Preparation of Conductive Polyester Fibers Using Continuous Two-Step Plating Silver
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Deposition of Silver on Polyester Fibers by Electroless Plating under the Dynamic Condition
2.3. Deposition of Silver by Continuous Electroplating
2.4. Characterization
3. Results and Discussion
3.1. Mechanism Discussion of Electroless Plating under the Dynamic Condition
3.1.1. Pretreatment on Polyester Fibers
3.1.2. Electroless Silver Plating on Polyester Fibers
3.1.3. Influence of the Transmission Condition on the Continuous Electroless Plating Silver
3.2. Influence of Electroplating Process Conditions on Deposition of Silver
3.2.1. Influence of Power Supply Method on Electroplating Silver
3.2.2. Influence of Control Voltage on Electroplating Silver
3.2.3. Influence of Electroplating Time on Electroplating Silver
3.3. Silver Composition Analysis
3.4. Mechanical Properties Analysis
3.5. Washing Fastness Analysis
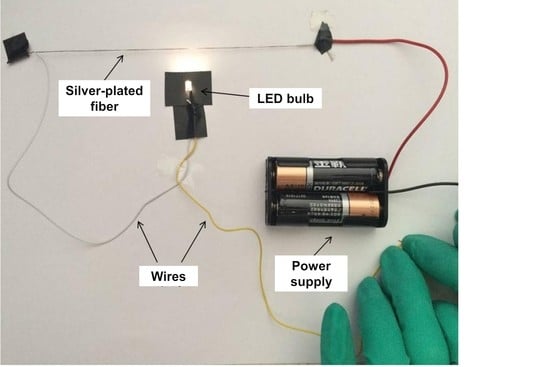
3.6. Conductive Properties Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Liu, X.Y.; Xie, M.M.; Li, Y.C.; Zhou, L.; Shao, J.Z. Study on the reduction properties of thiourea dioxide and its application in discharge printing of polyester fabrics. Fibers Polym. 2018, 19, 1237. [Google Scholar] [CrossRef]
- Chaudhary, H.; Gupta, D.; Gupta, C. Multifunctional dyeing and finishing of polyester with sericin and basic dyes. J. Text. Inst. 2017, 108, 314–324. [Google Scholar] [CrossRef]
- Kogo, A.A.; Ismail, I.M.; Yakasai, M.Y. Effects on the electro-mechanical properties of aniline-doped polyester fibric. Bayero J. Pure Appl. Sci. 2017, 10, 159–162. [Google Scholar] [CrossRef]
- Rojas, J.P.; Conchouso, D.; Arevalo, A.; Singh, D.; Foulds, I.G.; Hussain, M.M. Paper-based origami flexible and foldable thermoelectric nanogenerator. Nano Energy 2017, 31, 296–301. [Google Scholar] [CrossRef]
- Rastegar, L.; Montazer, M.; Gaminian, H. Multifunctional colored polyester fabric treated with dopamine hydrochloride at room temperature: Higher tensile, hydrophilicity and anti-bacterial properties along with aminolysis. Fibers Polym. 2017, 18, 1915–1923. [Google Scholar] [CrossRef]
- Yin, J.; Nysten, B. Contact electrification and charge decay on polyester fibres: A KPFM study. J. Electrostat. 2018, 96, 16–22. [Google Scholar] [CrossRef]
- Wang, C.; Guo, R.H.; Lan, J.W.; Tan, L.; Jiang, S.X.; Xiang, C. Preparation of multi-functional fabric via silver/reduced graphene oxide coating with poly(diallyldimethylammonium chloride) modification. J. Mater. Sci. Mater. Electron. 2018, 29, 8010–8019. [Google Scholar] [CrossRef]
- Kumar, N.; Ginting, R.T.; Ovhal, M.; Kang, J.W. All-solid-state flexible supercapacitor based on spray-printed polyester/PEDOT:PSS electrodes. Mol. Cryst. Liq. Cryst. 2018, 660, 135–142. [Google Scholar] [CrossRef]
- Smith, D.R.; Mock, J.J.; Starr, A.F.; Schurig, D. Gradient index metaterials. Phys. Rev. E 2005, 71, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, S.C.; Nagaraju, G.; Yu, J.S. Conductive silver nanowires-fenced carbon cloth fibers-supported layered double hydroxide nanosheets as a flexible and binder-free electrode for high-performance asymmetric supercapacitors. Nano Energy 2017, 36, 58–67. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Dou, J.X.; He, J.H.; Xiao, C.X.; Shen, L.Y.; Yang, J.H.; Wang, Y.; Zhou, Z.W. Electrically/infrared actuated shape memory composites based on a bio-based polyester blend and graphene nanoplatelets and their excellent self-driven ability. J. Mater. Chem. C 2017, 5, 4145–4158. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, J.W.; Yoon, S.; Kim, K.S.; Kim, J.H.; Park, S.; Kim, S.H.; Piao, L. High-performance stretchable conductive composite fibers from surface-modified silver nanowires and thermoplastic polyurethane by wet spinning. ACS Appl. Mater. Interfaces 2018, 10, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y.; Lin, C.X.; Guan, J.P.; Li, Y.J. Silver nanoparticle-immobilized porous POM/PLLA nanofibrous membranes: Efficient catalysts for reduction of 4-nitroaniline. RSC Adv. 2017, 7, 7460–7468. [Google Scholar] [CrossRef]
- Li, M.M.; Gong, Y.M.; Wang, W.H.; Xu, G.P.; Liu, Y.F.; Guo, J. In-situ reduced silver nanoparticles on populus fiber and the catalytic application. Appl. Surf. Sci. 2017, 394, 351–357. [Google Scholar] [CrossRef]
- Mahmud, S.; Sultana, M.Z.; Pervez, M.N.; Habib, M.A.; Liu, H.H. Surface Functionalization of “Rajshahi Silk” Using Green Silver Nanoparticles. Fibers 2017, 5, 35. [Google Scholar] [CrossRef]
- Li, X.Q.; Shi, C.; Wang, J.D.; Wang, J.; Li, M.J.; Hua, Q.; Hong, S.; Ogino, K. Polyaniline-doped TiO2/PLLA fibers with enhanced visible-light photocatalytic degradation performance. Fibers Polym. 2017, 18, 50–56. [Google Scholar] [CrossRef]
- Islam, S.; Sun, G. Thermodynamics, kinetics, and multifunctional finishing of textile materials with colorants extracted from natural renewable sources. ACS Sustain. Chem. Eng. 2017, 5, 7451–7466. [Google Scholar] [CrossRef]
- Gan, X.P.; Wu, Y.T.; Liu, L.; Shen, B.; Hu, W.B. Electroless copper plating on PET fabrics using hypophosphite asreducing agent. Surf. Coat. Technol. 2007, 201, 7018–7023. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, H.K.; Byun, S.W.; Jeong, S.H.; Hong, Y.K.; Joo, J.S.; Song, K.T.; Kim, K.; Lee, C.J.; Lee, J.Y. PET fabric/polypyrrole composite with high electrical conductivity for EMI shielding. Synth. Met. 2002, 126, 233–239. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Chen, H.W.; Wang, Y. Region-selective electroless gold plating on polycarbonate sheets by UV-patterning in combination with silver activating. Electrochim. Acta 2010, 55, 2542–2549. [Google Scholar] [CrossRef]
- Li, X.Q.; Wang, J.D.; Li, M.J.; Yang, J.; Gu, Z.J.; Liu, C.C.; Ogino, K. Fe-doped TiO2/SiO2 nanofibrous membranes with surface molecular imprinted modification for selective removing 4-Nitrophenol. Chin. Chem. Lett. 2018, 29, 527–530. [Google Scholar] [CrossRef]
- Chen, D.X.; Kang, Z.X. ABS plastic metallization through UV covalent grafting and layer-by-layer deposition. Surf. Coat. Technol. 2017, 328, 63–69. [Google Scholar] [CrossRef]
- Micheli, D.; Apollo, C.; Pastore, R.; Morles, R.B.; Laurenzi, S.; Marchetti, M. Nanostructured composite materials for electromagnetic interference shielding applications. Acta Astronaut. 2011, 102, 699. [Google Scholar] [CrossRef]
- Qin, X.; Wang, H.C.; Shan, R.F. Morphology-controlled synthesis of Ag nanoparticle decorated glassy carbon electrode and its electrochemical performance. Ionics 2018, 24, 1765. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, I.Y.; Kim, H.R. Production of electromagnetic shielding fabrics by optimization of electroless silver plating conditions for PET fabrics. J. Text. Inst. 2017, 108, 1065–1073. [Google Scholar] [CrossRef]
- Li, X.Q.; Shi, C.; Qiu, H.; Sun, H.; Ogino, K. Fabrication of fluorescent poly(L-lactide-co-caprolactone) fibers with quantum-dot incorporation from emulsion electrospinning for chloramphenicol detection. J. Appl. Polym. Sci. 2016, 133, 44584. [Google Scholar] [CrossRef]
- Fukuhara, C.; Ohkura, H.; Kamata, Y.; Murakami, Y.; Igarashi, A. Catalytic properties of plate-type copper-based catalysts, for steam reforming of methanol, on an aluminum plate prepared by electroless plating. Appl. Catal. A Gen. 2004, 273, 125–132. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Salgueiriño-Maceira, V.; Liz-Marzán, L.M. Deposition of silver nanoparticles on silica spheres by pretreatment steps in electroless plating. Chem. Mater. 2001, 5, 1630–1633. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, L.M.; Luo, J.; Wu, Y.C.; Li, J. Effect of electroless plating Ni–Cu–P layer on brazability of cemented carbide to steel. Surf. Coat. Technol. 2012, 8, 2521–2524. [Google Scholar] [CrossRef]
- Li, N.J.; Li, N.W.; Li, M.M.; Yi, D. The preparation and application of special performance ornamental alloy coating on plastic substrate. Appl. Mech. Mater. 2013, 423–426, 837–841. [Google Scholar] [CrossRef]
- Li, Q.L.; He, X.Y.; Zhang, Y.Q.; Yang, X.F. Preparation and Microwave Absorbing Properties of an Electroless Ni-Co Coating on Multiwall Carbon Nanotubes Using [Ag(NH3)2]+ as Activator. J. Nanomater. 2015, 404698. [Google Scholar] [CrossRef]
- Matijević, E.; Poskanzer, A.M.; Zuman, P. Characterization of the stannous chloride/Palladium chloride catalysts for electroless plating. Plat. Surf. Finish. 1975, 61, 958–965. [Google Scholar]
- Wang, X.W.; Ma, S.J.; Wang, X.H.; Ma, C.; Yuan, Z.H. Facile conversion of Zn nanowires to Zn nanotubes by heating-induced volatilization in nanopores of anodic aluminum oxide template. Vacuum 2016, 132, 86–90. [Google Scholar] [CrossRef]
- Wei, L.; Yu, J.; Hu, X.J.; Huang, Y. Facile surface modification of porous stainless steel substrate with TiO2 intermediate layer for fabrication of H2-permeable composite palladium membranes. Sep. Sci. Technol. 2016, 51, 998–1006. [Google Scholar] [CrossRef]
- Wei, L.; Yu, J.; Hu, X.J.; Wang, R.X.; Huang, Y. Effects of Sn residue on the high temperature stability of the H2-permeable palladium membranes prepared by electroless plating on Al2O3 substrate after SnCl2–PdCl2 process: A case study. Chin. J. Chem. Eng. 2016, 24, 1154–1160. [Google Scholar] [CrossRef]
- Wang, P.C.; Chang, C.P.; Youh, M.J.; Liu, Y.M.; Chu, C.M.; Ger, M.D. The preparation of pH-sensitive Pd catalyst ink for selective electroless deposition of copper on a flexible PET substrate. J. Taiwan Inst. Chem. E 2016, 60, 555–563. [Google Scholar] [CrossRef]
- Ang, L.M.; Hor, T.S.A.; Xu, G.Q.; Tung, C.H.; Zhao, S.P.; Wang, J.L.S. Electroless Plating of Metals onto Carbon Nanotubes Activated by a Single-Step Activation Method. Chem. Mater. 1999, 11, 2115–2118. [Google Scholar] [CrossRef]
- Absalan, G.; Akhond, M.; Soleimani, M.; Ershadifar, H. Efficient electrocatalytic oxidation and determination of isoniazid on carbon ionic liquid electrode modified with electrodeposited palladium nanoparticles. J. Electroanal. Chem. 2016, 761, 1–7. [Google Scholar] [CrossRef]
- Wataha, J.C.; Hanks, C.T. Biological effects of palladium and risk of using palladium in dental casting alloys. J. Oral Rehabil. 1996, 23, 309–320. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhan, L.; Wang, Q.; Song, S.; Chen, J.; Zhu, K.; Xu, X.H.; Liu, W.P. Increasing the activity and stability of chemi-deposited palladium catalysts on nickel foam substrate by electrochemical deposition of a middle coating of silver. Sep. Purif. Technol. 2011, 80, 526–532. [Google Scholar] [CrossRef]
- Shukla, S.; Seal, S.; Rahaman, Z.; Scammon, K. Electroless copper coating of cenospheres using silver nitrate activator. Mater. Lett. 2002, 57, 151–156. [Google Scholar] [CrossRef]
- Zoppas, F.M.; Marchesini, F.A.; Devard, A.; Bernardes, A.M.; Miró, E.E. Controlled deposition of Pd and In on carbon fibers by sequential electroless plating for the catalytic reduction of nitrate in water. Catal. Commun. 2016, 78, 59–63. [Google Scholar] [CrossRef]
- Shao, Q.S.; Bai, R.C.; Tang, Z.Y.; Pang, H.W.; Yan, W.; Sun, J.L.; Ren, M.S. Preparation of silver-deposited aromatic polysulfonamide fibers with excellent performance via electroless nanoplating using a chlorine-aided silver activation system. Ind. Eng. Chem. Res. 2015, 54, 11302–11311. [Google Scholar] [CrossRef]
- Fatema, U.K.; Gotoh, Y. A new electroless Ni plating procedure of iodine-treated aramid fiber. J. Coat. Technol. Res. 2013, 10, 415–425. [Google Scholar] [CrossRef]
- Shu, Z.; Wang, X. Environment-friendly Pd free surface activation technics for ABS surface. Appl. Surf. Sci. 2012, 258, 5328–5331. [Google Scholar] [CrossRef]
- Cha, S.H.; Koo, H.C.; Kim, J.J. The inhibition of silver agglomeration by gold activation in silver electroless plating. J. Electrochem. Soc. 2005, 152, C388–C391. [Google Scholar] [CrossRef]
- Okinaka, Y.; Hoshino, M. Some recent topics in gold plating for electronics applications. Gold Bull. 1998, 31, 3. [Google Scholar] [CrossRef]
- Lien, W.; Huang, P.; Tseng, S.; Cheng, C.H.; Lai, S.M.; Liaw, W.C. Electroless silver plating on tetraethoxy silane–bridged fiber glass. Appl. Surf. Sci. 2012, 258, 2246–2254. [Google Scholar] [CrossRef]
- Liu, C.C.; Cheng, J.; Li, X.Q.; Gu, Z.J.; Ogino, K. Laser-Induced Silver Seeding on Filter Paper for Selective Electroless Copper Plating. Materials 2018, 8, 1348. [Google Scholar] [CrossRef] [PubMed]
- De-Almeida, M.R.H.; Carlos, I.A.; Barbosa, L.L.; Carlos, R.M.; Lima-Neto, B.S.; Pallone, E.M.J.A. Voltammetric and morphological characterization of copper electrodeposition from non-cyanide electrolyte. J. Appl. Electrochem. 2002, 32, 763. [Google Scholar] [CrossRef]
- Varentsova, V.I.; Varenstov, V.K.; Bataev, I.A.; Yusin, S.I. Effect of surface state of carbon fiber electrode on copper electroplating from sulfate solution. Prot. Met. Phys. Chem. Surf. 2011, 47, 43–47. [Google Scholar] [CrossRef]
- Xie, B.G.; Sun, J.J.; Lin, Z.B.; Chen, G.N. Electrodeposition of mirror-bright silver in cyanide-free bath containing uracil as complexing agent without a separate strike plating process. J. Electrochem. Soc. 2009, 156, D79–D83. [Google Scholar] [CrossRef]
- Wang, L.Y.; Chen, X.G.; Cao, D.R. A cyanide-selective colorimetric “naked-eye” and fluorescent chemosensor based on a diketopyrrolopyrrole–hydrazone conjugate and its use for the design of a molecular-scale logic device. RSC Adv. 2016, 6, 96676–96685. [Google Scholar] [CrossRef]
- Kato, M.; Okinaka, Y. Some recent developments in non-cyanide gold plating for electronics applications. Gold Bull. 2004, 37, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Bomparola, R.; Caporali, S.; Lavacchi, A.; Bardi, U. Silver electrodeposition from air and water-stable ionic liquid: An environmentally friendly alternative to cyanide baths. Surf. Coat. Technol. 2007, 201, 9485–9490. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, C.; Jing, X.L. Adhesion improvement of electroless copper plating on phenolic resin matrix composite through a tin-free sensitization process. Appl. Surf. Sci. 2013, 271, 303–310. [Google Scholar] [CrossRef]
- Li, X.Q.; Wang, J.D.; Hu, Z.M.; Li, M.J.; Ogino, K. In situ polypyrrole polymerization enhances the photocatalytic activity of nanofibrous TiO2/SiO2 membranes. Chin. Chem. Lett. 2018, 29, 166–170. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, S.Q.; Wang, W.; Yu, D. Electroless silver plated flexible graphite felt prepared by dopamine functionalization and applied for electromagnetic interference shielding. Colloid. Surf. A 2018, 558, 538–547. [Google Scholar] [CrossRef]
- Chang, S.Y.; Wan, C.C.; Wang, Y.Y.; Shih, C.H.; Tsai, M.H.; Shue, S.L.; Yu, C.H.; Liang, M.S. Characterization of Pd-free electroless Co-based cap selectively deposited on Cu surface via borane-based reducing agent. Thin Solid Films 2006, 515, 1107–1111. [Google Scholar] [CrossRef]
- Guo, R.H.; Jiang, S.Q.; Yuen, C.W.M.; Ng, M.C.F. An alternative process for electroless copper plating on polyester fabric. J. Mater. Sci.: Mater. Electron. 2009, 20, 33. [Google Scholar] [CrossRef]
- Li, X.F.; Li, Y.Q.; Cai, J.; Zhan, D.Y. Metallization of bacteria cells. Sci. China Ser. E-Technol. Sci. 2003, 46, 161. [Google Scholar] [CrossRef]
- Yang, C.C.; Wang, Y.Y.; Wan, C.C. Synthesis and characterization of PVP stabilized Ag/Pd nanop-articles and its potential as an activator for electroless copper depositi-on. J. Electrochem. Soc. 2005, 152, C96–C100. [Google Scholar] [CrossRef]
- Dimeska, R.; Murray, P.S.; Ralph, S.F.; Wallace, G.G. Electroless recovery of silver by inherently conducting polymer powders, membranes and composite materials. Polymer 2006, 47, 4520–4530. [Google Scholar] [CrossRef]













| Sample | Breaking Strength (CN) | Elongation at Break (%) |
|---|---|---|
| Original polyester fiber | 1041.81 | 23.57 |
| Coarsening treated polyester fiber | 911.23 | 16.35 |
| Electroless silver-plated polyester fiber | 934.25 | 18.81 |
| Electroplated silver-coated polyester fiber | 950.31 | 19.24 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Li, X.; Li, X.; Xu, T.; Song, C.; Ogino, K.; Gu, Z. Preparation of Conductive Polyester Fibers Using Continuous Two-Step Plating Silver. Materials 2018, 11, 2033. https://doi.org/10.3390/ma11102033
Liu C, Li X, Li X, Xu T, Song C, Ogino K, Gu Z. Preparation of Conductive Polyester Fibers Using Continuous Two-Step Plating Silver. Materials. 2018; 11(10):2033. https://doi.org/10.3390/ma11102033
Chicago/Turabian StyleLiu, Changchun, Xuelian Li, Xiaoqiang Li, Tianze Xu, Chunyu Song, Kenji Ogino, and Zhijie Gu. 2018. "Preparation of Conductive Polyester Fibers Using Continuous Two-Step Plating Silver" Materials 11, no. 10: 2033. https://doi.org/10.3390/ma11102033