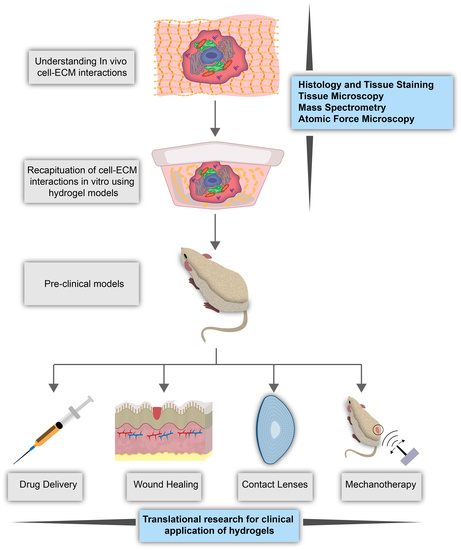
Biomimetic Hydrogels in the Study of Cancer Mechanobiology: Overview, Biomedical Applications, and Future Perspectives
Abstract
:1. Cellular Microenvironment
1.1. Extracellular Matrix
1.1.1. Cell-ECM Interactions
Mechanobiology of the Cellular Microenvironment

| Functional Category | Mechano-Transducers | Mechanical Signal | Examples of Cellular Responses |
|---|---|---|---|
| Cell Mechanical and Physical Properties | Integrins | Force | RhoA activation leading to increased cell stiffness [62,63] |
| Focal Adhesions | Force | Actin polymerization [55] | |
| Yes-associated protein (YAP) | Force | Oligodendrocyte morphology and maturation [40] | |
| Titin | Force | Implicated in development of mechanical unloading-induced diaphragm weakness [64] | |
| Stress Fibers (actin filaments, myosin II, etc.) | Force | Transmit tension to other proteins, regulate assembly of filaments [65] | |
| Vinculin | Force | Transmit tensile force [66] | |
| Myosin II | Force | Increased cortical tension and cell membrane fusion promotion [67] | |
| Vasodilator stimulated phosphoprotein (VASP), zyxin, and Testin LIM domain protein (TES) | Force | Regulate junction dynamics [68] | |
| Neurogenic locus notch homolog protein 1 (NOTCH1) | Shear Stress | Altered cell morphology [69] | |
| Piezo1 | Force | Vascular structure [70] | |
| Lamin A | Rigidity | Nuclear mechanics [71,72] | |
| Integrins | Force | Tyrosine Phosphorylation, MAPK signaling [15] | |
| Alters Signaling Pathways | Focal Adhesions | Force | Integrin convergence [73] |
| Fibronectin | Force | Altered integrin binding [74] | |
| T-cell receptor (TCR) | Force | T-cell calcium and IL-2 secretion [75] | |
| Talin | Force | Recruitment of vinculin to focal adhesion complexes [76] | |
| Piezo2 | Force | Serotonin release [77] | |
| Vinculin | Force | Enhanced PI3K activation [78] | |
| p130Cas | Force | Activation of Cas signaling pathway [79] | |
| Syndecan-1 | Force | Activation of pro-inflammatory and growth-stimulating pathways [80] | |
| Transient Receptor Potential Cation Channel Subfamily V Member 4 (TRPV4) | Force | Reorientation and flow-mediated nitric oxide production [81] | |
| Ion Channels | Force | Cell signaling [82] | |
| von Willebrand factor—glycoprotein Ib complex (VWF-GPIb) | Shear Stress | Enhanced calcium triggering in platelets and T cells [83] | |
| Platelet endothelial cell adhesion molecule-1 (PECAM-1) | Shear Stress | Tyrosine kinase Src and PI3K signaling activated [84] | |
| G-protein coupled receptor 68 (GPR68) | Shear Stress | Component in signaling for cardiovascular pathophysiology [85] | |
| β-catenin | Shear Stress | Activated expression of FOXC2 transcription factor [86] | |
| Caveolin-1 and β1 Integrin | Stiffness | FA assembly and turnover [62] | |
| rho-associated, coiled-coil-containing protein kinase (ROCK) 1 and 2 | Stiffness | Regulation of RhoA signaling pathways [87] | |
| YAP | Stiffness | Altered translocation depending on surrounding stiffness [88] | |
| Piezo1 | Force | Ion Permeation and selection [89] | |
| C-X-C motif chemokine receptor (CXCR1/2) | Shear Stress | Mediates laminar shear-stress-induced endothelial cell migration [90] | |
| Transforming growth factor beta 1 (TGFβ1) | Shear Stress | Collagenase-dependent fibroblast migration [91] | |
| Migration | RhoA | Force | Collective cell migration [92] |
| Vinculin and metavinculin | Force | Regulation of cell adhesion and motility [66] | |
| NOTCH1 | Shear Stress | Decreased proliferation [69] | |
| Caveolin 1 | Rigidity | Decreased proliferation [93] | |
| Cancer | YAP1 | Shear Stress Stiffness | Cancer cell motility [54] Nuclear localization of YAP1 [94] |
| TGFβ1 | Shear Stress | Human melanoma cell tumor invasiveness [91] | |
| PI3K/Akt pathway | Stiffness | Overexpression of VEGF in hepatocarcinoma cells [63] | |
| TRPV4 ion channel | Stiffness | Tumor vascularization through down-regulation of Rho kinase activity [95] | |
| microRNAs | Stiffness | Altered expression in different stiffness conditions [96] | |
| Twist1 | Stiffness | Induction of EMT and tumor metastasis [97] | |
| Myocardin related transcription factor A (MRTF-A) | Stiffness | Regulates miRNAs involved in myogenic differentiation [88] | |
| Differentiation | Focal Adhesions | Force | Osteogenic differentiation [98] Myofibroblastic differentiation [99] |
| Transient Receptor Potential Cation Channel Subfamily M Member 7 (TRPM7) | Shear Stress | Osteogenic differentiation of mesenchymal stromal cells [100] |
1.1.2. Cell-ECM Interactions in Cancer
1.2. Neighboring Cells and Secreted Factors
1.3. Hydrogels as In Vitro Models of the Cellular Microenvironment
1.3.1. Mimicking Cellular Microenvironment Biomechanics
1.3.2. Recapitulating Cellular Microenvironment Heterogeneity
2. Hydrogels and Their Applications
2.1. Types of Hydrogels
2.1.1. Natural Hydrogels
2.1.2. Synthetic Hydrogels
2.1.3. Hybrid Hydrogels
2.2. General Properties of Hydrogels
2.3. Research Applications of Hydrogels
2.4. Clinical Applications of Hydrogels
3. Cancer and the Tumor Microenvironment
3.1. Glioblastoma and the Tumor Microenvironment
3.1.1. The Blood-Brain Barrier
3.1.2. Extracellular Matrix of the Brain
3.1.3. Overview of Microenvironment and Biomechanics of Glioblastoma
Biomechanics of the Glioblastoma Extracellular Matrix
Modification of the Extracellular Matrix in the Brain by Glioblastoma Cells
3.1.4. Glioblastoma Migration, Invasion, and Mechanotransduction
3.1.5. In Vitro Studies of Mechanotransduction in Glioblastoma
3.1.6. Hydrogel Culture Methods
Collagen
Hyaluronan
Other Proteins
Composite Biomaterial Hydrogels
Synthetic Hydrogels
4. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Arg-Gly-Asp peptides | RGD |
| basic fibroblast growth factor | bFGF |
| blood-brain barrier | BBB |
| C-X-C motif chemokine receptor | CXCR |
| epidermal growth factor | EGF |
| epidermal growth factor receptor | EGFR |
| extracellular matrix | ECM |
| G-protein coupled receptor 68 | GPR68 |
| gelatin methacryloyl | GelMa |
| glioblastoma | GBM |
| human umbilical vein endothelial cells | HUVECs |
| hyaluronic acid | HA |
| linker of nucleo- and cyto-skeleton | LINC |
| matrix metalloproteinase | MMP |
| Myocardin related transcription factor A | MRTF-A |
| Neurogenic locus notch homolog protein 1 | NOTCH1 |
| phosphoinositide 3-kinase | PI3K |
| physically interacting cell sequencing | PIC-seq |
| platelet endothelial cell adhesion molecule-1 | PECAM-1 |
| polydimethylsiloxane | PDMS |
| polyethylene glycol | PEG |
| positron emission tomography | PET |
| rho-associated, coiled-coil-containing protein kinase | ROCK |
| T-cell receptor | TCR |
| Testin LIM domain protein | TES |
| Three-dimensional | 3D |
| Transforming growth factor beta 1 | TGF b1 |
| Transient Receptor Potential Cation Channel Subfamily M Member 7 | TRPM7 |
| Transient Receptor Potential Cation Channel Subfamily V Member 4 | TRPV4 |
| Two-dimensional | 2D |
| urokinase | uPA |
| urokinase receptor | uPAR |
| vascular endothelial growth factor | VEGF |
| vascular endothelial growth factor receptor | VEGFR |
| Vasodilator stimulated phosphoprotein | VASP |
| von Willebrand factor—glycoprotein Ib complex | VWF-GPIb |
| Yes-associated protein | YAP |
References
- Shakiba, D.; Babaei, B.; Saadat, F.; Thomopoulos, S.; Genin, G.M. The Fibrous Cellular Microenvironment, and How Cells Make Sense of a Tangled Web. Proc. Natl. Acad. Sci. USA 2017, 114, 5772–5774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. Noncollagen Components of the Extracellular Matrix. Molecular Cell Biology. 4th Edition. 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK21706/ (accessed on 1 March 2022).
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaffey, N. Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. and Walter, P. Molecular Biology of the Cell. 4th Edn. Ann. Bot. 2003, 91, 401. [Google Scholar] [CrossRef]
- Akhmanova, M.; Osidak, E.; Domogatsky, S.; Rodin, S.; Domogatskaya, A. Physical, Spatial, and Molecular Aspects of Extracellular Matrix of In Vivo Niches and Artificial Scaffolds Relevant to Stem Cells Research. Available online: https://www.hindawi.com/journals/sci/2015/167025/ (accessed on 25 May 2019).
- Muiznieks, L.D.; Keeley, F.W. Molecular Assembly and Mechanical Properties of the Extracellular Matrix: A Fibrous Protein Perspective. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Yue, B. Biology of the Extracellular Matrix: An Overview. J. Glaucoma 2014, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.K.; Basu, S.; Sharma, R.I. The Extracellular Matrix: Structure, Composition, Age-Related Differences, Tools for Analysis and Applications for Tissue Engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; Bissell, M.J. Of Extracellular Matrix, Scaffolds, and Signaling: Tissue Architecture Regulates Development, Homeostasis, and Cancer. Annu. Rev. Cell Dev. Biol. 2006, 22, 287–309. [Google Scholar] [CrossRef] [Green Version]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Ahmed, M.; ffrench-Constant, C. Extracellular Matrix Regulation of Stem Cell Behavior. Curr. Stem Cell Rep. 2016, 2, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R. Angiogenesis: Basement Membranes: Structure, Assembly and Role in Tumour Angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef]
- Malik, R.; Lelkes, P.I.; Cukierman, E. Biomechanical and Biochemical Remodeling of Stromal Extracellular Matrix in Cancer. Trends Biotechnol. 2015, 33, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, B.-Z.; Zamir, E.; Bershadsky, A.; Kam, Z.; Yamada, K.M.; Geiger, B.; Hynes, R. Physical State of the Extracellular Matrix Regulates the Structure and Molecular Composition of Cell-Matrix Adhesions. Mol. Biol. Cell 2000, 11, 1047–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mui, K.L.; Chen, C.S.; Assoian, R.K. The Mechanical Regulation of Integrin–Cadherin Crosstalk Organizes Cells, Signaling and Forces. J. Cell Sci. 2016, 129, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell Adhesion: Integrating Cytoskeletal Dynamics and Cellular Tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Latif, N.; Sarathchandra, P.; Taylor, P.M.; Antoniw, J.; Yacoub, M.H. Molecules Mediating Cell-ECM and Cell-Cell Communication in Human Heart Valves. Cell Biochem. Biophys. 2005, 43, 275–287. [Google Scholar] [CrossRef]
- Seong, J.; Wang, N.; Wang, Y. Mechanotransduction at Focal Adhesions: From Physiology to Cancer Development. J. Cell Mol. Med. 2013, 17, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Bernfield, M.; Götte, M.; Park, P.W.; Reizes, O.; Fitzgerald, M.L.; Lincecum, J.; Zako, M. Functions of Cell Surface Heparan Sulfate Proteoglycans. Annu. Rev. Biochem. 1999, 68, 729–777. [Google Scholar] [CrossRef]
- Salmivirta, M.; Jalkanen, M. Syndecan Family of Cell Surface Proteoglycans: Developmentally Regulated Receptors for Extracellular Effector Molecules. Experientia 1995, 51, 863–872. [Google Scholar] [CrossRef]
- Mythreye, K.; Blobe, G.C. Proteoglycan Signaling Co–Receptors: Roles in Cell Adhesion, Migration and Invasion. Cell. Signal. 2009, 21, 1548–1558. [Google Scholar] [CrossRef] [Green Version]
- Lin, X. Functions of Heparan Sulfate Proteoglycans in Cell Signaling during Development. Development 2004, 131, 6009–6021. [Google Scholar] [CrossRef] [Green Version]
- Kirkbride, K.C.; Ray, B.N.; Blobe, G.C. Cell-Surface Co-Receptors: Emerging Roles in Signaling and Human Disease. Trends Biochem. Sci. 2005, 30, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of Mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Wang, N. Review of Cellular Mechanotransduction. J. Phys. D Appl. Phys. 2017, 50, 233002. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Emon, M.A.B.; Staudacher, J.J.; Thomas, A.L.; Zessner-Spitzenberg, J.; Mancinelli, G.; Krett, N.; Saif, M.T.; Jung, B. Increased Stiffness of the Tumor Microenvironment in Colon Cancer Stimulates Cancer Associated Fibroblast-Mediated Prometastatic Activin A Signaling. Sci. Rep. 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Emon, B.; Bauer, J.; Jain, Y.; Jung, B.; Saif, T. Biophysics of Tumor Microenvironment and Cancer Metastasis-A Mini Review. Comput. Struct. Biotechnol. J. 2018, 16, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell Stiffness Is a Biomarker of the Metastatic Potential of Ovarian Cancer Cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Iwata, M. Stiffness of Cancer Cells Measured with an AFM Indentation Method. J. Mech. Behav. Biomed. Mater. 2015, 49, 105–111. [Google Scholar] [CrossRef]
- Raudenska, M.; Kratochvilova, M.; Vicar, T.; Gumulec, J.; Balvan, J.; Polanska, H.; Pribyl, J.; Masarik, M. Cisplatin Enhances Cell Stiffness and Decreases Invasiveness Rate in Prostate Cancer Cells by Actin Accumulation. Sci. Rep. 2019, 9, 1660. [Google Scholar] [CrossRef]
- Xiao, W.; Sohrabi, A.; Seidlits, S.K. Integrating the Glioblastoma Microenvironment into Engineered Experimental Models. Future Sci. OA 2017, 3, FSO189. [Google Scholar] [CrossRef] [Green Version]
- Pepin, K.M.; McGee, K.P.; Arani, A.; Lake, D.S.; Glaser, K.J.; Manduca, A.; Parney, I.F.; Ehman, R.L.; Huston, J. MR Elastography Analysis of Glioma Stiffness and IDH1-Mutation Status. Am. J. Neuroradiol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Streitberger, K.-J.; Reiss-Zimmermann, M.; Freimann, F.B.; Bayerl, S.; Guo, J.; Arlt, F.; Wuerfel, J.; Braun, J.; Hoffmann, K.-T.; Sack, I. High-Resolution Mechanical Imaging of Glioblastoma by Multifrequency Magnetic Resonance Elastography. PLoS ONE 2014, 9, e110588. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.D.; Cox, T.R. Relative Stiffness Measurements of Tumour Tissues by Shear Rheology. Bio-Protoc. 2017, 7, e2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Xiong, F.; Li, X.; Xiang, B.; Li, Z.; Wu, X.; Guo, C.; Li, X.; Li, Y.; Li, G.; et al. Application of Atomic Force Microscopy in Cancer Research. J. Nanobiotechnology 2018, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, E.J.; Sohn, Y.-M.; Seo, M. Tumor Stiffness Measured by Quantitative and Qualitative Shear Wave Elastography of Breast Cancer. Br. J. Radiol. 2018, 91. [Google Scholar] [CrossRef]
- Bondu, V.; Wu, C.; Cao, W.; Simons, P.C.; Gillette, J.; Zhu, J.; Erb, L.; Zhang, X.F.; Buranda, T. Low-Affinity Binding in Cis to P2Y2R Mediates Force-Dependent Integrin Activation during Hantavirus Infection. Mol. Biol. Cell 2017, 28, 2887–2903. [Google Scholar] [CrossRef]
- Nordenfelt, P.; Elliott, H.L.; Springer, T.A. Coordinated Integrin Activation by Actin-Dependent Force during T-Cell Migration. Nat. Commun. 2016, 7, 13119. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Springer, T.A. Integrin Extension Enables Ultrasensitive Regulation by Cytoskeletal Force. Proc. Natl. Acad. Sci. USA 2017, 114, 4685–4690. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Osanai, Y.; Tanaka, K.F.; Abe, M.; Natsume, R.; Sakimura, K.; Ikenaka, K. YAP Functions as a Mechanotransducer in Oligodendrocyte Morphogenesis and Maturation. Glia 2017, 65, 360–374. [Google Scholar] [CrossRef]
- Conway, D.E.; Schwartz, M.A. Mechanotransduction of Shear Stress Occurs through Changes in VE-Cadherin and PECAM-1 Tension: Implications for Cell Migration. Cell Adh. Migr. 2014, 9, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Kale, G.R.; Yang, X.; Philippe, J.-M.; Mani, M.; Lenne, P.-F.; Lecuit, T. Distinct Contributions of Tensile and Shear Stress on E-Cadherin Levels during Morphogenesis. Nat. Commun. 2018, 9, 5021. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-H.; Cherng, W.-J.; Verma, S. Drawbacks to Stem Cell Therapy in Cardiovascular Diseases. Future Cardiol. 2008, 4, 399–408. [Google Scholar] [CrossRef]
- Snyder, J.L.; McBeath, E.; Thomas, T.N.; Chiu, Y.J.; Clark, R.L.; Fujiwara, K. Mechanotransduction Properties of the Cytoplasmic Tail of PECAM-1. Biol. Cell 2017, 109, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliemann, L.; Rytter, N.; Piil, P.; Nilton, J.; Lind, T.; Nyberg, M.; Cocks, M.; Hellsten, Y. The Endothelial Mechanotransduction Protein Platelet Endothelial Cell Adhesion Molecule-1 Is Influenced by Aging and Exercise Training in Human Skeletal Muscle. Front. Physiol. 2018, 9, 1807. [Google Scholar] [CrossRef] [PubMed]
- Chachisvilis, M.; Zhang, Y.-L.; Frangos, J.A. G Protein-Coupled Receptors Sense Fluid Shear Stress in Endothelial Cells. Proc. Natl. Acad. Sci. USA 2006, 103, 15463–15468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckmann, R.; Houben, A.; Tohidnezhad, M.; Kweider, N.; Fragoulis, A.; Wruck, C.J.; Brandenburg, L.O.; Hermanns-Sachweh, B.; Goldring, M.B.; Pufe, T.; et al. Mechanical Forces Induce Changes in VEGF and VEGFR-1/SFlt-1 Expression in Human Chondrocytes. Int. J. Mol. Sci. 2014, 15, 15456–15474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, L.; Chen, Y.; Xue, L.; Du, X.; Zhu, C. Cooperative Unfolding of Distinctive Mechanoreceptor Domains Transduces Force into Signals. eLife 2016, 5. [Google Scholar] [CrossRef]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a Distance: Mechanically Coupling the Extracellular Matrix with the Nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef]
- Poh, Y.-C.; Na, S.; Chowdhury, F.; Ouyang, M.; Wang, Y.; Wang, N. Rapid Activation of Rac GTPase in Living Cells by Force Is Independent of Src. PLoS ONE 2009, 4, e7886. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Stoops, E.; CP, U.; Markus, B.; Reuveny, A.; Ordan, E.; Volk, T. Mechanotransduction via the LINC Complex Regulates DNA Replication in Myonuclei. J. Cell Biol. 2018, 217, 2005–2018. [Google Scholar] [CrossRef]
- del Alamo, J.C.; Norwich, G.N.; Li, Y.J.; Lasheras, J.C.; Chien, S. Anisotropic Rheology and Directional Mechanotransduction in Vascular Endothelial Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 15411–15416. [Google Scholar] [CrossRef] [Green Version]
- Na, S.; Collin, O.; Chowdhury, F.; Tay, B.; Ouyang, M.; Wang, Y.; Wang, N. Rapid Signal Transduction in Living Cells Is a Unique Feature of Mechanotransduction. Proc. Natl. Acad. Sci. USA 2008, 105, 6626–6631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Keely, P.J. Mechanical Signaling through the Cytoskeleton Regulates Cell Proliferation by Coordinated Focal Adhesion and Rho GTPase Signaling. J. Cell Sci. 2011, 124, 1195–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isenberg, B.C.; DiMilla, P.A.; Walker, M.; Kim, S.; Wong, J.Y. Vascular Smooth Muscle Cell Durotaxis Depends on Substrate Stiffness Gradient Strength. Biophys. J. 2009, 97, 1313–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouzés, E.; Farge, E. Interplay of Mechanical Deformation and Patterned Gene Expression in Developing Embryos. Curr. Opin. Genet. Dev. 2004, 14, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in Development: A Growing Role for Contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Mammoto, T.; Ingber, D.E. Mechanical Control of Tissue and Organ Development. Development 2010, 137, 1407–1420. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Matthews, B.D.; Overby, D.R.; Mannix, R.; Ingber, D.E. Cellular Adaptation to Mechanical Stress: Role of Integrins, Rho, Cytoskeletal Tension and Mechanosensitive Ion Channels. J. Cell Sci. 2006, 119, 508–518. [Google Scholar] [CrossRef] [Green Version]
- Yeh, Y.-C.; Ling, J.-Y.; Chen, W.-C.; Lin, H.-H.; Tang, M.-J. Mechanotransduction of Matrix Stiffness in Regulation of Focal Adhesion Size and Number: Reciprocal Regulation of Caveolin-1 and Β1 Integrin. Sci. Rep. 2017, 7, 15008. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Xie, X.; Wang, Z.; Hu, C.; Zheng, Q.; Wang, Y.; Chen, R.; Xue, T.; Chen, J.; Gao, D.; et al. Increasing Matrix Stiffness Upregulates Vascular Endothelial Growth Factor Expression in Hepatocellular Carcinoma Cells Mediated by Integrin Β1. Biochem. Biophys. Res. Commun. 2014, 444, 427–432. [Google Scholar] [CrossRef] [PubMed]
- van der Pijl, R.J.; Granzier, H.; Ottenheijm, C.A.C. Diaphragm Contractile Weakness Due to Altered Mechanical Loading: Role of Titin. Am. J. Physiol. Cell Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Wittchen, E.S. The Tension Mounts: Stress Fibers as Force-Generating Mechanotransducers. J. Cell Biol. 2013, 200, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Sharek, L.; O’Brien, E.T.; Urbina, F.L.; Gupton, S.L.; Superfine, R.; Burridge, K.; Campbell, S.L. Vinculin and Metavinculin Exhibit Distinct Effects on Focal Adhesion Properties, Cell Migration, and Mechanotransduction. PLoS ONE 2019, 14, e0221962. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.Y.; Ulrich, T.A.; Deleyrolle, L.P.; MacKay, J.L.; Lin, J.-M.G.; Martuscello, R.T.; Jundi, M.A.; Reynolds, B.A.; Kumar, S. Constitutive Activation of Myosin-Dependent Contractility Sensitizes Glioma Tumor-Initiating Cells to Mechanical Inputs and Reduces Tissue Invasion. Cancer Res. 2015, 75, 1113–1122. [Google Scholar] [CrossRef] [Green Version]
- Oldenburg, J.; van der Krogt, G.; Twiss, F.; Bongaarts, A.; Habani, Y.; Slotman, J.A.; Houtsmuller, A.; Huveneers, S.; de Rooij, J. VASP, Zyxin and TES Are Tension-Dependent Members of Focal Adherens Junctions Independent of the α-Catenin-Vinculin Module. Sci. Rep. 2015, 5, 17225. [Google Scholar] [CrossRef] [Green Version]
- Mack, J.J.; Mosqueiro, T.S.; Archer, B.J.; Jones, W.M.; Sunshine, H.; Faas, G.C.; Briot, A.; Aragón, R.L.; Su, T.; Romay, M.C.; et al. NOTCH1 Is a Mechanosensor in Adult Arteries. Nat. Commun. 2017, 8, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 Integration of Vascular Architecture with Physiological Force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.D.P.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.-W.; Tewari, M.; et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 2013, 341. [Google Scholar] [CrossRef] [Green Version]
- Lammerding, J.; Schulze, P.C.; Takahashi, T.; Kozlov, S.; Sullivan, T.; Kamm, R.D.; Stewart, C.L.; Lee, R.T. Lamin A/C Deficiency Causes Defective Nuclear Mechanics and Mechanotransduction. J. Clin. Investig. 2004, 113, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, M.A.; Modzelewska, K.; Kwong, L.; Keely, P.J. Focal Adhesion Regulation of Cell Behavior. Biochim. Biophys. Acta 2004, 1692, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Kubow, K.E.; Vukmirovic, R.; Zhe, L.; Klotzsch, E.; Smith, M.L.; Gourdon, D.; Luna, S.; Vogel, V. Mechanical Forces Regulate the Interactions of Fibronectin and Collagen I in Extracellular Matrix. Nat. Commun. 2015, 6, 8026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Chen, W.; Evavold, B.D.; Zhu, C. Accumulation of Dynamic Catch Bonds between TCR and Agonist Peptide-MHC Triggers T Cell Signaling. Cell 2014, 157, 357–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, S.; Ng, W.P.; Kumar, S. Contributions of Talin-1 to Glioma Cell–Matrix Tensional Homeostasis. J. R. Soc. Interface 2012, 9, 1311–1317. [Google Scholar] [CrossRef]
- Alcaino, C.; Knutson, K.R.; Treichel, A.J.; Yildiz, G.; Strege, P.R.; Linden, D.R.; Li, J.H.; Leiter, A.B.; Szurszewski, J.H.; Farrugia, G.; et al. A Population of Gut Epithelial Enterochromaffin Cells Is Mechanosensitive and Requires Piezo2 to Convert Force into Serotonin Release. Proc. Natl. Acad. Sci. USA 2018, 115, E7632–E7641. [Google Scholar] [CrossRef] [Green Version]
- Rubashkin, M.G.; Cassereau, L.; Bainer, R.; DuFort, C.C.; Yui, Y.; Ou, G.; Paszek, M.J.; Davidson, M.W.; Chen, Y.-Y.; Weaver, V.M. Force Engages Vinculin and Promotes Tumor Progression by Enhancing PI3K Activation of Phosphatidylinositol (3,4,5)-Triphosphate. Cancer Res. 2014, 74, 4597–4611. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Tamada, M.; Dubin-Thaler, B.J.; Cherniavskaya, O.; Sakai, R.; Tanaka, S.; Sheetz, M.P. Force Sensing by Mechanical Extension of the Src Family Kinase Substrate P130Cas. Cell 2006, 127, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Julien, M.A.; Haller, C.A.; Wang, P.; Wen, J.; Chaikof, E.L. Mechanical Strain Induces a Persistent Upregulation of Syndecan-1 Expression in Smooth Muscle Cells. J. Cell. Physiol. 2007, 211, 167–173. [Google Scholar] [CrossRef]
- Cappelli, H.C.; Kanugula, A.K.; Adapala, R.K.; Amin, V.; Sharma, P.; Midha, P.; Paruchuri, S.; Thodeti, C.K. Mechanosensitive TRPV4 Channels Stabilize VE-Cadherin Junctions to Regulate Tumor Vascular Integrity and Metastasis. Cancer Lett. 2019, 442, 15–20. [Google Scholar] [CrossRef]
- Martinac, B. Mechanosensitive Ion Channels: Molecules of Mechanotransduction. J. Cell Sci. 2004, 117, 2449–2460. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Deng, W.; Zhou, L.; Xu, Y.; Yang, W.; Liang, X.; Wang, Y.; Kulman, J.D.; Zhang, X.F.; Li, R. Identification of a Juxtamembrane Mechanosensitive Domain in the Platelet Mechanosensor Glycoprotein Ib-IX Complex. Blood 2015, 125, 562–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Science, A.A. for the A. of PECAM-1 as Fluid Shear Stress Sensor. Sci. Signal. 2005, 2005, tw333. [Google Scholar] [CrossRef]
- Xu, J.; Mathur, J.; Vessières, E.; Hammack, S.; Nonomura, K.; Favre, J.; Grimaud, L.; Petrus, M.; Francisco, A.; Li, J.; et al. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 2018, 173, 762–775.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, B.; Srinivasan, R.S. Mechanosensitive β-Catenin Signaling Regulates Lymphatic Vascular Development. BMB Rep. 2016, 49, 403–404. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Chen, Y.; Qin, X.; Li, S.; Liu, Y. Unveiling the Mechanotransduction Mechanism of Substrate Stiffness-Modulated Cancer Cell Motility via ROCK1 and ROCK2 Differentially Regulated Manner. FASEB J. 2019, 33, 644.4. [Google Scholar] [CrossRef]
- Hadden, W.J.; Young, J.L.; Holle, A.W.; McFetridge, M.L.; Kim, D.Y.; Wijesinghe, P.; Taylor-Weiner, H.; Wen, J.H.; Lee, A.R.; Bieback, K.; et al. Stem Cell Migration and Mechanotransduction on Linear Stiffness Gradient Hydrogels. Proc. Natl. Acad. Sci. USA 2017, 114, 5647–5652. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xiao, B. The Mechanosensitive Piezo1 Channel: Structural Features and Molecular Bases Underlying Its Ion Permeation and Mechanotransduction. J. Physiol. 2018, 596, 969–978. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Shen, Y.; Huang, X.-L.; Liu, X.-J.; Liu, X.-H. Roles of Mechanical Force and CXCR1/CXCR2 in Shear-Stress-Induced Endothelial Cell Migration. Eur. Biophys. J. 2012, 41, 13–25. [Google Scholar] [CrossRef]
- Shieh, A.C.; Rozansky, H.A.; Hinz, B.; Swartz, M.A. Tumor Cell Invasion Is Promoted by Interstitial Flow-Induced Matrix Priming by Stromal Fibroblasts. Cancer Res. 2011, 71, 790–800. [Google Scholar] [CrossRef] [Green Version]
- Burridge, K.; Monaghan-Benson, E.; Graham, D.M. Mechanotransduction: From the Cell Surface to the Nucleus via RhoA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374. [Google Scholar] [CrossRef]
- Chin, L.; Xia, Y.; Discher, D.E.; Janmey, P.A. Mechanotransduction in Cancer. Curr. Opin. Chem. Eng. 2016, 11, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, A.J.; Hicks, S.R.; Svec, K.V.; Naughton, H.; Edmunds, Z.L.; Howe, A.K. The Mechanical Microenvironment Regulates Ovarian Cancer Cell Morphology, Migration, and Spheroid Disaggregation. Sci. Rep. 2018, 8, 7228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoppil, R.J.; Cappelli, H.C.; Adapala, R.K.; Kanugula, A.K.; Paruchuri, S.; Thodeti, C.K. TRPV4 Channels Regulate Tumor Angiogenesis via Modulation of Rho/Rho Kinase Pathway. Oncotarget 2016, 7, 25849–25861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broders-Bondon, F.; Ho-Bouldoires, T.H.N.; Fernandez-Sanchez, M.-E.; Farge, E. Mechanotransduction in Tumor Progression: The Dark Side of the Force. J. Cell Biol. 2018, 217, 1571–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.C.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.J.; et al. Matrix Stiffness Drives Epithelial-Mesenchymal Transition and Tumour Metastasis through a TWIST1-G3BP2 Mechanotransduction Pathway. Nat. Cell Biol. 2015, 17, 678–688. [Google Scholar] [CrossRef]
- Biggs, M.J.P.; Dalby, M.J. Focal Adhesions in Osteoneogenesis. Proc. Inst. Mech Eng. H 2010, 224, 1441–1453. [Google Scholar] [CrossRef] [Green Version]
- Dugina, V.; Fontao, L.; Chaponnier, C.; Vasiliev, J.; Gabbiani, G. Focal Adhesion Features during Myofibroblastic Differentiation Are Controlled by Intracellular and Extracellular Factors. J. Cell Sci. 2001, 114, 3285–3296. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Liu, Y.-A.; Huang, C.-J.; Yen, M.-H.; Tseng, C.-T.; Chien, S.; Lee, O.K. Mechanosensitive TRPM7 Mediates Shear Stress and Modulates Osteogenic Differentiation of Mesenchymal Stromal Cells through Osterix Pathway. Sci. Rep. 2015, 5, 16522. [Google Scholar] [CrossRef] [Green Version]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction Gone Awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Pathak, A.; Kumar, S. Independent Regulation of Tumor Cell Migration by Matrix Stiffness and Confinement. Proc. Natl. Acad. Sci. USA 2012, 109, 10334–10339. [Google Scholar] [CrossRef] [Green Version]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maherally, Z.; Smith, J.R.; Ghoneim, M.K.; Dickson, L.; An, Q.; Fillmore, H.L.; Pilkington, G.J. Silencing of CD44 in Glioma Leads to Changes in Cytoskeletal Protein Expression and Cellular Biomechanical Deformation Properties as Measured by AFM Nanoindentation. BioNanoScience 2016, 6, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Prabhune, M.; Belge, G.; Dotzauer, A.; Bullerdiek, J.; Radmacher, M. Comparison of Mechanical Properties of Normal and Malignant Thyroid Cells. Micron 2012, 43, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.R.; Pabijan, J.; Garcia, R.; Lekka, M. The Softening of Human Bladder Cancer Cells Happens at an Early Stage of the Malignancy Process. Beilstein J. Nanotechnol. 2014, 5, 447–457. [Google Scholar] [CrossRef]
- Lekka, M.; Pabijan, J. Measuring Elastic Properties of Single Cancer Cells by AFM. Methods Mol. Biol. 2019, 1886, 315–324. [Google Scholar] [CrossRef]
- Plodinec, M.; Loparic, M.; Monnier, C.A.; Obermann, E.C.; Zanetti-Dallenbach, R.; Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; Lim, R.Y.H.; et al. The Nanomechanical Signature of Breast Cancer. Nat. Nanotechnol. 2012, 7, 757–765. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional Homeostasis and the Malignant Phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Nishida-Aoki, N.; Gujral, T.S. Emerging Approaches to Study Cell–Cell Interactions in Tumor Microenvironment. Oncotarget 2019, 10, 785–797. [Google Scholar] [CrossRef] [Green Version]
- Zervantonakis, I.K.; Poskus, M.D.; Scott, A.L.; Selfors, L.M.; Lin, J.-R.; Dillon, D.A.; Pathania, S.; Sorger, P.K.; Mills, G.B.; Brugge, J.S. Fibroblast–Tumor Cell Signaling Limits HER2 Kinase Therapy Response via Activation of MTOR and Antiapoptotic Pathways. Proc. Natl. Acad. Sci. USA 2020, 117, 16500–16508. [Google Scholar] [CrossRef]
- Mueller, M.; Rasoulinejad, S.; Garg, S.; Wegner, S.V. The Importance of Cell–Cell Interaction Dynamics in Bottom-Up Tissue Engineering: Concepts of Colloidal Self-Assembly in the Fabrication of Multicellular Architectures. Nano Lett. 2020, 20, 2257–2263. [Google Scholar] [CrossRef]
- Mayor, R.; Etienne-Manneville, S. The Front and Rear of Collective Cell Migration. Nat. Rev. Mol. Cell Biol. 2016, 17, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.S.; Tan, J.; Tien, J. Mechanotransduction at Cell-Matrix and Cell-Cell Contacts. Annu. Rev. Biomed. Eng. 2004, 6, 275–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giladi, A.; Cohen, M.; Medaglia, C.; Baran, Y.; Li, B.; Zada, M.; Bost, P.; Blecher-Gonen, R.; Salame, T.-M.; Mayer, J.U.; et al. Dissecting Cellular Crosstalk by Sequencing Physically Interacting Cells. Nat. Biotechnol. 2020, 38, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Melissaridou, S.; Wiechec, E.; Magan, M.; Jain, M.V.; Chung, M.K.; Farnebo, L.; Roberg, K. The Effect of 2D and 3D Cell Cultures on Treatment Response, EMT Profile and Stem Cell Features in Head and Neck Cancer. Cancer Cell Int. 2019, 19, 16. [Google Scholar] [CrossRef] [Green Version]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-Dimensional Cell Culture Matrices: State of the Art. Tissue Eng. Part. B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.-H.; Chen, Y.-D.; Huang, S.-F.; Wang, H.-M.; Wu, M.-H. The Effect of Primary Cancer Cell Culture Models on the Results of Drug Chemosensitivity Assays: The Application of Perfusion Microbioreactor System as Cell Culture Vessel. Biomed. Res. Int. 2015, 2015, 470283. [Google Scholar] [CrossRef]
- Bonnier, F.; Keating, M.E.; Wróbel, T.P.; Majzner, K.; Baranska, M.; Garcia-Munoz, A.; Blanco, A.; Byrne, H.J. Cell Viability Assessment Using the Alamar Blue Assay: A Comparison of 2D and 3D Cell Culture Models. Toxicol. Vitr. 2015, 29, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Petrie, R.J.; Yamada, K.M. At the Leading Edge of Three-Dimensional Cell Migration. J. Cell Sci. 2012, 125, 5917–5926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera-Perez, M.; Voytik-Harbin, S.L.; Rickus, J.L. Extracellular Matrix Properties Regulate the Migratory Response of Glioblastoma Stem Cells in Three-Dimensional Culture. Tissue Eng. Part A 2015, 21, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Petrie, R.J.; Gavara, N.; Chadwick, R.S.; Yamada, K.M. Nonpolarized Signaling Reveals Two Distinct Modes of 3D Cell Migration. J. Cell Biol. 2012, 197, 439–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.M.; Amann, A.; Koeck, S.; Lorenz, E.; Kelm, J.M.; Obexer, P.; Zwierzina, H.; Gamerith, G. Evaluation of Assays for Drug Efficacy in a Three-Dimensional Model of the Lung. J. Cancer Res. Clin. Oncol. 2016, 142, 1955–1966. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Sahan, A.Z.; Lim, E.; Suarez, K. The Potential Use of a Three Dimensional Cell Culture of Spheroids in the Study of Various Therapeutic Approaches. MOJ Immunol. 2016, 3, 11–12. [Google Scholar] [CrossRef]
- Barthes, J.; Özçelik, H.; Hindié, M.; Ndreu-Halili, A.; Hasan, A.; Vrana, N.E. Cell Microenvironment Engineering and Monitoring for Tissue Engineering and Regenerative Medicine: The Recent Advances. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Gentili, C.; Cancedda, R. Cartilage and Bone Extracellular Matrix. Curr. Pharm. Des. 2009, 15, 1334–1348. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable Hydrogels for Cartilage and Bone Tissue Engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Charrier, E.E.; Pogoda, K.; Wells, R.G.; Janmey, P.A. Control of Cell Morphology and Differentiation by Substrates with Independently Tunable Elasticity and Viscous Dissipation. Nat. Commun. 2018, 9, 449. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorthi, K.; Hara, J.; Ito, C.; Asuri, P. Role of Three-Dimensional Matrix Stiffness in Regulating the Response of Human Neural Cells to Toxins. Cell. Mol. Bioeng. 2014, 7, 278–284. [Google Scholar] [CrossRef]
- Pogoda, K.; Bucki, R.; Byfield, F.J.; Cruz, K.; Lee, T.; Marcinkiewicz, C.; Janmey, P.A. Soft Substrates Containing Hyaluronan Mimic the Effects of Increased Stiffness on Morphology, Motility, and Proliferation of Glioma Cells. Biomacromolecules 2017, 18, 3040–3051. [Google Scholar] [CrossRef] [PubMed]
- Koch, D.; Rosoff, W.J.; Jiang, J.; Geller, H.M.; Urbach, J.S. Strength in the Periphery: Growth Cone Biomechanics and Substrate Rigidity Response in Peripheral and Central Nervous System Neurons. Biophys. J. 2012, 102, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Fuente, G.; Mollinedo, P.; Grande, L.; Vazquez-Barquero, A.; Fernandez-Luna, J.L. Culture Dimensionality Influences the Resistance of Glioblastoma Stem-like Cells to Multikinase Inhibitors. Mol. Cancer 2014, 13, 1664–1672. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.; Guo, W.-H.; Wang, Y.-L. Fibroblasts Probe Substrate Rigidity with Filopodia Extensions before Occupying an Area. Proc. Natl. Acad. Sci. USA 2014, 111, 17176–17181. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.S.; Bentil, S.; DeJesus, J.; Larison, J.; Hissong, A.; Dupaix, R.; Sarkar, A.; Winter, J.O. Inherent Interfacial Mechanical Gradients in 3D Hydrogels Influence Tumor Cell Behaviors. PLoS ONE 2012, 7, e35852. [Google Scholar] [CrossRef]
- Stabenfeldt, S.E.; LaPlaca, M.C. Variations in Rigidity and Ligand Density Influence Neuronal Response in Methylcellulose–Laminin Hydrogels. Acta Biomater. 2011, 7, 4102–4108. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.F.; Bártolo, P.J. 3D Bioprinting of Photocrosslinkable Hydrogel Constructs. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef] [Green Version]
- Bessonov, I.V.; Rochev, Y.A.; Arkhipova, A.Y.; Kopitsyna, M.N.; Bagrov, D.V.; Karpushkin, E.A.; Bibikova, T.N.; Moysenovich, A.M.; Soldatenko, A.S.; Nikishin, I.I.; et al. Fabrication of Hydrogel Scaffolds via Photocrosslinking of Methacrylated Silk Fibroin. Biomed. Mater. 2019, 14, 034102. [Google Scholar] [CrossRef]
- Seiffert, S.; Oppermann, W.; Saalwächter, K. Hydrogel Formation by Photocrosslinking of Dimethylmaleimide Functionalized Polyacrylamide. Polymer 2007, 48, 5599–5611. [Google Scholar] [CrossRef]
- O’Connell, C.D.; Zhang, B.; Onofrillo, C.; Duchi, S.; Blanchard, R.; Quigley, A.; Bourke, J.; Gambhir, S.; Kapsa, R.; Bella, C.D.; et al. Tailoring the Mechanical Properties of Gelatin Methacryloyl Hydrogels through Manipulation of the Photocrosslinking Conditions. Soft Matter 2018, 14, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K. Cross-Linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Baday, M.; Ercal, O.; Sahan, A.Z.; Sahan, A.; Ercal, B.; Inan, H.; Demirci, U. Density Based Characterization of Mechanical Cues on Cancer Cells Using Magnetic Levitation. Adv. Healthc. Mater. 2019, 8, 1801517. [Google Scholar] [CrossRef] [PubMed]
- Akalin, O.B.; Bayraktar, H. Alteration of Cell Motility Dynamics through Collagen Fiber Density in Photopolymerized Polyethylene Glycol Hydrogels. Int. J. Biol. Macromol. 2020, 157, 414–423. [Google Scholar] [CrossRef]
- Ananthanarayanan, B.; Kim, Y.; Kumar, S. Elucidating the Mechanobiology of Malignant Brain Tumors Using a Brain Matrix-Mimetic Hyaluronic Acid Hydrogel Platform. Biomaterials 2011, 32, 7913–7923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samal, S.K.; Dash, M.; Dubruel, P.; Van Vlierberghe, S. Chapter 8—Smart Polymer Hydrogels: Properties, Synthesis and Applications. In Smart Polymers and their Applications; Aguilar, M.R., San Román, J., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 237–270. ISBN 978-0-85709-695-1. [Google Scholar]
- Kopecek, J. Hydrogel Biomaterials: A Smart Future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Faraj, Y.; Ju, X.-J.; Wang, W.; Xie, R.; Chu, L.-Y. Nanocomposite Smart Hydrogels with Improved Responsiveness and Mechanical Properties: A Mini Review. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1306–1313. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.L.; Hwang, Y.; Kar, M.; Varghese, S. Smart Hydrogels as Functional Biomimetic Systems. Biomater. Sci. 2014, 2, 603–618. [Google Scholar] [CrossRef]
- Dzobo, K.; Motaung, K.S.C.M.; Adesida, A. Recent Trends in Decellularized Extracellular Matrix Bioinks for 3D Printing: An Updated Review. Int. J. Mol. Sci. 2019, 20, 4628. [Google Scholar] [CrossRef] [Green Version]
- Tam, R.Y.; Smith, L.J.; Shoichet, M.S. Engineering Cellular Microenvironments with Photo- and Enzymatically Responsive Hydrogels: Toward Biomimetic 3D Cell Culture Models. Acc. Chem. Res. 2017, 50, 703–713. [Google Scholar] [CrossRef]
- DeQuach, J.A.; Mezzano, V.; Miglani, A.; Lange, S.; Keller, G.M.; Sheikh, F.; Christman, K.L. Simple and High Yielding Method for Preparing Tissue Specific Extracellular Matrix Coatings for Cell Culture. PLoS ONE 2010, 5, e13039. [Google Scholar] [CrossRef] [Green Version]
- Scherzer, M.T.; Waigel, S.; Donninger, H.; Arumugam, V.; Zacharias, W.; Clark, G.; Siskind, L.J.; Soucy, P.; Beverly, L. Fibroblast-Derived Extracellular Matrices: An Alternative Cell Culture System That Increases Metastatic Cellular Properties. PLoS ONE 2015, 10, e0138065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.W.; Kook, Y.-M.; Lee, H.J.; Park, H.; Koh, W.-G. A Three-Dimensional Co-Culture of HepG2 Spheroids and Fibroblasts Using Double-Layered Fibrous Scaffolds Incorporated with Hydrogel Micropatterns. RSC Adv. 2014, 4, 61005–61011. [Google Scholar] [CrossRef]
- Hunt, N.C.; Shelton, R.M.; Grover, L. An Alginate Hydrogel Matrix for the Localised Delivery of a Fibroblast/Keratinocyte Co-Culture. Biotechnol. J. 2009, 4, 730–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Ikada, Y.; Tabata, Y. Controlled Release of Growth Factors Based on Biodegradation of Gelatin Hydrogel. J. Biomater. Sci. Polym. Ed. 2001, 12, 77–88. [Google Scholar] [CrossRef]
- Tabata, Y. Tissue Regeneration Based on Growth Factor Release. Tissue Eng. 2003, 9 (Suppl. S1), S5–S15. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel Biomaterial Strategies for Controlled Growth Factor Delivery for Biomedical Applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design Properties of Hydrogel Tissue-Engineering Scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Saha, N.; Saarai, A.; Roy, N.; Kitano, T.; Saha, P. Polymeric Biomaterial Based Hydrogels for Biomedical Applications. J. Biomater. Nanobiotechnology 2011, 2, 85. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Mequanint, K. Hydrogel Biomaterials. Biomed. Eng.-Front. Chall. 2011. [Google Scholar] [CrossRef] [Green Version]
- Lutolf, M.P.; Lauer-Fields, J.L.; Schmoekel, H.G.; Metters, A.T.; Weber, F.E.; Fields, G.B.; Hubbell, J.A. Synthetic Matrix Metalloproteinase-Sensitive Hydrogels for the Conduction of Tissue Regeneration: Engineering Cell-Invasion Characteristics. Proc. Natl. Acad. Sci. USA 2003, 100, 5413–5418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Illeperuma, W.R.K.; Suo, Z.; Vlassak, J.J. Hybrid Hydrogels with Extremely High Stiffness and Toughness. ACS Macro Lett. 2014, 3, 520–523. [Google Scholar] [CrossRef]
- Dehne, H.; Hecht, F.M.; Bausch, A.R. The Mechanical Properties of Polymer–Colloid Hybrid Hydrogels. Soft Matter 2017, 13, 4786–4790. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.R.; Patel, S.; Singh, D. Chapter 9—Natural Polymer-Based Hydrogels as Scaffolds for Tissue Engineering. In Nanobiomaterials in Soft Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 231–260. ISBN 978-0-323-42865-1. [Google Scholar]
- Vieira, S.; da Silva Morais, A.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Natural-Based Hydrogels: From Processing to Applications. In Encyclopedia of Polymer Science and Technology; American Cancer Society: Atlanta, GA, USA, 2017; pp. 1–27. ISBN 978-0-471-44026-0. [Google Scholar]
- Caliari, S.R.; Burdick, J.A. A Practical Guide to Hydrogels for Cell Culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746. [Google Scholar] [CrossRef]
- Rao, S.S.; DeJesus, J.; Short, A.R.; Otero, J.J.; Sarkar, A.; Winter, J.O. Glioblastoma Behaviors in Three-Dimensional Collagen-Hyaluronan Composite Hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 9276–9284. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A Complex Protein Mixture Required for Optimal Growth of Cell Culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Grundy, T.J.; De Leon, E.; Griffin, K.R.; Stringer, B.W.; Day, B.W.; Fabry, B.; Cooper-White, J.; O’Neill, G.M. Differential Response of Patient-Derived Primary Glioblastoma Cells to Environmental Stiffness. Sci. Rep. 2016, 6, 23353. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Acuña, R.; Quirós, M.; Farkas, A.E.; Dedhia, P.H.; Huang, S.; Siuda, D.; García-Hernández, V.; Miller, A.J.; Spence, J.R.; Nusrat, A.; et al. Synthetic Hydrogels for Human Intestinal Organoid Generation and Colonic Wound Repair. Nat. Cell Biol. 2017, 19, 1326–1335. [Google Scholar] [CrossRef] [Green Version]
- Sawhney, A.S.; Pathak, C.P.; Hubbell, J.A. Bioerodible Hydrogels Based on Photopolymerized Poly(Ethylene Glycol)-Co-Poly(.Alpha.-Hydroxy Acid) Diacrylate Macromers. Macromolecules 1993, 26, 581–587. [Google Scholar] [CrossRef]
- Chirila, T.V.; Constable, I.J.; Crawford, G.J.; Vijayasekaran, S.; Thompson, D.E.; Chen, Y.C.; Fletcher, W.A.; Griffin, B.J. Poly(2-Hydroxyethyl Methacrylate) Sponges as Implant Materials: In Vivo and in Vitro Evaluation of Cellular Invasion. Biomaterials 1993, 14, 26–38. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Tong, X.; Yang, F. Bioengineered 3D Brain Tumor Model to Elucidate the Effects of Matrix Stiffness on Glioblastoma Cell Behavior Using PEG-Based Hydrogels. Mol. Pharm. 2014, 11, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, P.; Dong, C.; Jiang, H.; Xue, B.; Gao, X.; Qin, M.; Wang, W.; Chen, B.; Cao, Y. Rationally Designed Synthetic Protein Hydrogels with Predictable Mechanical Properties. Nat. Commun. 2018, 9, 620. [Google Scholar] [CrossRef] [Green Version]
- Langer, R.; Tirrell, D.A. Designing Materials for Biology and Medicine. Nature 2004, 428, 487. [Google Scholar] [CrossRef]
- Thomas, T.W.; DiMilla, P.A. Spreading and Motility of Human Glioblastoma Cells on Sheets of Silicone Rubber Depend on Substratum Compliance. Med. Biol. Eng. Comput. 2000, 38, 360–370. [Google Scholar] [CrossRef]
- Gurski, L.A.; Jha, A.K.; Zhang, C.; Jia, X.; Farach-Carson, M.C. Hyaluronic Acid-Based Hydrogels as 3D Matrices for in Vitro Evaluation of Chemotherapeutic Drugs Using Poorly Adherent Prostate Cancer Cells. Biomaterials 2009, 30, 6076–6085. [Google Scholar] [CrossRef] [Green Version]
- Gill, B.J.; Gibbons, D.L.; Roudsari, L.C.; Saik, J.E.; Rizvi, Z.H.; Roybal, J.D.; Kurie, J.M.; West, J.L. A Synthetic Matrix with Independently Tunable Biochemistry and Mechanical Properties to Study Epithelial Morphogenesis and EMT in a Lung Adenocarcinoma Model. Cancer Res. 2012, 72, 6013–6023. [Google Scholar] [CrossRef] [Green Version]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where We Have Been and Where We Are Going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S. Synthetic Hydrogels for Biomedical Applications. In Hydrogels for Medical and Related Applications; ACS Symposium Series; American Chemical Society: Washington, NY, USA, 1976; Volume 31, pp. 1–36. ISBN 978-0-8412-0338-9. [Google Scholar]
- Zheng, S.Y.; Ding, H.; Qian, J.; Yin, J.; Wu, Z.L.; Song, Y.; Zheng, Q. Metal-Coordination Complexes Mediated Physical Hydrogels with High Toughness, Stick–Slip Tearing Behavior, and Good Processability. Macromolecules 2016, 49, 9637–9646. [Google Scholar] [CrossRef]
- Ulrich, T.A.; de Juan Pardo, E.M.; Kumar, S. The Mechanical Rigidity of the Extracellular Matrix Regulates the Structure, Motility, and Proliferation of Glioma Cells. Cancer Res. 2009, 69, 4167–4174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.S.; Nelson, M.T.; Xue, R.; DeJesus, J.K.; Viapiano, M.S.; Lannutti, J.J.; Sarkar, A.; Winter, J.O. Mimicking White Matter Tract Topography Using Core-Shell Electrospun Nanofibers to Examine Migration of Malignant Brain Tumors. Biomaterials 2013, 34, 5181–5190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedron, S.; Becka, E.; Harley, B.A.C. Regulation of Glioma Cell Phenotype in 3D Matrices by Hyaluronic Acid. Biomaterials 2013, 34, 7408–7417. [Google Scholar] [CrossRef]
- Shibayama, M.; Tanaka, T. Volume Phase Transition and Related Phenomena of Polymer Gels. In Responsive Gels: Volume Transitions I; Dušek, K., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1993; pp. 1–62. ISBN 978-3-540-47737-2. [Google Scholar]
- Taraban, M.B.; Feng, Y.; Hammouda, B.; Hyland, L.L.; Yu, Y.B. Chirality-Mediated Mechanical and Structural Properties of Oligopeptide Hydrogels. Chem. Mater. 2012, 24, 2299–2310. [Google Scholar] [CrossRef]
- Hydrogel Sensors and Actuators: Engineering and Technology; Gerlach, G.; Arndt, K.-F. (Eds.) Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-540-75644-6. [Google Scholar]
- Kizilay, M.Y.; Okay, O. Effect of Initial Monomer Concentration on Spatial Inhomogeneity in Poly(Acrylamide) Gels. Macromolecules 2003, 36, 6856–6862. [Google Scholar] [CrossRef]
- Lindemann, B.; Schröder, U.P.; Oppermann, W. Influence of the Cross-Linker Reactivity on the Formation of Inhomogeneities in Hydrogels. Macromolecules 1997, 30, 4073–4077. [Google Scholar] [CrossRef]
- Oldenziel, W.H.; Beukema, W.; Westerink, B.H.C. Improving the Reproducibility of Hydrogel-Coated Glutamate Microsensors by Using an Automated Dipcoater. J. Neurosci. Methods 2004, 140, 117–126. [Google Scholar] [CrossRef]
- Petersen, O.W.; Rønnov-Jessen, L.; Howlett, A.R.; Bissell, M.J. Interaction with Basement Membrane Serves to Rapidly Distinguish Growth and Differentiation Pattern of Normal and Malignant Human Breast Epithelial Cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9064–9068. [Google Scholar] [CrossRef] [Green Version]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, P.; Chirasatitsin, S.; Ngamkham, K.; Engler, A.J.; Battaglia, G. Cell Instructive Microporous Scaffolds through Interface Engineering. J. Am. Chem. Soc. 2012, 134, 20103–20109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.-Y.; Zhu, Y.-J.; Li, H.; Zhang, Y.-G.; Shen, Y.-Q.; Sun, T.-W.; Chen, F. Preparation and Enhanced Mechanical Properties of Hybrid Hydrogels Comprising Ultralong Hydroxyapatite Nanowires and Sodium Alginate. J. Colloid Interface Sci. 2017, 497, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Suggitt, M.; Bibby, M.C. 50 Years of Preclinical Anticancer Drug Screening: Empirical to Target-Driven Approaches. Clin. Cancer Res. 2005, 11, 971–981. [Google Scholar] [CrossRef]
- Singh, M.; Close, D.A.; Mukundan, S.; Johnston, P.A.; Sant, S. Production of Uniform 3D Microtumors in Hydrogel Microwell Arrays for Measurement of Viability, Morphology, and Signaling Pathway Activation. Assay Drug Dev. Technol. 2015, 13, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Putnam, A.J.; Mooney, D.J. Tissue Engineering Using Synthetic Extracellular Matrices. Nat. Med. 1996, 2, 824–826. [Google Scholar] [CrossRef]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting Three-Dimensional Cell-Laden Tissue Constructs with Controllable Degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Ahn, S.; Kim, Y.; Cho, Y.; Chun, W. Coaxial Structured Collagen–Alginate Scaffolds: Fabrication, Physical Properties, and Biomedical Application for Skin Tissue Regeneration. J. Mater. Chem. 2011, 21, 6165–6172. [Google Scholar] [CrossRef]
- Tan, Z.; Parisi, C.; Silvio, L.D.; Dini, D.; Forte, A.E. Cryogenic 3D Printing of Super Soft Hydrogels. Sci. Rep. 2017, 7, 16293. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable Natural Polymer Hydrogels for Articular Cartilage Tissue Engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable Hydrogels as Unique Biomedical Materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, K.; You, X.; Huang, B.; Wu, J.; Gu, Z. Hybrid Hydrogels with High Strength and Biocompatibility for Bone Regeneration. Int. J. Biol. Macromol. 2017, 104, 1143–1149. [Google Scholar] [CrossRef]
- Wu, D.-Q.; Chu, C.-C. Biodegradable Hydrophobic-Hydrophilic Hybrid Hydrogels: Swelling Behavior and Controlled Drug Release. J. Biomater. Sci. Polym. Ed. 2008, 19, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable Hydrogels for Sustained Release of Therapeutic Agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.S.; Cho, J.; Thambi, T.; Giang Phan, V.H.; Kwon, I.; Lee, D.S. Bioengineered Robust Hybrid Hydrogels Enrich the Stability and Efficacy of Biological Drugs. J. Control. Release 2017, 267, 119–132. [Google Scholar] [CrossRef]
- Kang, C.E.; Poon, P.C.; Tator, C.H.; Shoichet, M.S. A New Paradigm for Local and Sustained Release of Therapeutic Molecules to the Injured Spinal Cord for Neuroprotection and Tissue Repair. Tissue Eng. Part A 2008, 15, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.K.; Lee, D.S. Injectable Biodegradable Hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef]
- Wang, Y.; Lapitsky, Y.; Kang, C.E.; Shoichet, M.S. Accelerated Release of a Sparingly Soluble Drug from an Injectable Hyaluronan-Methylcellulose Hydrogel. J. Control. Release 2009, 140, 218–223. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable Block Copolymers as Injectable Drug-Delivery Systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-Sensitive Hydrogels for Drug Delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Yubao, G.; Hecheng, M.; Jianguo, L. Controlled WISP-1 ShRNA Delivery Using Thermosensitive Biodegradable Hydrogel in the Treatment of Osteoarthritis. J. Bionic Eng. 2015, 12, 285–293. [Google Scholar] [CrossRef]
- Lee, P.Y.; Cobain, E.; Huard, J.; Huang, L. Thermosensitive Hydrogel PEG–PLGA–PEG Enhances Engraftment of Muscle-Derived Stem Cells and Promotes Healing in Diabetic Wound. Mol. Ther. 2007, 15, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Tam, R.Y.; Zahir, T.; Tator, C.H.; Shoichet, M.S. Repair of the Injured Spinal Cord by Transplantation of Neural Stem Cells in a Hyaluronan-Based Hydrogel. Biomaterials 2013, 34, 3775–3783. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Agaoglu, S.; Diep, P.; Martini, M.; Kt, S.; Baday, M.; Emre Araci, I. Ultra-Sensitive Microfluidic Wearable Strain Sensor for Intraocular Pressure Monitoring. Lab A Chip 2018, 18, 3471–3483. [Google Scholar] [CrossRef]
- Lloyd, A.W.; Faragher, R.G.; Denyer, S.P. Ocular Biomaterials and Implants. Biomaterials 2001, 22, 769–785. [Google Scholar] [CrossRef]
- Salmon, D.; Roussel, L.; Gilbert, E.; Kirilov, P.; Pirot, F. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin, Germany, 2015. [Google Scholar]
- Qi, C.; Xu, L.; Deng, Y.; Wang, G.; Wang, Z.; Wang, L. Sericin Hydrogels Promote Skin Wound Healing with Effective Regeneration of Hair Follicles and Sebaceous Glands after Complete Loss of Epidermis and Dermis. Biomater. Sci. 2018, 6, 2859–2870. [Google Scholar] [CrossRef]
- Baker, E.L.; Lu, J.; Yu, D.; Bonnecaze, R.T.; Zaman, M.H. Cancer Cell Stiffness: Integrated Roles of Three-Dimensional Matrix Stiffness and Transforming Potential. Biophys. J. 2010, 99, 2048–2057. [Google Scholar] [CrossRef] [Green Version]
- Gkretsi, V.; Stylianopoulos, T. Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef]
- Quan, F.-S.; Kim, K.S. Medical Applications of the Intrinsic Mechanical Properties of Single Cells. Acta Biochim. Biophys. Sin. 2016, 48, 865–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wullkopf, L.; West, A.-K.V.; Leijnse, N.; Cox, T.R.; Madsen, C.D.; Oddershede, L.B.; Erler, J.T. Cancer Cells’ Ability to Mechanically Adjust to Extracellular Matrix Stiffness Correlates with Their Invasive Potential. Mol. Biol. Cell 2018, 29, 2378–2385. [Google Scholar] [CrossRef]
- Boyd, N.F.; Li, Q.; Melnichouk, O.; Huszti, E.; Martin, L.J.; Gunasekara, A.; Mawdsley, G.; Yaffe, M.J.; Minkin, S. Evidence That Breast Tissue Stiffness Is Associated with Risk of Breast Cancer. PLoS ONE 2014, 9, e100937. [Google Scholar] [CrossRef] [PubMed]
- Fenner, J.; Stacer, A.C.; Winterroth, F.; Johnson, T.D.; Luker, K.E.; Luker, G.D. Macroscopic Stiffness of Breast Tumors Predicts Metastasis. Sci. Rep. 2014, 4, 5512. [Google Scholar] [CrossRef] [PubMed]
- Olcum, M.; Ozcivici, E. Daily Application of Low Magnitude Mechanical Stimulus Inhibits the Growth of MDA-MB-231 Breast Cancer Cells in Vitro. Cancer Cell Int. 2014, 14, 102. [Google Scholar] [CrossRef] [Green Version]
- Joyce, M.H.; Lu, C.; James, E.R.; Hegab, R.; Allen, S.C.; Suggs, L.J.; Brock, A. Phenotypic Basis for Matrix Stiffness-Dependent Chemoresistance of Breast Cancer Cells to Doxorubicin. Front. Oncol. 2018, 8, 337. [Google Scholar] [CrossRef] [Green Version]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am. J. Pathol 2011, 178, 1221–1232. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in Adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) Consensus Review on Current Management and Future Directions. Neuro-Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Blanchette, M.; Daneman, R. Formation and Maintenance of the BBB. Mech. Dev. 2015, 138, 8–16. [Google Scholar] [CrossRef]
- Tajes, M.; Ramos-Fernández, E.; Weng-Jiang, X.; Bosch-Morató, M.; Guivernau, B.; Eraso-Pichot, A.; Salvador, B.; Fernàndez-Busquets, X.; Roquer, J.; Muñoz, F.J. The Blood-Brain Barrier: Structure, Function and Therapeutic Approaches to Cross It. Mol. Membr. Biol. 2014, 31, 152–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolburg, H.; Wolburg-Buchholz, K.; Kraus, J.; Rascher-Eggstein, G.; Liebner, S.; Hamm, S.; Duffner, F.; Grote, E.-H.; Risau, W.; Engelhardt, B. Localization of Claudin-3 in Tight Junctions of the Blood-Brain Barrier Is Selectively Lost during Experimental Autoimmune Encephalomyelitis and Human Glioblastoma Multiforme. Acta Neuropathol. 2003, 105, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Fischmann, A.; Rascher, G.; Duffner, F.; Grote, E.H.; Kalbacher, H.; Wolburg, H. Claudin-1 and Claudin-5 Expression and Tight Junction Morphology Are Altered in Blood Vessels of Human Glioblastoma Multiforme. Acta Neuropathol. 2000, 100, 323–331. [Google Scholar] [CrossRef]
- Bellail, A.C.; Hunter, S.B.; Brat, D.J.; Tan, C.; Van Meir, E.G. Microregional Extracellular Matrix Heterogeneity in Brain Modulates Glioma Cell Invasion. Int. J. Biochem. Cell Biol. 2004, 36, 1046–1069. [Google Scholar] [CrossRef]
- Demuth, T.; Berens, M.E. Molecular Mechanisms of Glioma Cell Migration and Invasion. J. Neurooncol. 2004, 70, 217–228. [Google Scholar] [CrossRef]
- Mahesparan, R.; Read, T.-A.; Lund-Johansen, M.; Skaftnesmo, K.O.; Bjerkvig, R.; Engebraaten, O. Expression of Extracellular Matrix Components in a Highly Infiltrative in Vivo Glioma Model. Acta Neuropathol. 2003, 105, 49–57. [Google Scholar] [CrossRef]
- Payne, L.S.; Huang, P.H. The Pathobiology of Collagens in Glioma. Mol. Cancer Res. 2013, 11, 1129–1140. [Google Scholar] [CrossRef] [Green Version]
- Lim, E.-J.; Suh, Y.; Yoo, K.-C.; Lee, J.-H.; Kim, I.-G.; Kim, M.-J.; Chang, J.H.; Kang, S.-G.; Lee, S.-J. Tumor-Associated Mesenchymal Stem-like Cells Provide Extracellular Signaling Cue for Invasiveness of Glioblastoma Cells. Oncotarget 2017, 8, 1438–1448. [Google Scholar] [CrossRef] [Green Version]
- Gilbertson, R.J.; Rich, J.N. Making a Tumour’s Bed: Glioblastoma Stem Cells and the Vascular Niche. Nat. Rev. Cancer 2007, 7, 733–736. [Google Scholar] [CrossRef]
- Heddleston, J.M.; Li, Z.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. The Hypoxic Microenvironment Maintains Glioblastoma Stem Cells and Promotes Reprogramming towards a Cancer Stem Cell Phenotype. Cell Cycle 2009, 8, 3274–3284. [Google Scholar] [CrossRef] [Green Version]
- Ciasca, G.; Sassun, T.E.; Minelli, E.; Antonelli, M.; Papi, M.; Santoro, A.; Giangaspero, F.; Delfini, R.; Spirito, M.D. Nano-Mechanical Signature of Brain Tumours. Nanoscale 2016, 8, 19629–19643. [Google Scholar] [CrossRef]
- Diao, W.; Tong, X.; Yang, C.; Zhang, F.; Bao, C.; Chen, H.; Liu, L.; Li, M.; Ye, F.; Fan, Q.; et al. Behaviors of Glioblastoma Cells in in Vitro Microenvironments. Sci. Rep. 2019, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Umesh, V.; Rape, A.D.; Ulrich, T.A.; Kumar, S. Microenvironmental Stiffness Enhances Glioma Cell Proliferation by Stimulating Epidermal Growth Factor Receptor Signaling. PLoS ONE 2014, 9, e101771. [Google Scholar] [CrossRef] [Green Version]
- Memmel, S.; Sisario, D.; Zöller, C.; Fiedler, V.; Katzer, A.; Heiden, R.; Becker, N.; Eing, L.; Ferreira, F.L.R.; Zimmermann, H.; et al. Migration Pattern, Actin Cytoskeleton Organization and Response to PI3K-, MTOR-, and Hsp90-Inhibition of Glioblastoma Cells with Different Invasive Capacities. Oncotarget 2017, 8, 45298–45310. [Google Scholar] [CrossRef] [Green Version]
- Masoumi, S.; Harisankar, A.; Gracias, A.; Bachinger, F.; Fufa, T.; Chandrasekar, G.; Gaunitz, F.; Walfridsson, J.; Kitambi, S.S. Understanding Cytoskeleton Regulators in Glioblastoma Multiforme for Therapy Design. Drug Des. Devel. 2016, 10, 2881–2897. [Google Scholar] [CrossRef] [Green Version]
- Efremov, Y.M.; Dokrunova, A.A.; Efremenko, A.V.; Kirpichnikov, M.P.; Shaitan, K.V.; Sokolova, O.S. Distinct Impact of Targeted Actin Cytoskeleton Reorganization on Mechanical Properties of Normal and Malignant Cells. Biochim. Biophys. Acta 2015, 1853, 3117–3125. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.S.; Lee, G.Y.H.; Ong, C.N.; Lim, C.T. AFM Indentation Study of Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2008, 374, 609–613. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The Extracellular Matrix: A Dynamic Niche in Cancer Progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Miroshnikova, Y.A.; Mouw, J.K.; Barnes, J.M.; Pickup, M.W.; Lakins, J.N.; Kim, Y.; Lobo, K.; Persson, A.I.; Reis, G.F.; McKnight, T.R.; et al. Tissue Mechanics Promote IDH1-Dependent HIF1α-Tenascin C Feedback to Regulate Glioblastoma Aggression. Nat. Cell Biol. 2016, 18, 1336–1345. [Google Scholar] [CrossRef]
- Stewart, D.C.; Rubiano, A.; Dyson, K.; Simmons, C.S. Mechanical Characterization of Human Brain Tumors from Patients and Comparison to Potential Surgical Phantoms. PLoS ONE 2017, 12, e0177561. [Google Scholar] [CrossRef] [Green Version]
- Barnes, J.M.; Przybyla, L.; Weaver, V.M. Tissue Mechanics Regulate Brain Development, Homeostasis and Disease. J. Cell Sci. 2017, 130, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Wiranowska, M.; Rojiani, M.V. Extracellular Matrix Microenvironment in Glioma Progression. Glioma-Explor. Its Biol. Pract. Relev. 2011. [Google Scholar] [CrossRef] [Green Version]
- Brat, D.J.; Castellano-Sanchez, A.A.; Hunter, S.B.; Pecot, M.; Cohen, C.; Hammond, E.H.; Devi, S.N.; Kaur, B.; Van Meir, E.G. Pseudopalisades in Glioblastoma Are Hypoxic, Express Extracellular Matrix Proteases, and Are Formed by an Actively Migrating Cell Population. Cancer Res. 2004, 64, 920–927. [Google Scholar] [CrossRef] [Green Version]
- Nakada, M.; Okada, Y.; Yamashita, J. The Role of Matrix Metalloproteinases in Glioma Invasion. Front. Biosci. 2003, 8, e261–e269. [Google Scholar] [CrossRef] [Green Version]
- Schuler, P.J.; Bendszus, M.; Kuehnel, S.; Wagner, S.; Hoffmann, T.K.; Goldbrunner, R.; Vince, G.H. Urokinase Plasminogen Activator, UPAR, MMP-2, and MMP-9 in the C6-Glioblastoma Rat Model. In Vivo 2012, 26, 571–576. [Google Scholar]
- Mikkelsen, T.; Yan, P.-S.; Ho, K.-L.; Sameni, M.; Sloane, B.F.; Rosenblum, M.L. Immunolocalization of Cathepsin B in Human Glioma: Implications for Tumor Invasion and Angiogenesis. J. Neurosurg. 1995, 83, 285–290. [Google Scholar] [CrossRef]
- Kobayashi, T.; Honke, K.; Gasa, S.; Fujii, T.; Maguchi, S.; Miyazaki, T.; Makita, A. Proteolytic Processing Sites Producing the Mature Form of Human Cathepsin D. Int. J. Biochem. 1992, 24, 1487–1491. [Google Scholar] [CrossRef]
- Rempel, S.A.; Rosenblum, M.L.; Mikkelsen, T.; Yan, P.S.; Ellis, K.D.; Golembieski, W.A.; Sameni, M.; Rozhin, J.; Ziegler, G.; Sloane, B.F. Cathepsin B Expression and Localization in Glioma Progression and Invasion. Cancer Res. 1994, 54, 6027–6031. [Google Scholar]
- Armento, A.; Ehlers, J.; Schötterl, S.; Naumann, U. Molecular Mechanisms of Glioma Cell Motility. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017; ISBN 978-0-9944381-2-6. [Google Scholar]
- Schnell, O.; Krebs, B.; Carlsen, J.; Miederer, I.; Goetz, C.; Goldbrunner, R.H.; Wester, H.-J.; Haubner, R.; Pöpperl, G.; Holtmannspötter, M.; et al. Imaging of Integrin Alpha(v)Beta(3) Expression in Patients with Malignant Glioma by [18F] Galacto-RGD Positron Emission Tomography. Neuro-Oncol. 2009, 11, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhao, X.; Zhang, L.; Li, F.; Ji, N.; Gao, Z.; Wang, J.; Kang, P.; Liu, Z.; Shi, J.; et al. 68Ga-PRGD2 PET/CT in the Evaluation of Glioma: A Prospective Study. Mol. Pharm. 2014, 11, 3923–3929. [Google Scholar] [CrossRef] [Green Version]
- Iagaru, A.; Mosci, C.; Mittra, E.; Zaharchuk, G.; Fischbein, N.; Harsh, G.; Li, G.; Nagpal, S.; Recht, L.; Gambhir, S.S. Glioblastoma Multiforme Recurrence: An Exploratory Study of (18)F FPPRGD2 PET/CT. Radiology 2015, 277, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Ellert-Miklaszewska, A.; Poleszak, K.; Pasierbinska, M.; Kaminska, B. Integrin Signaling in Glioma Pathogenesis: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 888. [Google Scholar] [CrossRef] [Green Version]
- Fillmore, H.L.; Chasiotis, I.; Cho, S.W.; Gillies, G.T. Atomic Force Microscopy Observations of Tumour Cell Invadopodia: Novel Cellular Nanomorphologies on Collagen Substrates. Nanotechnology 2002, 14, 73–76. [Google Scholar] [CrossRef]
- Kim, H.-D.; Guo, T.W.; Wu, A.P.; Wells, A.; Gertler, F.B.; Lauffenburger, D.A. Epidermal Growth Factor–Induced Enhancement of Glioblastoma Cell Migration in 3D Arises from an Intrinsic Increase in Speed But an Extrinsic Matrix- and Proteolysis-Dependent Increase in Persistence. Mol. Biol. Cell 2008, 19, 4249–4259. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, L.J.; Brangwynne, C.P.; Kasza, K.E.; Filippidi, E.; Gordon, V.D.; Deisboeck, T.S.; Weitz, D.A. Glioma Expansion in Collagen I Matrices: Analyzing Collagen Concentration-Dependent Growth and Motility Patterns. Biophys. J. 2005, 89, 635–650. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Motte, S.; Kaufman, L.J. Pore Size Variable Type I Collagen Gels and Their Interaction with Glioma Cells. Biomaterials 2010, 31, 5678–5688. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Kolettis, N.; Yang, M.; Gillard, E.R.; Sanchez, E.; Sun, C.-H.; Tromberg, B.J.; Krasieva, T.B.; Lyubovitsky, J.G. Multiphoton Imaging of Actin Filament Formation and Mitochondrial Energetics of Human ACBT Gliomas. Photochem. Photobiol. 2011, 87, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Bach, C.T.; Shum, M.S.Y.; O’Neill, G.M. NEDD9 Regulates 3D Migratory Activity Independent of the Rac1 Morphology Switch in Glioma and Neuroblastoma. Mol. Cancer Res. 2014, 12, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Ardebili, S.Y.; Zajc, I.; Gole, B.; Campos, B.; Herold-Mende, C.; Drmota, S.; Lah, T.T. CD133/Prominin1 Is Prognostic for GBM Patient’s Survival, but Inversely Correlated with Cysteine Cathepsins’ Expression in Glioblastoma Derived Spheroids. Radiol. Oncol. 2011, 45, 102–115. [Google Scholar] [CrossRef]
- Chigurupati, S.; Venkataraman, R.; Barrera, D.; Naganathan, A.; Madan, M.; Paul, L.; Pattisapu, J.V.; Kyriazis, G.A.; Sugaya, K.; Bushnev, S.; et al. Receptor Channel TRPC6 Is a Key Mediator of Notch-Driven Glioblastoma Growth and Invasiveness. Cancer Res. 2010, 70, 418–427. [Google Scholar] [CrossRef] [Green Version]
- Chonan, Y.; Taki, S.; Sampetrean, O.; Saya, H.; Sudo, R. Endothelium-Induced Three-Dimensional Invasion of Heterogeneous Glioma Initiating Cells in a Microfluidic Coculture Platform. Int. Biol. 2017, 9, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. Weinh. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Ouasti, S.; Donno, R.; Cellesi, F.; Sherratt, M.J.; Terenghi, G.; Tirelli, N. Network Connectivity, Mechanical Properties and Cell Adhesion for Hyaluronic Acid/PEG Hydrogels. Biomaterials 2011, 32, 6456–6470. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, J.; Wang, Y.; Cui, F.-Z. Hyaluronic Acid-Based Scaffold for Central Neural Tissue Engineering. Interface Focus 2012, 2, 278–291. [Google Scholar] [CrossRef] [Green Version]
- Borzacchiello, A.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Ambrosio, L. Hyaluronic Acid Based Hydrogels for Regenerative Medicine Applications. Available online: https://www.hindawi.com/journals/bmri/2015/871218/ (accessed on 17 June 2020).
- Florczyk, S.J.; Wang, K.; Jana, S.; Wood, D.L.; Sytsma, S.K.; Sham, J.; Kievit, F.M.; Zhang, M. Porous Chitosan-Hyaluronic Acid Scaffolds as a Mimic of Glioblastoma Microenvironment ECM. Biomaterials 2013, 34, 10143–10150. [Google Scholar] [CrossRef] [Green Version]
- Pedron, S.; Harley, B.A.C. Impact of the Biophysical Features of a 3D Gelatin Microenvironment on Glioblastoma Malignancy. J. Biomed. Mater. Res. Part A 2013, 101, 3404–3415. [Google Scholar] [CrossRef]
- Wan, Y.; Mahmood, M.A.I.; Li, N.; Allen, P.B.; Kim, Y.; Bachoo, R.; Ellington, A.D.; Iqbal, S.M. Nano-Textured Substrates with Immobilized Aptamers for Cancer Cell Isolation and Cytology. Cancer 2012, 118, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.J.; Fisher, C.; Scheffler, K.; Wan, R.; Maleki, H.; Liu, H.; Sun, Y.; A Simmons, C.; Birngruber, R.; Lilge, L. Polyacrylamide Gel Substrates That Simulate the Mechanical Stiffness of Normal and Malignant Neuronal Tissues Increase Protoporphyin IX Synthesis in Glioma Cells. J. Biomed. Opt. 2015, 20, 098002. [Google Scholar] [CrossRef]
- Cruz-Acuña, R.; García, A.J. Synthetic Hydrogels Mimicking Basement Membrane Matrices to Promote Cell-Matrix Interactions. Matrix Biol. 2017, 57–58, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, S.; Gonzalez-Garcia, C.; Rico, P.; Reid, A.; Windmill, J.; Dalby, M.J.; Salmeron-Sanchez, M. Engineered 3D Hydrogels with Full-Length Fibronectin That Sequester and Present Growth Factors. Biomaterials 2020, 252, 120104. [Google Scholar] [CrossRef]
- Huang, C.; Holfeld, J.; Schaden, W.; Orgill, D.; Ogawa, R. Mechanotherapy: Revisiting Physical Therapy and Recruiting Mechanobiology for a New Era in Medicine. Trends Mol. Med. 2013, 19, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Tadeo, I.; Berbegall, A.P.; Escudero, L.M.; Alvaro, T.; Noguera, R. Biotensegrity of the Extracellular Matrix: Physiology, Dynamic Mechanical Balance, and Implications in Oncology and Mechanotherapy. Front. Oncol. 2014, 4, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vining, K.H.; Mooney, D.J. Mechanical Forces Direct Stem Cell Behaviour in Development and Regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C. Pancreatic Cancer Provides Testbed for First Mechanotherapeutics. Nat. Biotechnol. 2019, 37, 829–831. [Google Scholar] [CrossRef]






| 2-Dimensional Culture | 3-Dimensional Culture | ||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
| Simple | Does not mimic in vivo structure | More like in vivo structure | Expensive |
| Reproducible | Fewer interactions with environment | Niches are available | Time consuming |
| Inexpensive | Access to unlimited amount of nutrients from media | Access to nutrients is not unlimited, varies | Less reproducible |
| Less diverse phenotype and polarity | Can form organs or spheroid clusters of cells | More complex and difficult to carry out | |
| Altered cell morphology | Allows study of cell-cell and cell-ECM interactions | Fewer interactions with environment | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahan, A.Z.; Baday, M.; Patel, C.B. Biomimetic Hydrogels in the Study of Cancer Mechanobiology: Overview, Biomedical Applications, and Future Perspectives. Gels 2022, 8, 496. https://doi.org/10.3390/gels8080496
Sahan AZ, Baday M, Patel CB. Biomimetic Hydrogels in the Study of Cancer Mechanobiology: Overview, Biomedical Applications, and Future Perspectives. Gels. 2022; 8(8):496. https://doi.org/10.3390/gels8080496
Chicago/Turabian StyleSahan, Ayse Z., Murat Baday, and Chirag B. Patel. 2022. "Biomimetic Hydrogels in the Study of Cancer Mechanobiology: Overview, Biomedical Applications, and Future Perspectives" Gels 8, no. 8: 496. https://doi.org/10.3390/gels8080496