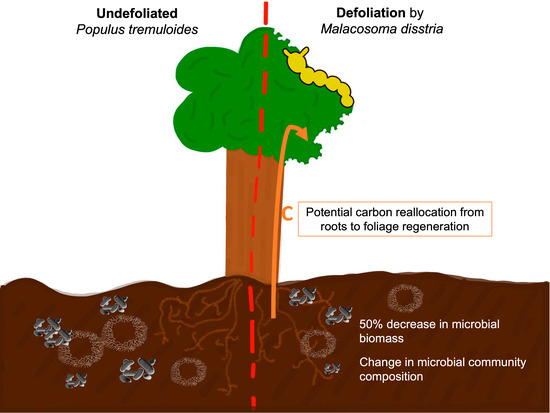
Decreased Soil Microbial Biomass and Changed Microbial Community Composition following a Defoliation Event by the Forest Tent Caterpillar
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Soil Microbial Biomass

3.2. Soil Microbial Community
3.3. Fungal Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree Mortality from Drought, Insects, and Their Interactions in a Changing Climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Ramsfield, T.D.; Bentz, B.J.; Faccoli, M.; Jactel, H.; Brockerhoff, E.G. Forest Health in a Changing World: Effects of Globalization and Climate Change on Forest Insect and Pathogen Impacts. Forestry 2016, 89, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.G. Disturbance and Landscape Dynamics in a Changing World. Ecology 2010, 91, 2833–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Ebata, T.; Safranyik, L. Mountain Pine Beetle and Forest Carbon Feedback to Climate Change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef]
- Mitton, J.B.; Ferrenberg, S.M. Mountain Pine Beetle Develops an Unprecedented Summer Generation in Response to Climate Warming. Am. Nat. 2012, 179, E163–E171. [Google Scholar] [CrossRef] [Green Version]
- Uelmen, J.A.; Lindroth, R.L.; Tobin, P.C.; Reich, P.B.; Schwartzberg, E.G.; Raffa, K.F. Effects of Winter Temperatures, Spring Degree-Day Accumulation, and Insect Population Source on Phenological Synchrony between Forest Tent Caterpillar and Host Trees. For. Ecol. Manag. 2016, 362, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Silfver, T.; Heiskanen, L.; Aurela, M.; Myller, K.; Karhu, K.; Meyer, N.; Tuovinen, J.-P.; Oksanen, E.; Rousi, M.; Mikola, J. Insect Herbivory Dampens Subarctic Birch Forest C Sink Response to Warming. Nat. Commun. 2020, 11, 2529. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Roques, A.; Battisti, A. Forest Insects and Climate Change. Curr. For. Rep. 2018, 4, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Bardgett, R.D.; Wardle, D.A. Herbivore-Mediated Linkages between Aboveground and Belowground Communities. Ecology 2003, 84, 2258–2268. [Google Scholar] [CrossRef]
- Hunter, M.D. Insect Population Dynamics Meets Ecosystem Ecology: Effects of Herbivory on Soil Nutrient Dynamics: Insect Population Dynamics Meets Ecosystem Ecology. Agric. For. Entomol. 2001, 3, 77–84. [Google Scholar] [CrossRef]
- Grüning, M.M.; Beule, L.; Meyer, S.; Karlovsky, P.; I.-M.-Arnold, A. The Abundance of Fungi, Bacteria and Denitrification Genes during Insect Outbreaks in Scots Pine Forests. Forests 2018, 9, 497. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, J.R.; Lindroth, R.L. Genetics, Environment, and Their Interaction Determine Efficacy of Chemical Defense in Trembling Aspen. Ecology 2007, 88, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.Å.; Rousk, J.; Metcalfe, D.B. Below-ground Responses to Insect Herbivory in Ecosystems with Woody Plant Canopies: A Meta-analysis. J. Ecol. 2020, 108, 917–930. [Google Scholar] [CrossRef]
- Niklaus, P.A.; Wardle, D.A.; Tate, K.R. Effects of Plant Species Diversity and Composition on Nitrogen Cycling and the Trace Gas Balance of Soils. Plant Soil 2006, 282, 83–98. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect Canopy Herbivory and Frass Deposition Affect Soil Nutrient Dynamics and Export in Oak Mesocosms. Ecology 2004, 85, 3335–3347. [Google Scholar] [CrossRef]
- le Mellec, A.; Gerold, G.; Michalzik, B. Insect Herbivory, Organic Matter Deposition and Effects on Belowground Organic Matter Fluxes in a Central European Oak Forest. Plant Soil 2011, 342, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Streminska Microbial Abundance and Some of Their Physiological Activities in Soil Organic Horizon of Pine Forest Affected by Insect Herbivory. Pol. J. Environ. Stud. 2006, 15, 905–914.
- Grüning, M.M.; Simon, J.; Rennenberg, H.; I.-M.-Arnold, A. Defoliating Insect Mass Outbreak Affects Soil N Fluxes and Tree N Nutrition in Scots Pine Forests. Front. Plant Sci. 2017, 8, 954. [Google Scholar] [CrossRef] [Green Version]
- Lovett, G.M.; Ruesink, A.E. Carbon and Nitrogen Mineralization from Decomposing Gypsy Moth Frass. Oecologia 1995, 104, 133–138. [Google Scholar] [CrossRef]
- Reynolds, B.C.; Hunter, M.D. Responses of Soil Respiration, Soil Nutrients, and Litter Decomposition to Inputs from Canopy Herbivores. Soil Biol. Biochem. 2001, 33, 1641–1652. [Google Scholar] [CrossRef]
- Baranchikov, Y.N.; Perevoznikova, V.D.; Vishnyakova, Z.V. Carbon Emission by Soils in Forests Damaged by the Siberian Moth. Russ. J. Ecol. 2002, 33, 398. [Google Scholar] [CrossRef]
- le Mellec, A.; Habermann, M.; Michalzik, B. Canopy Herbivory Altering C to N Ratios and Soil Input Patterns of Different Organic Matter Fractions in a Scots Pine Forest. Plant Soil 2009, 325, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Morehouse, K.; Johns, T.; Kaye, J.; Kaye, M. Carbon and Nitrogen Cycling Immediately Following Bark Beetle Outbreaks in Southwestern Ponderosa Pine Forests. For. Ecol. Manag. 2008, 255, 2698–2708. [Google Scholar] [CrossRef]
- Baldrian, P. Forest Microbiome: Diversity, Complexity and Dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [Green Version]
- Classen, A.; Demarco, J.; Hart, S.; Whitham, T.; Cobb, N.; Koch, G. Impacts of Herbivorous Insects on Decomposer Communities during the Early Stages of Primary Succession in a Semi-Arid Woodland. Soil Biol. Biochem. 2006, 38, 972–982. [Google Scholar] [CrossRef]
- Castaño, C.; Camarero, J.J.; Zas, R.; Sampedro, L.; Bonet, J.A.; Alday, J.G.; Oliva, J. Insect Defoliation Is Linked to a Decrease in Soil Ectomycorrhizal Biomass and Shifts in Needle Endophytic Communities. Tree Physiol. 2020, 40, 1712–1725. [Google Scholar] [CrossRef]
- Oneț, A.; Teușdea, A.; Boja, N.; Domuța, C.; Oneț, C. Effects of Common Oak (Quercus robur L.) Defolition on the Soil Properties of an Oak Forest in Western Plain of Romania. Ann. For. Res. 2016, 59, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Strickland, M.S.; Rousk, J. Considering Fungal: Bacterial Dominance in Soils—Methods, Controls, and Ecosystem Implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Mattson, W.J.; Herms, D.A.; Witter, J.A.; Allen, D.C. Woody Plant Grazing Systems: North American Outbreak Folivores and Their Host Plants; General technical report NE; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1991.
- Schowalter, T.D. Biology and Management of the Forest Tent Caterpillar (Lepidoptera: Lasiocampidae). J. Integr. Pest Manag. 2017, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Cooke, B.J.; Nealis, V.G.; Régnière, J. 15—Insect Defoliators as Periodic Disturbances in Northern Forest Ecosystems. In Plant Disturbance Ecology; Johnson, E.A., Miyanishi, K., Eds.; Academic Press: Burlington, MA, USA, 2007; pp. 487–525. ISBN 978-0-12-088778-1. [Google Scholar] [CrossRef]
- Cooke, B.J.; Lorenzetti, F. The Dynamics of Forest Tent Caterpillar Outbreaks in Québec, Canada. For. Ecol. Manag. 2006, 226, 110–121. [Google Scholar] [CrossRef]
- Sutton, A.S.; Tardif, J.C.T.C. Dendrochronological Reconstruction of Forest Tent Caterpillar Outbreaks in Time and Space, Western Manitoba, Canada. Can. J. For. Res. 2007, 37, 1643–1657. [Google Scholar] [CrossRef]
- Baranchikov, Y.N.; Mattson, W.J.; Hain, F.P.; Payne, T.L. Forest Insect Guilds: Patterns of Interaction with Host Trees, Proceedings of the Joint IUFRO Working Party Symposium, Abakan, USSR, 13–17 August 1989; NE-GTR-153; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1991.
- Moulinier, J.; Lorenzetti, F.; Bergeron, Y. Effects of a Forest Tent Caterpillar Outbreak on the Dynamics of Mixedwood Boreal Forests of Eastern Canada. Écoscience 2013, 20, 182–193. [Google Scholar] [CrossRef]
- Bergeron, Y. Species and Stand Dynamics in the Mixed Woods of Quebec’s Soutern Boreal Forest. Ecology 2000, 81, 1500–1516. [Google Scholar] [CrossRef]
- Spencer, K.L.; Harvey, G.L. Understanding System Disturbance and Ecosystem Services in Restored Saltmarshes: Integrating Physical and Biogeochemical Processes. Estuar. Coast. Shelf Sci. 2012, 106, 23–32. [Google Scholar] [CrossRef]
- Vincent, J.-S.; Hardy, L. L’évolution et l’extension Des Lacs Glaciaires Barlow et Ojibway En Territoire Québécois. GPQ 2011, 31, 357–372. [Google Scholar] [CrossRef] [Green Version]
- Government of Canada. Canadian Climate Normals—Climate—Environment and Climate Change Canada. Available online: https://climate.weather.gc.ca/climate_normals/ (accessed on 16 December 2022).
- Ministère de la Faune, des Forêts et des Parc (MFFP). Aires Infestées Par La Livrées Des Forêts Au Québec En 2016; Gouvernement du Québec, Direction de la Protection des Forêts: Québec, QC, Canada, 2016; p. 10.
- Ministère de la Faune, des Forêts et des Parc (MFFP). Aires Infestées Par La Livrée Des Forêts Au Québec En 2017; Gouvernement du Québec, Direction de la Protection des Forêts: Québec, QC, Canada, 2017; p. 14.
- Ministère de la Faune, des Forêts et des Parc (MFFP). Aires Infestées Par La Livrée Des Forêts Au Québec En 2018; Gouvernement du Québec, Direction de la Protection des Forêts: Québec, QC, Canada, 2018; p. 16.
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A Rapid Microtiter Plate Method To Measure Carbon Dioxide Evolved from Carbon Substrate Amendments so as To Determine the Physiological Profiles of Soil Microbial Communities by Using Whole Soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [Green Version]
- Sassi, M.B.; Dollinger, J.; Renault, P.; Tlili, A.; Bérard, A. The FungiResp Method: An Application of the MicroRespTM Method to Assess Fungi in Microbial Communities as Soil Biological Indicators. Ecol. Indic. 2012, 23, 482–490. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H. A Physiological Method for the Quantitative Measurement of Microbial Biomass in Soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Campbell, C.D.; Grayston, S.J.; Hirst, D.J. Use of Rhizosphere Carbon Sources in Sole Carbon Source Tests to Discriminate Soil Microbial Communities. J. Microbiol. Methods 1997, 30, 33–41. [Google Scholar] [CrossRef]
- le Mellec, A.; Michalzik, B. Impact of a Pine Lappet (Dendrolimus pini) Mass Outbreak on C and N Fluxes to the Forest Floor and Soil Microbial Properties in a Scots Pine Forest in Germany. Can. J. For. Res. 2008, 38, 1829–1841. [Google Scholar] [CrossRef]
- Kristensen, J.A. The Biogeochemical Consequences of Litter Transformation by Insect Herbivory in the Subarctic: A Microcosm Simulation Experiment. Biogeochemistry 2018, 138, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, J.R.; Lindroth, R.L. Effects of Variable Phytochemistry and Budbreak Phenology on Defoliation of Aspen during a Forest Tent Caterpillar Outbreak. Agric. For. Entomol. 2008, 10, 399–410. [Google Scholar] [CrossRef]
- Hagerman, A.E. Recent Advances in Polyphenol Research; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Coq, S.; Souquet, J.-M.; Meudec, E.; Cheynier, V.; Hättenschwiler, S. Interspecific Variation in Leaf Litter Tannins Drives Decomposition in a Tropical Rain Forest of French Guiana. Ecology 2010, 91, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Joanisse, G.D.; Bradley, R.L.; Preston, C.M.; Bending, G.D. Sequestration of Soil Nitrogen as Tannin–Protein Complexes May Improve the Competitive Ability of Sheep Laurel (Kalmia angustifolia) Relative to Black Spruce (Picea mariana). New Phytol. 2009, 181, 187–198. [Google Scholar] [CrossRef]
- Adamczyk, B.; Sietiö, O.-M.; Biasi, C.; Heinonsalo, J. Interaction between Tannins and Fungal Necromass Stabilizes Fungal Residues in Boreal Forest Soils. New Phytol. 2019, 223, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Hättenschwiler, S.; Sun, T.; Coq, S. The Chitin Connection of Polyphenols and Its Ecosystem Consequences. New Phytol. 2019, 223, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Schimel, J.P.; Cates, R.G.; Zou, J. Infuence of Balsam Poplar Tannin Fractions on Carbon and Nitrogen Dynamics in Alaskan Taiga foodplain Soils. Soil Biol. 2001, 33, 1827–1839. [Google Scholar] [CrossRef]
- Schimel, J.P.; Cleve, K.V.; Cates, R.G.; Clausen, T.P.; Reichardt, P.B. Effects of Balsam Poplar (Populus balsamifera) Tannins and Low Molecular Weight Phenolics on Microbial Activity in Taiga Floodplain Soil: Implications for Changes in N Cycling during Succession. Can. J. Bot. 1996, 74, 84–90. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen Additions and Microbial Biomass: A Meta-analysis of Ecosystem Studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Parker, T.C.; Sadowsky, J.; Dunleavy, H.; Subke, J.-A.; Frey, S.D.; Wookey, P.A. Slowed Biogeochemical Cycling in Sub-Arctic Birch Forest Linked to Reduced Mycorrhizal Growth and Community Change after a Defoliation Event. Ecosystems 2017, 20, 316–330. [Google Scholar] [CrossRef] [Green Version]
- Saravesi, K.; Aikio, S.; Wäli, P.R.; Ruotsalainen, A.L.; Kaukonen, M.; Huusko, K.; Suokas, M.; Brown, S.P.; Jumpponen, A.; Tuomi, J.; et al. Moth Outbreaks Alter Root-Associated Fungal Communities in Subarctic Mountain Birch Forests. Microb. Ecol. 2015, 69, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.R.; Lieffers, V.J.; Landhäusser, S.M.; Comeau, P.G.; Greenway, K.J. An Analysis of Sucker Regeneration of Trembling Aspen. Can. J. For. Res. 2003, 33, 1169–1179. [Google Scholar] [CrossRef]
- Chodak, M.; Klimek, B.; Niklińska, M. Composition and Activity of Soil Microbial Communities in Different Types of Temperate Forests. Biol. Fertil. Soils 2016, 52, 1093–1104. [Google Scholar] [CrossRef]
- Gartzia-Bengoetxea, N.; Kandeler, E.; Martínez de Arano, I.; Arias-González, A. Soil Microbial Functional Activity Is Governed by a Combination of Tree Species Composition and Soil Properties in Temperate Forests. Appl. Soil Ecol. 2016, 100, 57–64. [Google Scholar] [CrossRef]
- Paré, D.; Bernier, P.; Lafleur, B.; Titus, B.D.; Thiffault, E.; Maynard, D.G.; Guo, X. Estimating Stand-Scale Biomass, Nutrient Contents, and Associated Uncertainties for Tree Species of Canadian Forests. Can. J. For. Res. 2013, 43, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Carter, M.R. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-429-12622-2. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dansereau-Macias, É.; Despland, E.; Handa, I.T. Decreased Soil Microbial Biomass and Changed Microbial Community Composition following a Defoliation Event by the Forest Tent Caterpillar. Forests 2023, 14, 792. https://doi.org/10.3390/f14040792
Dansereau-Macias É, Despland E, Handa IT. Decreased Soil Microbial Biomass and Changed Microbial Community Composition following a Defoliation Event by the Forest Tent Caterpillar. Forests. 2023; 14(4):792. https://doi.org/10.3390/f14040792
Chicago/Turabian StyleDansereau-Macias, Éléonore, Emma Despland, and Ira Tanya Handa. 2023. "Decreased Soil Microbial Biomass and Changed Microbial Community Composition following a Defoliation Event by the Forest Tent Caterpillar" Forests 14, no. 4: 792. https://doi.org/10.3390/f14040792