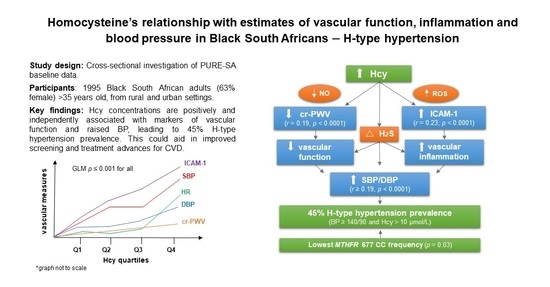
H-Type Hypertension among Black South Africans and the Relationship between Homocysteine, Its Genetic Determinants and Estimates of Vascular Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Questionnaires
2.3. Anthropometric Measurements
2.4. Cardiovascular Marker Measurements
2.5. Laboratory Biochemical Measurements
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Homocysteine and Blood Pressure—H-Type Hypertension
4.2. H-Type Hypertension and Genetic Determinants
4.3. Hcy with Vascular Function and Inflammation Markers
4.4. Treatment and Recommendations
4.5. Limitations, Strengths and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, F.; Zhuang, L.; Wang, Y.; Ma, Y. Homocysteine levels and risk of essential hypertension: A meta-analysis of published epidemiological studies. Clin. Exp. Hypertens. 2017, 39, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, Y.; Luo, D.; Deng, S.; Wu, B.; Hu, Y. Evidence on the causal link between homocysteine and hypertension from a meta-analysis of 40 173 individuals implementing Mendelian randomization. J. Clin. Hypertens. 2019, 21, 1879–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towfighi, A.; Markovic, D.; Ovbiagele, B. Pronounced association of elevated serum homocysteine with stroke in subgroups of individuals: A nationwide study. J. Neurol. Sci. 2010, 298, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, S.; Zhang, Y.; Tang, G.; Wang, Y.; Mao, G.; Li, Z.; Xu, X.; Wang, B.; Huo, Y. H-type hypertension and risk of stroke in chinese adults: A prospective, nested case–control study. J. Transl. Intern. Med. 2015, 3, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Huang, S.; Yang, Y.; He, X.; Fei, L.; Xing, Y. Association Between MTHFR Polymorphisms and the Risk of Essential Hypertension: An Updated Meta-analysis. Front. Genet. 2021, 12, 698590. [Google Scholar] [CrossRef]
- E Schutte, A.; Botha, S.; Fourie, C.M.T.; Gafane-Matemane, L.F.; Kruger, R.; Lammertyn, L.; Malan, L.; Mels, C.M.C.; Schutte, R.; Smith, W.; et al. Recent advances in understanding hypertension development in sub-Saharan Africa. J. Hum. Hypertens. 2017, 31, 491–500. [Google Scholar] [CrossRef] [Green Version]
- E Schutte, A.; Schutte, R.; Huisman, H.W.; van Rooyen, J.M.; Fourie, C.M.; Malan, N.T.; Malan, L.; MC Mels, C.; Smith, W.; Moss, S.J.; et al. Are behavioural risk factors to be blamed for the conversion from optimal blood pressure to hypertensive status in Black South Africans? A 5-year prospective study. Int. J. Epidemiol. 2012, 41, 1114–1123. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.D.; Refsum, H. Homocysteine—From disease biomarker to disease prevention. J. Intern. Med. 2021, 290, 826–854. [Google Scholar] [CrossRef]
- Nienaber-Rousseau, C.; Ellis, S.M.; Moss, S.J.; Melse-Boonstra, A.; Towers, G.W. Gene–environment and gene–gene interactions of specific MTHFR, MTR and CBS gene variants in relation to homocysteine in black South Africans. Gene 2013, 530, 113–118. [Google Scholar] [CrossRef]
- Teo, K.; Chow, C.K.; Vaz, M.; Rangarajan, S.; Yusuf, S. The Prospective Urban Rural Epidemiology (PURE) study: Examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am. Heart J. 2009, 158, 1–7. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Zhao, L.; Wang, H.; Song, B.; Li, Z.; Gao, Y.; Lu, J.; Chandra, A.; Xu, Y. H-type Hypertension and Recurrence of Ischemic Stroke. Life Sci. J. 2011, 8, 460–463. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- du Plessis, J.P.; Melse-Boonstra, A.; Zandberg, L.; Nienaber-Rousseau, C. Gene interactions observed with the HDL-c blood lipid, intakes of protein, sugar and biotin in relation to circulating homocysteine concentrations in a group of black South Africans. Mol. Genet. Metab. Rep. 2019, 22, 100556. [Google Scholar] [CrossRef] [PubMed]
- Nienaber-Rousseau, C.; Pisa, P.T.; Venter, C.S.; Ellis, S.M.; Kruger, A.; Moss, S.J.; Melse-Boonstra, A.; Towers, G.W. Nutritional Genetics: The Case of Alcohol and the MTHFR C677T Polymorphism in Relation to Homocysteine in a Black South African Population. Lifestyle Genom. 2013, 6, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Nygård, O.; Vollset, S.E.; Refsum, H.; Stensvold, I.; Tverdal, A.; Nordrehaug, J.E.; Ueland, P.M.; Kvåle, G. Total Plasma Homocysteine and Cardiovascular Risk Profile. JAMA 1995, 274, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Kahleová, R.; Palyzová, D.; Zvára, K.; Zvarova, J.; Hrach, K.; Nováková, I.; Hyánek, J.; Bendlová, B.; Kožich, V. Essential hypertension in adolescents: Association with insulin resistance and with metabolism of homocysteine and vitamins. Am. J. Hypertens. 2002, 15, 857–864. [Google Scholar] [CrossRef] [Green Version]
- du Plessis, J.P.; Nienaber-Rousseau, C.; Lammertyn, L.; Schutte, A.E.; Pieters, M.; Kruger, H.S. The Relationship of Circulating Homocysteine with Fibrinogen, Blood Pressure, and Other Cardiovascular Measures in African Adolescents. J. Pediatr. 2021, 234, 158–163. [Google Scholar] [CrossRef]
- Qian, X.-L.; Cao, H.; Zhang, J.; Gu, Z.-H.; Tang, W.-Q.; Shen, L.; Hu, J.-L.; Yao, Z.-F.; Zhang, L.; Tang, M.-N.; et al. The prevalence, relative risk factors and MTHFR C677T genotype of H type hypertension of the elderly hypertensives in Shanghai, China: A cross-section study. BMC Cardiovasc. Disord. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Zhong, C.; Lv, L.; Liu, C.; Zhao, L.; Zhou, M.; Sun, W.; Xu, T.; Tong, W. High Homocysteine and Blood Pressure Related to Poor Outcome of Acute Ischemia Stroke in Chinese Population. PLoS ONE 2014, 9, e107498. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Guo, S.; Ma, R.; He, J.; Yan, Y.; Zhang, X.; Wang, X.; Cao, B.; Guo, H. Association between methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and H-type hypertension: A systematic review and meta-analysis. Ann. Hum. Genet. 2022. e-pub ahead of print. [Google Scholar] [CrossRef]
- Candrasatria, R.M.; Adiarto, S.; Sukmawan, R. Methylenetetrahydrofolate Reductase C677T Gene Polymorphism as a Risk Factor for Hypertension in a Rural Population. Int. J. Hypertens. 2020, 2020, 4267246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, T.; Wang, H.; Tang, J.; Hou, A.; Yan, X.; Yu, B.; Ran, S.; Luo, M.; Tang, Y.; et al. Effects of individualized administration of folic acid on prothrombotic state and vascular endothelial function with H-type hypertension. Medicine 2022, 101, e28628. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Strain, J.; Hughes, C.F.; Ward, M. Riboflavin, MTHFR genotype and blood pressure: A personalized approach to prevention and treatment of hypertension. Mol. Asp. Med. 2017, 53, 2–9. [Google Scholar] [CrossRef]
- Wilson, C.P.; McNulty, H.; Ward, M.; Strain, J.; Trouton, T.G.; Hoeft, B.A.; Weber, P.; Roos, F.F.; Horigan, G.; McAnena, L.; et al. Blood Pressure in Treated Hypertensive Individuals with the MTHFR 677TT Genotype Is Responsive to Intervention with Riboflavin. Hypertension 2013, 61, 1302–1308. [Google Scholar] [CrossRef] [Green Version]
- Bia, D.; Zócalo, Y. Physiological Age- and Sex-Related Profiles for Local (Aortic) and Regional (Carotid-Femoral, Carotid-Radial) Pulse Wave Velocity and Center-to-Periphery Stiffness Gradient, with and without Blood Pressure Adjustments: Reference Intervals and Agreement between Methods in Healthy Subjects (3–84 Years). J. Cardiovasc. Dev. Dis. 2021, 8, 3. [Google Scholar] [CrossRef]
- Rothlein, R.; Mainolfi, E.A.; Czajkowski, M.; Marlin, S.D. A form of circulating ICAM-1 in human serum. J. Immunol. 1991, 147, 3788–3793. [Google Scholar]
- Esse, R.; Barroso, M.; De Almeida, I.T.; Castro, R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-N.; Ye, P.; Cao, R.-H.; Yang, X.; Xiao, W.-K.; Zhang, Y.; Bai, Y.-Y.; Wu, H.-M. Plasma Homocysteine is a Predictive Factor for Arterial Stiffness: A Community-Based 4.8-Year Prospective Study. J. Clin. Hypertens. 2015, 17, 594–600. [Google Scholar] [CrossRef]
- Barroso, M.; Kao, D.; Blom, H.J.; Tavares de Almeida, I.; Castro, R.; Loscalzo, J.; Handy, D.E. S-adenosylhomocysteine induces inflammation through NFkB: A possible role for EZH2 in endothelial cell activation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, X.; Liu, J.; Zhao, J.; Mao, J.; Zhang, X.; Feng, L.; Han, C.; Li, M.; Wang, S.; Wu, D. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Atherosclerosis 2014, 236, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Durga, J.; Van Tits, L.J.H.; Schouten, E.G.; Kok, F.J.; Verhoef, P. Effect of Lowering of Homocysteine Levels on Inflammatory Markers. Arch. Intern. Med. 2005, 165, 1388–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Seung-Hye, C.; Smi, C.-K.; Chung-Sil, K.; Haeyoung, L. The Effects of Korean DASH Diet Education Program on Oxidative Stress, Antioxidant Capacity, and Serum Homocysteine Level among Elderly Korean Women. J. Korean Biol. Nurs. Sci. 2017, 19, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Elias, M.F.; Brown, C.J. New Evidence for Homocysteine Lowering for Management of Treatment-Resistant Hypertension. Am. J. Hypertens. 2022, 35, 303–305. [Google Scholar] [CrossRef]

| Median (25th–75th) or n (%) | ||||||
|---|---|---|---|---|---|---|
| Whole Group (n = 1995) | Normal BP (n = 1044) | Hypertension (n = 526) | H-Type Hypertension * (n = 425) | Differences Among Groups # p Value | ||
| Age (years) | 48.0 (41.0–56.0) | 45.0 (40.0–53.0) @ | 49.0 (43.0–57.0) @ | 53.0 (46.0–61.0) @ | <0.0001 | |
| Sex | Male | 743 | 393 (52.9) @ | 160 (21.5) @ | 190 (25.6) @ | <0.0001 |
| Female | 1252 | 651 (52.0) @ | 366 (29.2) @ | 235 (18.8) @ | ||
| Urbanization level | Urban | 996 | 456 (45.8) @ | 330 (33.1) @ | 210 (21.1) @ | <0.0001 |
| Rural | 999 | 588 (58.9) @ | 196 (19.6) @ | 215 (21.5) @ | ||
| Tobacco use | Current | 1033 | 544 (52.7) | 272 (26.3) | 217 (21.0) | 0.71 |
| Former | 77 | 39 (50.6) | 19 (24.7) | 19 (24.7) | ||
| Never | 875 | 455 (52.0) | 231 (26.4) | 189 (21.6) | ||
| Anthro markers | BMI (kg/m2) | 23.0 (19.3–28.9) | 22.1 (18.9–27.5) @,† | 25.3 (20.2–31.7) @,$ | 23.1 (19.5–28.8) $,† | <0.0001 |
| Waist circumference (cm) | 77.45 (70.2–87.7) | 74.7 (68.8–84.3) @ | 81.9 (72.5–92.7) @ | 80.1 (72.2–89.1) @ | <0.0001 | |
| Hip circumference (cm) | 93.13 (84.8–106) | 91.4 (83.8–103) @ | 97.3 (87.4–111) @ | 92.2 (85.2–104) @ | <0.0001 | |
| Waist-to-hip ratio | 0.83 (0.78–0.88) | 0.82 (0.77–0.87) @ | 0.83 (0.78–0.88) @ | 0.85 (0.81–0.90) @ | <0.0001 | |
| Biochemical markers | HIV sero-negative | 1655 | 820 (49.5) @ | 448 (27.1) @ | 387 (23.4) @ | <0.0001 |
| HIV sero-positive | 324 | 215 (66.4) @ | 73 (22.5) @ | 36 (11.1) @ | ||
| HIV status unknown | 14 | 9 (64.3) | 4 (28.6) | 1 (7.10) | ||
| TC (mmol/L) | 4.82 (4.01–5.87) | 4.68 (3.87–5.67) @ | 5.02 (4.22–6.12) @ | 4.96 (4.15–6.06) @ | <0.0001 | |
| LDL-C (mmol/L) | 2.77 (2.07–3.63) | 2.73 (2.06–3.52) $ | 2.91 (2.20–3.82) $ | 2.77 (1.97–3.67) | 0.01 | |
| HDL-C (mmol/L) | 1.42 (1.06–1.87) | 1.35 (1.01–1.80) @ | 1.37 (1.05–1.85) @ | 1.60 (1.18–2.11) @ | <0.0001 | |
| Triglycerides (mmol/L) | 1.08 (0.82–1.55) | 1.01 (0.79–1.40) @ | 1.18 (0.86–1.70) @, $ | 1.12 (0.82–1.70) $ | <0.0001 | |
| Fasting glucose (mmol/L) | 4.80 (4.30–5.30) | 4.80 (4.30–5.20) † | 4.80 (4.30–5.40) | 4.90 (4.40–5.45) † | 0.01 | |
| HbA1c (%) | 5.50 (5.30–5.80) | 5.50 (5.30–5.80) † | 5.60 (5.30–5.90) † | 5.50 (5.20–5.85) | 0.02 | |
| GGT (µkat/L) | 46.0 (29.7–88.0) | 40.4 (28.0–69.0) @,$ | 49.5 (30.8–82.3) $ | 57.4 (33.8–139.8) @ | <0.0001 | |
| Hcy (µmol/L) | 9.18 (7.50–12.1) | 8.78 (7.16–11.0) @ | 8.00 (6.78–8.99) @ | 12.8 (11.4–15.9) @ | <0.0001 | |
| CRP (mg/L) | 3.29 (0.96–9.34) | 2.80 (0.73–9.29) @ | 3.77 (1.39–9.08) † | 3.76 (1.16–9.67) | <0.0001 | |
| Cardiovascular markers | SBP (mmHg) | 130 (116–147) | 117 (109–126) @ | 146 (136–160) @ | 135 (120–152) @ | <0.0001 |
| DBP (mmHg) | 87.0 (78.0–97.0) | 78.0 (72.0–84.0) @ | 97.0 (92.0–105) @ | 89.0 (80.0–99.0) @ | <0.0001 | |
| Pulse pressure (mmHg) | 43.0 (35.0–53.0) | 39.0 (33.0–45.0) @ | 50.0 (40.0–61.0) @ | 45.0 (36.0–56.0) @ | <0.0001 | |
| Heart rate (bpm) | 72.0 (62.0–84.0) | 71.0 (62.0–84.0) @ | 71.0 (61.0–81.0) @ | 74.0 (63.0–87.0) @ | <0.0001 | |
| cr-PWV (m/s) | 10.9 (9.55–12.2) | 10.5 (9.17–11.8) @ | 11.2 (9.98–12.3) @,† | 11.2 (10.0–12.8) @,† | <0.0001 | |
| ICAM-1 (ng/mL) | 371 (234–507) | 377 (247–510) | 489 (327–805) | 440 (286–548) | 0.83 | |
| VCAM-1 (ng/mL) | 693 (390–1279) | 679 (384–1295) | 751 (357–1308) | 662 (449–1139) | 0.99 | |
| Anti-hypertensives use n (%) | 283 | 82 (29.0) @ | 122 (43.1) @ | 79 (27.9) @ | <0.0001 | |
| Variables | Normal vs. HTN | Normal vs. H-Type HTN | HTN vs. H-Type HTN * | ||||
|---|---|---|---|---|---|---|---|
| p Value | Effect Size | p Value | Effect Size | p Value | Effect Size | ||
| Continuous Variables | |||||||
| Age (years) | <0.0001 | 0.36 | <0.0001 | 0.69 | <0.0001 | 0.33 | |
| Anthro markers | BMI (kg/m2) | <0.0001 | 0.40 | 0.58 | 0.17 | 0.001 | 0.21 |
| Waist circumference (cm) | <0.0001 | 0.45 | <0.001 | 0.35 | 0.16 | 0.10 | |
| Hip circumference (cm) | <0.0001 | 0.37 | 0.55 | 0.09 | <0.0001 | 0.26 | |
| Waist-to-hip ratio | <0.0001 | 0.16 | <0.0001 | 0.45 | <0.0001 | 0.27 | |
| Biochemical markers | TC (mmol/L) | <0.0001 | 0.24 | <0.01 | 0.26 | 0.31 | 0.01 |
| LDL-C (mmol/L) | 0.04 | 0.15 | 0.34 | 0.03 | 0.89 | 0.11 | |
| HDL-C (mmol/L) | <0.0001 | 0.11 | <0.0001 | 0.37 | 0.001 | 0.26 | |
| Triglycerides (mmol/L) | <0.0001 | 0.79 | 0.03 | 0.23 | 0.23 | 0.08 | |
| Fasting glucose (mmol/L) | 0.001 | 0.23 | 0.05 | 0.13 | 0.32 | 0.10 | |
| HbA1c (%) | 0.04 | 0.88 | 0.41 | 0.07 | 0.11 | 0.15 | |
| GGT (µkat/L) | <0.0001 | 0.11 | <0.0001 | 0.33 | 0.03 | 0.15 | |
| Hcy (µmol/L) | <0.0001 | 0.56 | <0.0001 | 1.13 | <0.0001 | 1.87 | |
| CRP (mg/L) | <0.01 | 0.09 | 0.28 | 0.05 | 0.86 | 0.05 | |
| Cardiovascular markers | SBP (mmHg) | <0.0001 | 2.10 | <0.0001 | 2.42 | 0.53 | 0.11 |
| DBP (mmHg) | <0.0001 | 2.35 | <0.0001 | 2.38 | 0.57 | 0.09 | |
| Pulse pressure (mmHg) | <0.0001 | 1.00 | <0.0001 | 1.19 | 0.67 | 0.08 | |
| Heart rate (bpm) | 0.45 | 0.13 | <0.0001 | 0.22 | <0.0001 | 0.34 | |
| cr-PWV (m/s) | <0.0001 | 0.33 | <0.0001 | 0.49 | 0.04 | 0.17 | |
| ICAM-1 (ng/mL) | 0.14 | 0.02 | 0.03 | 0.03 | 0.21 | 0.51 | |
| VCAM-1 (ng/mL) | 0.30 | 0.06 | 0.01 | 0.14 | 0.86 | 0.17 | |
| Categorical Variables | |||||||
| Sex | Male/Female | 0.01 | 0.07 | 0.01 | 0.07 | <0.0001 | 0.15 |
| Urbanization level | Urban/Rural | <0.0001 | 0.18 | 0.05 | 0.05 | <0.0001 | 0.14 |
| Tobacco use | Current/Former/Never | 0.96 | 0.01 | 0.40 | 0.05 | 0.27 | 0.06 |
| HIV status | Sero-negative/-positive/Unknown | 0.01 | 0.08 | <0.0001 | 0.16 | 0.02 | 0.09 |
| Anti-hypertensives use | Yes/No | <0.0001 | 0.18 | <0.0001 | 0.21 | 0.27 | 0.04 |
| Variables | Unadjusted | Adjusted | Sensitivity Analysis | ||||
|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | ||
| Age (year) | 0.28 | <0.0001 | 0.27 | <0.0001 | 0.27 | <0.0001 | |
| Anthropometrical Markers | |||||||
| BMI (kg/m2) | −0.13 | <0.0001 | −0.04 | 0.35 | −0.16 | <0.0001 | |
| Waist circumference (cm) | −0.03 | 0.24 | 0.02 | 0.69 | −0.04 | 0.09 | |
| Hip circumference (cm) | −0.14 | <0.0001 | 0.01 | 0.82 | −0.16 | <0.0001 | |
| Waist-to-hip ratio | 0.17 | <0.0001 | −0.02 | 0.67 | 0.17 | <0.0001 | |
| Biochemical Markers | |||||||
| TC (mmol/L) | 0.05 | 0.02 | 0.10 | 0.03 | 0.05 | 0.06 | |
| LDL-C (mmol/L) | −0.05 | 0.03 | 0.01 | 0.91 | −0.07 | <0.01 | |
| HDL-C (mmol/L) | 0.19 | <0.0001 | 0.20 | <0.0001 | 0.20 | <0.0001 | |
| Triglycerides (mmol/L) | 0.001 | 0.96 | −0.06 | 0.16 | −0.02 | 0.54 | |
| Fasting glucose (mmol/L) | 0.002 | 0.92 | −0.07 | 0.10 | −0.02 | 0.34 | |
| HbA1c (%) | −0.05 | 0.02 | −0.02 | 0.64 | −0.06 | 0.01 | |
| GGT (µkat/L) | 0.24 | <0.0001 | 0.24 | <0.0001 | 0.23 | <0.0001 | |
| CRP (mg/L) | 0.03 | 0.15 | 0.01 | 0.86 | 0.01 | 0.60 | |
| Cardiovascular Markers | |||||||
| Blood pressure (mmHg) | SBP | 0.19 | <0.0001 | 0.07 | 0.14 | 0.19 | <0.0001 |
| DBP | 0.16 | <0.0001 | 0.08 | 0.09 | 0.15 | <0.0001 | |
| Pulse pressure (mmHg) | 0.14 | <0.0001 | 0.01 | 0.76 | 0.14 | <0.0001 | |
| Heart rate (bpm) | 0.11 | <0.0001 | 0.12 | 0.01 | 0.09 | <0.001 | |
| cr-PWV (m/s) | 0.19 | <0.0001 | 0.04 | 0.32 | 0.21 | <0.0001 | |
| ICAM-1 (ng/mL) | 0.23 | <0.0001 | –0.02 | 0.68 | 0.21 | <0.0001 | |
| VCAM-1 (ng/mL) | 0.04 | 0.35 | 0.03 | 0.53 | 0.05 | 0.23 | |
| CVD Marker | Homocysteine (Adjusted Means with 95% CI Determined through GLM) | |||||
|---|---|---|---|---|---|---|
| Quartile 1 (n = 464) <7.44 µmol/L | Quartile 2 (n = 467) ≥7.45 to <9.17 µmol/L | Quartile 3 (n = 469) ≥9.18 to <12.04 µmol/L | Quartile 4 (n = 467) >12.05 µmol/L | GLM p Value | Sensitivity p Value | |
| CRP (mg/L) | 8.12 (6.97–9.27) | 8.17 (7.05–9.29) | 8.16 (7.04–9.29) | 8.94 (7.78–10.1) | 0.73 | 0.88 |
| Fasting glucose (µmol/L) | 5.05 (4.90–5.21) | 4.95 (4.81–5.10) | 5.01 (4.86–5.15) | 4.90 (4.75–5.06) | 0.58 | 0.11 |
| SBP (mmHg) | 131 (129–133) c,d,f | 133 (131–135) c,g | 133 (130–135) d,h | 137 (135–139) f,g,h | 0.001 | 0.01 |
| DBP (mmHg) | 85.4 (84.1–86.7) c,f | 85.9 (84.6–87.2) g | 87.5 (86.2–88.8) c,d | 90.9 (89.6–92.2) f,d,g | <0.0001 | <0.0001 |
| PP (mmHg) | 45.6 (44.3–46.9) | 46.9 (45.6–48.2) | 45.0 (43.8–46.3) | 46.1 (44.8–47.4) | 0.21 | 0.37 |
| HR (bpm) | 71.9 (70.4–73.3) g | 71.6 (70.3–73.0) f | 73.3 (71.9–74.7) h | 77.5 (76.1–79.0) f,g,h | <0.0001 | <0.0001 |
| cr-PWV (m/s) | 10.7 (10.5–10.9) a,f,g | 10.9 (10.7–11.2) a,h | 11.1 (10.9–11.3) f,b | 11.3 (11.1–11.5) g,h,b | <0.0001 | 0.001 |
| ICAM-1 (ng/mL) | 335 (310–360) a,g | 351 (326–377) c | 383 (356–409) a | 420 (393–449) c,g | <0.0001 | 0.001 |
| VCAM-1 (ng/mL) | 1053 (853–1254) | 1127 (914–1340) | 1042 (814–1269) | 909 (661–1157) | 0.63 | 0.59 |
| TC (mmol/L) | 4.87 (4.74–5.00) c | 5.14 (5.02–5.26) c | 5.02 (4.89–5.14) | 5.04 (4.91–5.17) | 0.03 | 0.07 |
| LDL-C (mmol/L) | 2.90 (2.80–3.01) | 3.03 (2.93–3.14) c | 2.92 (2.82–3.03) | 2.80 (2.69–2.91) c | 0.001 | 0.04 |
| HDL-C (mmol/L) | 1.38 (1.32–1.44) c,d,g | 1.52 (1.46–1.57) c,f | 1.52 (1.47–1.58) d,e | 1.66 (1.60–1.71) g,f,e | <0.0001 | <0.0001 |
| Triglicerides (mmol/L) | 1.29 (1.22–1.36) | 1.29 (1.23–1.36) | 1.26 (1.19–1.33) | 1.29 (1.22–1.36) | 0.89 | 0.65 |
| Variables | SBP (R2 = 0.18) | |||
|---|---|---|---|---|
| B | SE | β | p | |
| Age | 0.71 | 0.05 | 0.30 | <0.0001 |
| Sex | 5.34 | 1.21 | 0.11 | <0.0001 |
| BMI (kg/m2) | 0.41 | 0.09 | 0.12 | <0.0001 |
| GGT (μkat/L) | 0.004 | 0.003 | 0.03 | 0.13 |
| Hcy (μmol/L) | 0.43 | 0.12 | 0.08 | <0.0001 |
| Anti-hypertensive use | 8.92 | 1.51 | 0.14 | <0.0001 |
| DBP (R2 = 0.10) | ||||
| B | SE | β | p | |
| Age | 0.17 | 0.03 | 0.12 | <0.0001 |
| Sex | 0.21 | 0.75 | 0.01 | 0.79 |
| BMI (kg/m2) | 0.33 | 0.05 | 0.16 | <0.0001 |
| GGT (μkat/L) | 0.01 | 0.002 | 0.09 | <0.0001 |
| Hcy (μmol/L) | 0.38 | 0.08 | 0.12 | <0.0001 |
| Anti-hypertensive use | 5.13 | 0.94 | 0.13 | <0.0001 |
| PP (R2 = 0.19) | ||||
| B | SE | β | p | |
| Age | 0.54 | 0.03 | 0.37 | <0.0001 |
| Sex | 5.14 | 0.74 | 0.16 | <0.0001 |
| BMI (kg/m2) | 0.08 | 0.05 | 0.04 | 0.11 |
| GGT (μkat/L) | –0.002 | 0.002 | –0.03 | 0.19 |
| Hcy (μmol/L) | 0.04 | 0.07 | 0.01 | 0.57 |
| Anti-hypertensive use | 3.80 | 0.92 | 0.09 | <0.0001 |
| HR (R2 = 0.10) | ||||
| B | SE | β | p | |
| Age | –0.20 | 0.04 | –0.13 | <0.0001 |
| Sex | –8.25 | 0.83 | –0.25 | <0.0001 |
| BMI (kg/m2) | –0.21 | 0.06 | –0.09 | <0.0001 |
| GGT (μkat/L) | 0.01 | 0.002 | 0.13 | <0.0001 |
| Hcy (μmol/L) | 0.56 | 0.08 | 0.16 | <0.0001 |
| Anti-hypertensive use | 2.40 | 1.04 | 0.06 | 0.02 |
| cr-PWV (R2 = 0.15) | ||||
| B | SE | β | p | |
| Age | 0.01 | 0.01 | 0.05 | 0.04 |
| Sex | 0.77 | 0.12 | 0.16 | <0.0001 |
| BMI (kg/m2) | –0.08 | 0.01 | –0.25 | <0.0001 |
| GGT (μkat/L) | 0.001 | <0.0001 | 0.05 | 0.02 |
| Hcy (μmol/L) | 0.04 | 0.01 | 0.09 | <0.0001 |
| Anti-hypertensive use | 0.20 | 0.15 | 0.03 | 0.17 |
| ICAM-1 (R2 = 0.08) | ||||
| B | SE | β | p | |
| Age | 2.27 | 2.04 | 0.05 | 0.27 |
| Sex | −5.73 | 45.9 | −0.01 | 0.90 |
| BMI (kg/m2) | −3.34 | 3.20 | −0.05 | 0.30 |
| GGT (μkat/L) | 0.71 | 0.11 | 0.28 | <0.0001 |
| Hcy (μmol/L) | −11.7 | 4.58 | -0.11 | 0.01 |
| Anti-hypertensive use | 95.1 | 57.2 | 0.07 | 0.10 |
| VCAM (R2 = 0.01) | ||||
| B | SE | β | p | |
| Age | 4.51 | 5.61 | 0.04 | 0.42 |
| Sex | 59.11 | 126 | 0.02 | 0.64 |
| BMI (kg/m2) | −7.08 | 8.79 | −0.04 | 0.42 |
| GGT (μkat/L) | 0.02 | 0.29 | 0.003 | 0.95 |
| Hcy (μmol/L) | −15.0 | 12.6 | −0.05 | 0.23 |
| Anti-hypertensive use | −57.0 | 157 | −0.02 | 0.71 |
| Gene; SNP ID (rs Number; Location) | Whole Group | Normotensive | Hypertension | H-Type Hypertension |
|---|---|---|---|---|
| Genotype Counts (Frequencies %) | ||||
| MTHFR; C677T Ala222Val (rs1801133; 1:11796321) | CC 1561 (84.0) | 821 (83.3) | 404 (87.1) | 336 (82.4) |
| CT 282 (15.2) | 156 (15.8) | 58 (12.5) | 68 (16.7) | |
| TT 15 (0.80) | 9 (0.90) | 2 (0.40) | 4 (0.90) | |
| Phi = 0.08 | ||||
| Chi-square p = 0.03 | ||||
| MTR; A2756G Asp919Gly (rs1805087; 1:236885200) | AA 1181 (63.7) | 621 (63.3) | 284 (61.2) | 276 (67.7) |
| AG 583 (31.5) | 315 (32.1) | 154 (33.2) | 114 (27.9) | |
| GG 89 (4.80) | 45 (4.60) | 26 (5.60) | 18 (4.40) | |
| Phi = 0.06 | ||||
| Chi-square p = 0.28 | ||||
| CBS; T833C Ile278Thr (rs5742905; 21:43063074) | TT 984 (52.9) | 520 (52.7) | 246 (52.9) | 218 (53.2) |
| TC 740 (39.8) | 396 (40.2) | 187 (40.2) | 157 (38.3) | |
| CC 137 (7.30) | 70 (7.10) | 32 (6.90) | 35 (8.50) | |
| Phi = 0.05 | ||||
| Chi-square p = 0.55 | ||||
| CBS; 844ins68 indel (no rs#) | WT 985 (52.9) | 521 (52.8) | 245 (52.7) | 219 (53.3) |
| HT 742 (39.8) | 396 (40.2) | 188 (40.4) | 158 (38.4) | |
| MT 135 (7.30) | 69 (7.00) | 32 (6.90) | 34 (8.30) | |
| Phi = 0.04 | ||||
| Chi-square p = 0.80 | ||||
| CBS; G9276A (novel SNP no rs#; 21:43071860) | GG 966 (51.9) | 517 (52.4) | 232 (50.0) | 217 (52.9) |
| GA 750 (40.3) | 391 (39.7) | 199 (42.9) | 160 (39.0) | |
| AA 144 (7.80) | 78 (7.90) | 33 (7.10) | 33 (8.10) | |
| Phi = 0.04 | ||||
| Chi-square p = 0.73 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
du Plessis, J.P.; Lammertyn, L.; Schutte, A.E.; Nienaber-Rousseau, C. H-Type Hypertension among Black South Africans and the Relationship between Homocysteine, Its Genetic Determinants and Estimates of Vascular Function. J. Cardiovasc. Dev. Dis. 2022, 9, 447. https://doi.org/10.3390/jcdd9120447
du Plessis JP, Lammertyn L, Schutte AE, Nienaber-Rousseau C. H-Type Hypertension among Black South Africans and the Relationship between Homocysteine, Its Genetic Determinants and Estimates of Vascular Function. Journal of Cardiovascular Development and Disease. 2022; 9(12):447. https://doi.org/10.3390/jcdd9120447
Chicago/Turabian Styledu Plessis, Jacomina P., Leandi Lammertyn, Aletta E. Schutte, and Cornelie Nienaber-Rousseau. 2022. "H-Type Hypertension among Black South Africans and the Relationship between Homocysteine, Its Genetic Determinants and Estimates of Vascular Function" Journal of Cardiovascular Development and Disease 9, no. 12: 447. https://doi.org/10.3390/jcdd9120447