guo, H.L.; Zhao, S.F.; Wu, X.X.; Qi, H. Fabrication and characterization of TiO2/ZrO2 ceramic membranes for nanofiltration. Micropor. Mesopor. Mat. 2016, 260, 125–131. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Han, W.; Liu, K.Q.; Ao, W.Q.; Si, K.K. Influence of bonding phases on properties of in-situ bonded porous SiC membrane supports. Ceramics International. Ceram. Int. 2019, 46, 8536–8542. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Kadir, S.H.S.A.L.; Sokri, M.N.M.; Tai, Z.S.; Iwamoto, Y.; Tanemura, M.; Honda, S.; Puteh, M.H.; Rahman, M.A. Influence of the Natural Zeolite Particle Size Toward the Ammonia Adsorption Activity in Ceramic Hollow Fiber Membrane. Membranes 2020, 10, 63. [Google Scholar] [CrossRef]
- Gu, Q.L.; Albert Ng, T.C.; Bao, Y.P.; Yong, H.; Tan, S.C.; Wang, J. Developing better ceramic membranes for water and wastewater Treatment: Where microtructure integrates with chemistry and functionalities. Chem. Eng. J. 2020, 428, 130456. [Google Scholar] [CrossRef]
- Chu, K.H.; Fathizadeh, M.; Yu, M.; Flora, J.R.V.; Jang, A.; Jang, M.; Park, C.M.; Yoo, S.S.; Her, N.; Yoon, Y. Evaluation of Removal Mechanisms in a Graphene Oxide-Coated Ceramic Ultrafiltration Membrane for Retention of Natural Organic Matter, Pharmaceuticals, and Inorganic Salts. ACS Appl. Mater. Inter. 2017, 9, 40369–40377. [Google Scholar] [CrossRef] [PubMed]
- Karan, S.; Jiang, Z.W.; Livingston, A.G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science. 2015, 348, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, A.; Tay, S.W.; Hong, L. Zeolite Composite Membranes with a Nanoporous Fluorinated Carbonaceous Sheath for Organic Solvent Filtration. ACS Appl. Nano Mater. 2021, 4, 2783–2794. [Google Scholar] [CrossRef]
- Justino, N.M.; Vicentini, D.S.; Ranjbari, K.; Bellier, M.; Nogueira, D.J.; Matias, W.G.; Perreault, F. Nanoparticle-templated polyamide membranes for improved biofouling resistance. Environ. Sci-Nano 2021, 8, 565–579. [Google Scholar] [CrossRef]
- Chee, D.N.A.; Ismail, A.F.; Aziz, F.; Amin, M.A.M.; Abdullah, N. The influence of alumina particle size on the properties and performance of alumina hollow fiber as support membrane for protein separation. Sep. Purif. Technol. 2020, 250, 117147. [Google Scholar] [CrossRef]
- Zhou, M.; Nabavi, M.S.; Hedlund, J. Influence of support surface roughness on zeolite membrane quality. Micropor. Mesopor. Mat. 2020, 308, 110546. [Google Scholar] [CrossRef]
- Araya, M.V.; Oeltze, H.; Radeva, J.; Roth, A.G.; Gobbert, C.; Pahl, R.N.; Dahne, L.; Wiese, J. Operation of Hybrid Membranes for the Removal of Pharmaceuticals and Pollutants from Water and Wastewater. Membranes 2022, 12, 502. [Google Scholar] [CrossRef]
- Hammad, I.; Dornier, M.; Lebrun, M.; Maraval, I.; Poucheret, P.; Mayer, C.D. Impact of crossflow microfiltration on aroma and sensory profiles of a potential functional citrus-based food. J. Sci. Food Agr. 2022, 102, 5768–5777. [Google Scholar] [CrossRef]
- Alawy, R.M.J.A.; Abod, B.M.; Kamar, F.H.; Nechifor, A.C. Removal of Dyes from Wastewater by Ceramic Membrane. Rev. Chim. Bucharest. 2019, 70, 1715–1719. [Google Scholar] [CrossRef]
- Changmai, M.; Mondal, P.; Sinha, A.P.B.; Biswas, P.; Sarkar, S.; Purkait, M.K. Metal removal efficiency of novel LD-slag-incorporated ceramic membrane from steel plant wastewater. Int. J. Environ. An. Ch. 2022, 102, 1078–1094. [Google Scholar] [CrossRef]
- Bian, W.J.; Wang, B.M.; Tang, W.; Zhou, M.; Jin, C.R.; Ding, H.P.; Fan, W.W.; Dong, Y.H.; Li, J.; Ding, D. Revitalizing interface in protonic ceramic cells by acid etch. Nature 2022, 604, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Lin, H.P.; Fan, Z.L.; Ma, L.; Shao, Q.; Ji, Y.J.; Zeng, F.F.; Geng, S.Z.; Yang, S.Z.; Kong, N.N.; et al. Iridium metallene oxide for acidic oxygen evolution catalysis. Nat. Commun. 2021, 12, 6007. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Du, B.; He, C.; Zeng, S.H.; Hua, K.H.; Xi, X.; Luo, B.Y.; Shui, A.Z.; Tian, W. Corrosion Resistance Properties of Porous Alumina-Mullite Ceramic Membrane Supports. Adv. Eng. Mater. 2022, 22, 1901442. [Google Scholar] [CrossRef]
- Ferdowsi, S.; Salem, A.; Salem, S. Spectrophotometrical analysis for fabrication of pH-independent nano-sized gamma-alumina by dealumination of kaolin and precipitation in the presence of surfactant composites. Spectrochim. Acta. A. 2019, 218, 109–118. [Google Scholar] [CrossRef]
- Schacht, M.; Boukis, N.; Dinjus, E. Corrosion of alumina ceramics in acidic aqueous solutions at high temperatures and pressures. J. Mater. Sci. 2000, 35, 6251–6258. [Google Scholar] [CrossRef]
- Lin, G.S.; Liu, Y.C.; Anbarasan, R.; Naakagawa, K.; Yoshioka, T.; Matsuyama, H.; Tseng, H.; Tung, K.L. Silica gel-coated silicon carbide layer deposited by atmospheric plasma spraying. J. Taiwan Ins. Chem. E. 2020, 110, 173–181. [Google Scholar] [CrossRef]
- Tuci, G.L.; Liu, Y.F.; Rossin, A.; Guo, X.Y.; Pham, C.; Giambastiani, G.; Cuong, P. Porous Silicon Carbide (SiC): A Chance for Improving Catalysts or Just Another Active-Phase Carrier? Chem. Rev. 2021, 17, 10559–10665. [Google Scholar] [CrossRef]
- Muthu, M.; Santhanam, M. Effect of reduced graphene oxide, alumina and silica nanoparticles on the deterioration characteristics of Portland cement paste exposed to acidic environment. Cement Concrete Comp. 2018, 91, 118–137. [Google Scholar] [CrossRef]
- Chen, H.J.; Zhang, Z.B.; Zhong, X.; Zhuo, Z.J.; Tian, S.L.; Fu, S.Y.; Chen, Y.; Liu, Y.H. Constructing MoS2/Lignin-derived carbon nanocomposites for highly efficient removal of Cr (VI) from aqueous environment. J. Hazard. Mater. 2021, 408, 124847. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Frenzel, L.M.; Roland, U.; Kopinke, F.D. Coating of solid substrates with carbon via hydrothermal carbonization. Mater. Lett. 2021, 288, 129315. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.J.; Wang, C.; Deng, C.M.; Mao, J.; Tan, X.; Li, W.; Zhang, P.; Wang, Q.W. Self-lubricating design strategy for thermally sprayed ceramic coatings by in-situ synthesis of carbon spheres. Surf. Coat. Tech. 2022, 446, 128759. [Google Scholar] [CrossRef]
- Hofmann, S.; Koch, D.; Baranger, E.; Lamon, J. Predicting the mechanical behavior of carbon fiber reinforced silicon carbide with interlaminar manufacturing defects. Clin. Neuroradiol. 2015, 29, 12. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.H.; Lin, P.P.; Ma, G.L.; Lin, T.S.; He, P.; Long, W.M.; Zhang, Q.G. Design of multi-layered architecture in dissimilar ceramic/metal joints with reinforcements clustering away from both substrates. Mater. Design 2021, 198, 109379. [Google Scholar] [CrossRef]
- Feng, Q.H.; Lin, P.P.; Lin, T.S.; He, P.; Liu, Y.; Long, W.M.; Li, J. Controllable distribution of reinforcements for reducing the strain energy in dissimilar ceramic/metal joints. J. Eur. Ceram. Soc. 2021, 41, 1076–1087. [Google Scholar] [CrossRef]
- Ouyang, H.B.; Li, G.B.; Li, C.Y.; Huang, J.F.; Fei, J.; Lu, J. Microstructure and ablation properties of C/C-Zr-Si-O composites prepared by carbothermal reduction of hydrothermal co-deposited oxides. Mater. Design 2018, 159, 145–154. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Q.; Meng, X.L.; Yan, L.S.; Cui, H. Adhesive joint properties of advanced carbon/ceramic composite and tungsten-copper alloy for the hybrid rocket nozzle. Int. J. Adhes. Adhes. 2020, 102, 102670. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Sazali, N.; Ismail, A.F. Effect of intermediate layer on gas separation performance of disk supported carbon membrane. Sep. Sci. Technol. 2017, 52, 2137–2149. [Google Scholar] [CrossRef]
- Wang, C.; Ling, L.; Huang, Y.; Yao, Y.G.; Song, Q. Decoration of porous ceramic substrate with pencil for enhanced gas separation performance of carbon membrane. Carbon 2015, 84, 151–159. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Zhang, X.; Chen, W.; Yang, H.P.; Chen, H.P. The structure evolution of biochar from biomass pyrolysis and its correlation with gas pollutant adsorption performance. Bioresource Technol. 2017, 246, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.P.; Xia, Q.; Han, L.F.; Zhao, Y.L.; Huang, N.; Ren, Q.X.; Zhang, X.; Ru, H.Q. Fabrication of carbon-coated boron carbide particle and its role in the reaction bonding of boron carbide by silicon infiltration. J. Eur. Ceram. Soc. 2022, 42, 860–868. [Google Scholar] [CrossRef]
- Xu, R.L.; Bian, D.; Aradhyula, T.V.; Chavali, M.; Zhao, Y.W. Preparation and corrosion behavior studies of chemically bonded phosphate ceramic coating reinforced with modified multi-walled carbon nanotubes (MWCNTs). Ann. Am. Thorac. Soc. 2019, 16, 923–930. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wang, N.N.; Sun, C.H.; Lu, Z.X.; Xue, P.; Tang, B.; Bai, Z.C.; Dou, S.X. 3D spongy CoS2 nanoparticles/carbon composite as high-performance anode material for lithium/sodium ion batteries. Chem. Eng. J. 2018, 332, 370–376. [Google Scholar] [CrossRef]
- Wang, X.H.; Jiang, C.L.; Hou, B.X.; Wang, Y.Y.; Hao, C.; Wu, J.B. Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef]
- Liu, Y.J.; Ren, D.Z.; Song, Z.Y.; Wan, X.Y.; Zhang, C.T.; Jin, F.M.; Huo, Z.B. A novel method to prepare a magnetic carbon-based adsorbent with sugar-containing water as the carbon source and DETA as the modifying reagent. Environ. Sci. Pollut. R. 2018, 25, 13645–13659. [Google Scholar] [CrossRef]
- Mujmle, R.B.; Chung, W.J.; Kim, H. Chemical fixation of carbon dioxide catalyzed via hydroxyl and carboxyl-rich glucose carbonaceous material as a heterogeneous catalyst. Chem. Eng. J. 2020, 395, 125164. [Google Scholar] [CrossRef]
- Cui, K.J.; Li, P.; Zhang, R.; Cao, B. Preparation of pervaporation membranes by interfacial polymerization for acid wastewater purification. Chem. Eng. Res. Des. 2020, 156, 171–179. [Google Scholar] [CrossRef]
- Webber, J.; Zorzi, J.E.; Perottoni, C.A.; Silva, S.M.E.; Cruz, R. Identification of α-Al2O3 surface sites and their role in the adsorption of stearic acid. J. Mater. Sci. 2016, 51, 5170–5184. [Google Scholar] [CrossRef]
- Bowers, A.; Huang, C.P. Adsorption characteristics of polyacetic amino acids onto hydrous γ-Al2O3. J. Colloid Inter. Sci. 1985, 105, 197–215. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Y.; Feng, Y.; Wang, P.; Li, X.Y.; Shih, K.M. Fabrication of reactive flat-sheet ceramic membranes for oxidative degradation of ofloxacin by peroxymonosulfate. J. Mem. Sci. 2020, 611, 118302. [Google Scholar] [CrossRef]
- Li, X.B.; Zhou, Z.H.; Chen, L.; Kong, C.L.; Du, H.B. Design and Synthesis of Acid-resistant Zeolite T/NaY Composite Membrane for Water/Ethanol Separation. B. Korean Chem. Soc. 2019, 40, 511–516. [Google Scholar] [CrossRef]
- Zhang, S.B.; Zhong, J.X.; Zhao, S.K.; Suo, N.; Liu, G.C.; Xie, K.; Wang, F.C.; Liu, Y.Y.; Ju, W.P. Operational optimization control and membrane fouling mechanism analysis of ceramic membrane treating secondary treated effluent. Chin. J. Environ. Eng. 2021, 15, 3233–3243. [Google Scholar] [CrossRef]
- Qin, H.; Guo, W.M.; Gao, P.Z.; Xiao, H.N. Spheroidization of low-cost alumina powders for the preparation of high-flux flat-sheet ceramic membranes. Ceram. Int. 2020, 46, 13189–13197. [Google Scholar] [CrossRef]
- Song, I.H.; Bae, B.S.; Ha, J.H.; Lee, J.M. Effect of hydraulic pressure on alumina coating on pore characteristics of flat-sheet ceramic membrane. Ceram. Int. 2017, 43, 10502–10507. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, A.K.; Ghosal, P.S.; Varma, M. A review on hospital wastewater treatment: A special emphasis on occurrence and removal of pharmaceutically active compounds, resistant microorganisms, and SARS-CoV-2. J. Environ. Eng. 2021, 9, 104812. [Google Scholar] [CrossRef]
- Ji, B.; Zhu, L.; Wang, S.L.; Liu, Y. Temperature-effect on the performance of non-aerated microalgal bacterial granular sludge process in municipal wastewater treatment. J. Environ. Manag. 2021, 282, 111955. [Google Scholar] [CrossRef]












| Material | Position | Al a | O b | C c | Total |
|---|---|---|---|---|---|
| ACM | Surface | 43.23% | 53.99% | 2.78% | 100% |
| Cross-section | 44.54% | 54.64% | 0.82% | 100% | |
| C@ACM | Surface | 36.60% | 47.15% | 16.25% | 100% |
| Cross-section | 34.56% | 55.84% | 9.62% | 100% | |
| Hydrothermal precipitate | Surface | 0 | 24.72% | 75.28% | 100% |
| Membranes | Al 2p | O 1s | C 1s |
|---|---|---|---|
| ACM | 29.09% | 49.56% | 21.35% |
| C@ACM | 1.04% | 22.50% | 76.46% |
| Origin | Number | Milluiken Charge a |
|---|---|---|
| Molecular A | O19 | −0.6405 |
| O22 | −0.4108 | |
| O23 | −0.5408 | |
| O24 | −0.5318 | |
| Molecular B | O20 | −0.5611 |
| O35 | −0.3591 | |
| O36 | −0.4079 | |
| O37 | −0.5479 | |
| O45 | −0.3839 |
| Adsorption Isotherm Model | Parameters | Adsorption | |
|---|---|---|---|
| ACM | C@ACM | ||
| Pseudo first order | K1 | 1.275 × 10−2 | 1.002 × 10−2 |
| R2 | 0.9939 | 0.9929 | |
| Pseudo second order | K2 | 1884.000 | 6926.471 |
| R2 | 0.9994 | 0.9992 | |
| The Weber and Morris model | Kip | 1.472 × 10−5 | 3.540 × 10−6 |
| C | 2.438 × 10−4 | 7.306 × 10−5 | |
| R2 | 0.6931 | 0.6435 | |
| Elovich model | α | 1.235 × 10−4 | 3.681 × 10−5 |
| β | 1.445 × 104 | 5.541 × 104 | |
| R2 | 0.8710 | 0.7532 | |
| Adsorption Isotherm Model | Parameters | Adsorption | |
|---|---|---|---|
| ACM | C@ACM | ||
| Henry | H | 1.080 × 10−3 | 2.138 × 10−4 |
| R2 | 0.5878 | 0.5913 | |
| Langmuir | qmax | 1.650 × 10−3 | 3.334 × 10−4 |
| KL | 19.645 | 13.962 × 10−2 | |
| R2 | 0.9987 | 0.9975 | |
| Freundlich | n | 2.040 | 1.961 |
| KF | 1.878 × 10−3 | 3.821 × 10−4 | |
| R2 | 0.8894 | 0.80372 | |
| Tempkin | A | 1.570 × 10−3 | 3.150 × 10−4 |
| B | 2.515 × 10−4 | 5.512 × 10−5 | |
| R2 | 0.9074 | 0.9311 | |
| Radke–Prausnitz | a | 1.183 × 10−2 | 1.920 × 10−3 |
| b | 10.957 | 40.231 | |
| β | −19.956 | −25.322 | |
| R2 | 0.9906 | 0.9659 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Zhang, H.; Zhou, Y.; Tang, Z.; Li, B.; Fu, T.; Zhang, Y.; Zhu, H. Core-Shell Structured Carbon@Al2O3 Membrane with Enhanced Acid Resistance for Acid Solution Treatment. Membranes 2022, 12, 1246. https://doi.org/10.3390/membranes12121246
Wu Q, Zhang H, Zhou Y, Tang Z, Li B, Fu T, Zhang Y, Zhu H. Core-Shell Structured Carbon@Al2O3 Membrane with Enhanced Acid Resistance for Acid Solution Treatment. Membranes. 2022; 12(12):1246. https://doi.org/10.3390/membranes12121246
Chicago/Turabian StyleWu, Qianlian, Huimiao Zhang, Yi Zhou, Zhishu Tang, Bo Li, Tingming Fu, Yue Zhang, and Huaxu Zhu. 2022. "Core-Shell Structured Carbon@Al2O3 Membrane with Enhanced Acid Resistance for Acid Solution Treatment" Membranes 12, no. 12: 1246. https://doi.org/10.3390/membranes12121246