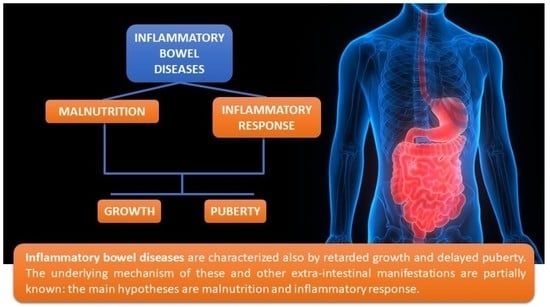
Growth and Puberty in Children with Inflammatory Bowel Diseases
Abstract
:1. Introduction
2. Research Strategies
3. Growth Failure and Delayed Puberty in Inflammatory Bowel Diseases (IBD)
3.1. Growth Failure
3.2. Delayed Puberty
4. Treatment of IBD and Its Effects on Growth Failure and Delayed Puberty
4.1. Glucocorticoid Therapy
4.2. Aminosalicylates
4.3. Immunosoppressive Drugs
4.4. Methotrexate (MTX)
4.5. Exclusive Enteral Nutrition (EEN)
4.6. Biologic Drugs
4.7. Growth Hormone
4.8. Surgery Options
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olén, O.; Askling, J.; Sachs, M.C.; Frumento, P.; Neovius, M.; Smedby, K.E.; Ekbom, A.; Malmborg, P.; Ludvigsson, J.F. Childhood Onset Inflammatory Bowel Disease and Risk of Cancer: A Swedish Nationwide Cohort Study 1964–2014. BMJ 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouratidou, N.; Malmborg, P.; Sachs, M.C.; Askling, J.; Ekbom, A.; Neovius, M.; Smedby, K.E.; Sävendahl, L.; Ludvigsson, J.F.; Olén, O. Adult Height in Patients with Childhood-Onset Inflammatory Bowel Disease: A Nationwide Population-Based Cohort Study. Aliment. Pharmacol. Ther. 2020, 51, 789–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, S.B.; Monteiro, I.M. Diagnosis and Management of Inflammatory Bowel Disease in Children. BMJ 2017, 357, j2083. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Bernstein, C.N.; Bitton, A.; Carroll, M.W.; Singh, H.; Otley, A.R.; Vutcovici, M.; El-Matary, W.; Nguyen, G.C.; Griffiths, A.M.; et al. Trends in Epidemiology of Pediatric Inflammatory Bowel Disease in Canada: Distributed Network Analysis of Multiple Population-Based Provincial Health Administrative Databases. Am. J. Gastroenterol. 2017, 112, 1120–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Andrade Lima Simões Ferreira, P.V.; de Cavalcanti, A.S.; da Silva, G.A.P. Linear Growth and Bone Metabolism in Pediatric Patients with Inflammatory Bowel Disease. J. Pediatr. Rio J. 2019, 95 (Suppl. 1), 59–65. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care Clin. Off. Pract. 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Abraham, B.P.; Mehta, S.; El-Serag, H.B. Natural History of Pediatric-Onset Inflammatory Bowel Disease: A Systematic Review. J. Clin. Gastroenterol. 2012, 46, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.A.; Back, S.J.; Ruchelli, E.D.; Markowitz, J.; Mascarenhas, M.; Verma, R.; Piccoli, D.A.; Baldassano, R.N. Lamina Propria and Circulating Interleukin-6 in Newly Diagnosed Pediatric Inflammatory Bowel Disease Patients. Am. J. Gastroenterol. 2002, 97, 2603–2608. [Google Scholar] [CrossRef]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of Endoscopic Brush Samples Identified Mucosa-Associated Dysbiosis in Inflammatory Bowel Disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut Microbiota in the Pathogenesis of Inflammatory Bowel Disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gosiewski, T.; Strus, M.; Fyderek, K.; Kowalska-Duplaga, K.; Wedrychowicz, A.; Jedynak-Wasowicz, U.; Sladek, M.; Pieczarkowski, S.; Adamski, P.; Heczko, P.B. Horizontal Distribution of the Fecal Microbiota in Adolescents with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 20–27. [Google Scholar] [CrossRef]
- Zacharopoulou, E.; Craviotto, V.; Fiorino, G.; Furfaro, F.; Zilli, A.; Gilardi, D.; Peyrin-Biroulet, L.; Danese, S.; Allocca, M. Targeting the Gut Layers in Crohn’s Disease: Mucosal or Transmural Healing? Expert Rev. Gastroenterol. Hepatol. 2020, 1–13. [Google Scholar] [CrossRef]
- Park, J.H.; Peyrin-Biroulet, L.; Eisenhut, M.; Shin, J.I. IBD Immunopathogenesis: A Comprehensive Review of Inflammatory Molecules. Autoimmun. Rev. 2017, 16, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Kusugami, K.; Ina, K.; Ando, T.; Shinoda, M.; Imada, A.; Ohsuga, M.; Sakai, T.; Matsuura, T.; Ito, K.; et al. Interleukin-6 and Soluble Interleukin-6 Receptor in the Colonic Mucosa of Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 1999, 14, 987–996. [Google Scholar] [CrossRef]
- Toptygina, A.P.; Semikina, E.L.; Bobyleva, G.V.; Miroshkina, L.V.; Petrichuk, S.V. Cytokine Profile in Children with Inflammatory Bowel Disease. Biochem 2014, 79, 1371–1375. [Google Scholar] [CrossRef]
- Koukos, G.; Polytarchou, C.; Kaplan, J.L.; Morley-Fletcher, A.; Gras-Miralles, B.; Kokkotou, E.; Baril-Dore, M.; Pothoulakis, C.; Winter, H.S.; Iliopoulos, D. MicroRNA-124 Regulates STAT3 Expression and Is down-Regulated in Colon Tissues of Pediatric Patients with Ulcerative Colitis. Gastroenterology 2013, 145, 842–852. [Google Scholar] [CrossRef] [Green Version]
- Tili, E.; Michaille, J.J.; Piurowski, V.; Rigot, B.; Croce, C.M. MicroRNAs in Intestinal Barrier Function, Inflammatory Bowel Disease and Related Cancers—Their Effects and Therapeutic Potentials. Curr. Opin. Pharmacol. 2017, 37, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Zikusoka, M.; Trindade, A.; Dassopoulos, T.; Harris, M.L.; Bayless, T.M.; Brant, S.R.; Chakravarti, S.; Kwon, J.H. MicroRNAs Are Differentially Expressed and Alter Expression of Macrophage Inflammatory Peptide-2alpha. Gastroenterology 2008, 135, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Feria, M.; Romero-García, T.; Caballero-Rico, J.Á.F.; Ramírez, H.P.; Avilés-Recio, M.; Castro-Fernandez, M.; Porcuna, N.C.; Romero-Gόmez, M.; García, F.; Grande, L.; et al. Modulation of Faecal Metagenome in Crohn’s Disease: Role of MicroRNAs as Biomarkers. World J. Gastroenterol. 2018, 24, 5223–5233. [Google Scholar] [CrossRef]
- Cao, B.; Zhou, X.; Ma, J.; Zhou, W.; Yang, W.; Fan, D.; Hong, L. Role of MiRNAs in Inflammatory Bowel Disease. Dig. Dis. Sci. 2017, 62, 1426–1438. [Google Scholar] [CrossRef]
- Liu, J.Z.; Anderson, C.A. Genetic Studies of Crohn’s Disease: Past, Present and Future. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 373–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.C.; MacRae, V.E.; McGrogan, P.; Ahmed, S.F. The Role of Pro-Inflammatory Cytokines in Inflammatory Bowel Disease Growth Retardation. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992. [Google Scholar] [CrossRef] [Green Version]
- Goodman, W.A.; Erkkila, I.P.; Pizarro, T.T. Sex Matters: Impact on Pathogenesis, Presentation and Treatment of Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. Laboratory Markers in IBD: Useful, Magic, or Unnecessary Toys? Gut 2006, 55, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, F.S.; Burri, E.; Beglinger, C. The Role and Utility of Faecal Markers in Inflammatory Bowel Disease. Therap. Adv. Gastroenterol. 2015, 8, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Israeli, E.; Grotto, I.; Gilburd, B.; Balicer, R.D.; Goldin, E.; Wiik, A.; Shoenfeld, Y. Anti-Saccharomyces Cerevisiae and Antineutrophil Cytoplasmic Antibodies as Predictors of Inflammatory Bowel Disease. Gut 2005, 54, 1232–1236. [Google Scholar] [CrossRef]
- Differentiating Ulcerative Colitis from Crohn Disease in Children and Young Adults: Report of a Working Group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 653–674. [CrossRef] [Green Version]
- Ezri, J.; Marques-Vidal, P.; Nydegger, A. Impact of Disease and Treatments on Growth and Puberty of Pediatric Patients with Inflammatory Bowel Disease. Digestion 2012, 85, 308–319. [Google Scholar] [CrossRef]
- Griffiths, A.M. Specificities of Inflammatory Bowel Disease in Childhood. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 509–523. [Google Scholar] [CrossRef]
- Sauer, C.G.; Kugathasan, S. Pediatric Inflammatory Bowel Disease: Highlighting Pediatric Differences in IBD. Med. Clin. N. Am. 2010, 94, 35–52. [Google Scholar] [CrossRef]
- Sawczenko, A.; Ballinger, A.B.; Savage, M.O.; Sanderson, I.R. Clinical Features Affecting Final Adult Height in Patients with Pediatric-Onset Crohn’s Disease. Pediatrics 2006, 118, 124–129. [Google Scholar] [CrossRef]
- Laakso, S.; Valta, H.; Verkasalo, M.; Toiviainen-Salo, S.; Mäkitie, O. Compromised Peak Bone Mass in Patients with Inflammatory Bowel Disease-a Prospective Study. J. Pediatr. 2014, 164, 1436–1443. [Google Scholar] [CrossRef] [Green Version]
- Ballinger, A.B.; Savage, M.O.; Sanderson, I.R. Delayed Puberty Associated with Inflammatory Bowel Disease. Pediatr. Res. 2003, 53, 205–210. [Google Scholar] [CrossRef]
- Zeferino, A.M.B.; Barros Filho, A.A.; Bettiol, H.; Barbieri, M.A. Acompanhamento Do Crescimento. J. Pediatr. Rio J. 2003, 79 (Suppl. 1), S23–S32. [Google Scholar] [CrossRef]
- Marchand, V.; Boctor, D.L.; Critch, J.N.; Gowrishankar, M.; Roth, D.; Unger, S.L.; Williams, R.C.; Bhatia, J.; Courant, G.; Davidson, A.G.F.; et al. The Toddler Who Is Falling off the Growth Chart. Paediatr. Child Health 2012, 17, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Heuschkel, R.; Salvestrini, C.; Beattie, R.M.; Hildebrand, H.; Walters, T.; Griffiths, A. Guidelines for the Management of Growth Failure in Childhood Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2008, 14, 839–849. [Google Scholar] [CrossRef]
- Hildebrand, H.; Karlberg, J.; Kristiansson, B. Longitudinal Growth in Children and Adolescents with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 165–173. [Google Scholar] [CrossRef]
- Gupta, N.; Bostrom, A.G.; Kirschner, B.S.; Ferry, G.D.; Winter, H.S.; Baldassano, R.N.; Gold, B.D.; Abramson, O.; Smith, T.; Cohen, S.A.; et al. Gender Differences in Presentation and Course of Disease in Pediatric Patients with Crohn Disease. Pediatrics 2007, 120, e1418–e1425. [Google Scholar] [CrossRef]
- Gupta, N.; Lustig, R.H.; Andrews, H.; Sylvester, F.; Keljo, D.; Goyal, A.; Gokhale, R.; Patel, A.S.; Guthery, S.; Leu, C.-S. Introduction to and Screening Visit Results of the Multicenter Pediatric Crohn’s Disease Growth Study. Inflamm. Bowel Dis. 2020. [Google Scholar] [CrossRef]
- Ishige, T. Growth Failure in Pediatric Onset Inflammatory Bowel Disease: Mechanisms, Epidemiology, and Management. Transl. Pediatr. 2019, 8, 16–22. [Google Scholar] [CrossRef]
- Conklin, L.S.; Oliva-Hemker, M. Nutritional Considerations in Pediatric Inflammatory Bowel Disease. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Mouli, V.P.; Ananthakrishnan, A.N. Review Article: Vitamin D and Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2014, 39, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggan, P.; O’Brien, M.; Kiely, M.; McCarthy, J.; Shanahan, F.; Cashman, K.D. Vitamin K Status in Patients with Crohn’s Disease and Relationship to Bone Turnover. Am. J. Gastroenterol. 2004, 99, 2178–2185. [Google Scholar] [CrossRef]
- Griffin, I.J.; Kim, S.C.; Hicks, P.D.; Liang, L.K.; Abrams, S.A. Zinc Metabolism in Adolescents with Crohn’s Disease. Pediatr. Res. 2004, 56, 235–239. [Google Scholar] [CrossRef] [Green Version]
- Ballinger, A.; El-Haj, T.; Perrett, D.; Turvill, J.; Obeid, O.; Dryden, S.; Williams, G.; Farthing, M.J.G. The Role of Medial Hypothalamic Serotonin in the Suppression of Feeding in a Rat Model of Colitis. Gastroenterology 2000, 118, 544–553. [Google Scholar] [CrossRef]
- Shamir, R. Nutritional Aspects in Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2009, 48 (Suppl. 2), S86–S88. [Google Scholar] [CrossRef] [PubMed]
- El-Haj, T.; Poole, S.; Farthing, M.J.G.; Ballinger, A.B. Anorexia in a Rat Model of Colitis: Interaction of Interleukin-1 and Hypothalamic Serotonin. Brain Res. 2002, 927, 1–7. [Google Scholar] [CrossRef]
- Gryboski, J.D.; Burger, J.A.; McCallum, R.; Lange, R. Gastric Emptying in Childhood Inflammatory Bowel Disease: Nutritional and Pathologic Correlates. Am. J. Gastroenterol. 1992, 87, 1148–1153. [Google Scholar] [CrossRef]
- Sanderson, I.R. Growth Problems in Children with IBD. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 601–610. [Google Scholar] [CrossRef]
- Shamir, R.; Phillip, M.; Levine, A. Growth Retardation in Pediatric Crohn’s Disease: Pathogenesis and Interventions. Inflamm. Bowel Dis. 2007, 13, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. Int. J. Endocrinol. 2014, 2014, 235060. [Google Scholar] [CrossRef] [Green Version]
- Hunziker, E.B.; Wagner, J.; Zapf, J. Differential Effects of Insulin-like Growth Factor I and Growth Hormone on Developmental Stages of Rat Growth Plate Chondrocytes in Vivo. J. Clin. Investig. 1994, 93, 1078–1086. [Google Scholar] [CrossRef] [Green Version]
- Koniaris, S.G.; Fisher, S.E.; Rubin, C.T.; Chawla, A. Experimental Colitis Impairs Linear Bone Growth Independent of Nutritional Factors. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 137–141. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Clayton, P.E.; Miller, V.; Hall, C.M.; Thomas, A.G. Low Serum Concentrations of Insulin-like Growth Factor-I in Children with Active Crohn Disease Effect of Enteral Nutritional Support and Glutamine Supplementation. Scand. J. Gastroenterol. 2002, 37, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, A.B.; Azooz, O.; El-Haj, T.; Poole, S.; Farthing, M.J.G. Growth Failure Occurs through a Decrease in Insulin-like Growth Factor 1 Which Is Independent of Undernutrition in a Rat Model of Colitis. Gut 2000, 46, 694–700. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Lustig, R.H.; Kohn, M.A.; McCracken, M.; Vittinghoff, E. Sex Differences in Statural Growth Impairment in Crohn’s Disease: Role of IGF-1. Inflamm. Bowel Dis. 2011, 17, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Tenore, A.; Berman, W.F.; Parks, J.S.; Bongiovanni, A.M. Basal and Stimulated Serum Growth Hormone Concentrations in Inflammatory Bowel Disease. J. Clin. Endocrinol. Metab. 1977, 44, 622–628. [Google Scholar] [CrossRef]
- Braegger, C.P.; Torresani, T.; Murch, S.H.; Savage, M.O.; Walker-Smith, J.A.; MacDonald, T.T. Urinary Growth Hormone in Growth-Impaired Children with Chronic Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 1993, 16, 49–52. [Google Scholar] [CrossRef]
- Farthing, M.J.G.; Campbell, C.A.; Walker-Smith, J.; Edwards, C.R.; Rees, L.H.; Dawson, A.M. Nocturnal Growth Hormone and Gonadotrophin Secretion in Growth Retarded Children with Crohn’s Disease. Gut 1981, 22, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Oguchi, S.; Walker, W.A.; Sanderson, I.R. Profile of IGF-Binding Proteins Secreted by Intestinal Epithelial Cells Changes with Differentiation. Am. J. Physiol. 1994, 267, G843–G850. [Google Scholar] [CrossRef]
- Savage, M.O.; Blair, J.C.; Jorge, A.J.; Street, M.E.; Ranke, M.B.; Camacho-Hübner, C. IGFs and IGFBPs in GH Insensitivity. Endocrin. Dev. 2005, 9, 100–106. [Google Scholar] [CrossRef]
- Bannerjee, K.; Camacho-Hübner, C.; Babinska, K.; Dryhurst, K.M.; Edwards, R.; Savage, M.O.; Sanderson, I.R.; Croft, N.M. Anti-Inflammatory and Growth-Stimulating Effects Precede Nutritional Restitution During Enteral Feeding in Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 270–275. [Google Scholar] [CrossRef]
- Beattie, R.M.; Camacho-Hübner, C.; Wacharasindhu, S.; Cotterill, A.M.; Walker-Smith, J.A.; Savage, M.O. Responsiveness of IGF-I and IGFBP-3 to Therapeutic Intervention in Children and Adolescents with Crohn’s Disease. Clin. Endocrinol. 1998, 49, 483–489. [Google Scholar] [CrossRef]
- Wine, E.; Reif, S.S.; Leshinsky-Silver, E.; Weiss, B.; Shaoul, R.R.; Shamir, R.; Wasserman, D.; Lerner, A.; Boaz, M.; Levine, A. Pediatric Crohn’s Disease and Growth Retardation: The Role of Genotype, Phenotype, and Disease Severity. Pediatrics 2004, 114, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Essers, J.B.; Kugathasan, S.; Escher, J.C.; Lettre, G.; Butler, J.L.; Stephens, M.C.; Ramoni, M.F.; Grand, R.J.; Hirschhorn, J. Association of Linear Growth Impairment in Pediatric Crohn’s Disease and a Known Height Locus: A Pilot Study. Ann. Hum. Genet. 2010, 74, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Russell, R.K.; Drummond, H.E.; Nimmo, E.R.; Anderson, N.H.; Noble, C.L.; Wilson, D.C.; Gillett, P.M.; McGrogan, P.; Hassan, K.; Weaver, L.T.; et al. Analysis of the Influence of OCTN1/2 Variants within the IBD5 Locus on Disease Susceptibility and Growth Indices in Early Onset Inflammatory Bowel Disease. Gut 2006, 55, 1114–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawczenko, A.; Sandhu, B.K. Presenting Features of Inflammatory Bowel Disease in Great Britain and Ireland. Arch. Dis. Child. 2003, 88, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Timmer, A.; Behrens, R.; Buderus, S.; Findeisen, A.; Hauer, A.; Keller, K.M.; Kliemann, G.; Lang, T.; Lohr, W.; Rzehak, P.; et al. Childhood Onset Inflammatory Bowel Disease: Predictors of Delayed Diagnosis from the CEDATA German-Language Pediatric Inflammatory Bowel Disease Registry. J. Pediatr. 2011, 158, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kritsch, K.R.; Murali, S.; Adamo, M.L.; Ney, D.M. Dexamethasone Decreases Serum and Liver IGF-I and Maintains Liver IGF-I MRNA in Parenterally Fed Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R528–R536. [Google Scholar] [CrossRef] [Green Version]
- Giustina, A.; Wehrenberg, W.B. The Role of Glucocorticoids in the Regulation of Growth Hormone Secretion Mechanisms and Clinical Significance. Trends Endocrinol. Metab. 1992, 3, 306–311. [Google Scholar] [CrossRef]
- Wong, S.C.; Dobie, R.; Altowati, M.A.; Werther, G.A.; Farquharson, C.; Ahmed, S.F. Growth and the Growth Hormone-Insulin like Growth Factor 1 Axis in Children with Chronic Inflammation: Current Evidence, Gaps in Knowledge, and Future Directions. Endocr. Rev. 2016, 37, 62–110. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.; Sedgwick, D.M. Juvenile Onset Inflammatory Bowel Disease: Height and Body Mass Index in Adult Life. BMJ 1994, 308, 1259–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowitz, J.; Grancher, K.; Rosa, J.; Aiges, H.; Daum, F. Growth Failure in Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 1993, 16, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Herzog, D.; Fournier, N.; Buehr, P.; Koller, R.; Rueger, V.; Heyland, K.; Nydegger, A.; Spalinger, J.; Schibli, S.; Braegger, C. Early-Onset Crohn’s Disease Is a Risk Factor for Smaller Final Height. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1234–1239. [Google Scholar] [CrossRef] [Green Version]
- Ghersin, I.; Khateeb, N.; Katz, L.H.; Daher, S.; Shamir, R.; Assa, A. Anthropometric Measures in Adolescents with Inflammatory Bowel Disease: A Population-Based Study. Inflamm. Bowel Dis. 2019, 25, 1061–1065. [Google Scholar] [CrossRef]
- Thébaut, A.; Amouyal, M.; Besançon, A.; Collet, M.; Selbonne, E.; Valentin, C.; Vonthron, M.; Zakariya, M.; Linglart, A. Puberty, Fertility and Chronic Diseases. Arch. Pediatr. 2013, 20, 673–684. [Google Scholar] [CrossRef]
- Palmert, M.R.; Dunkel, L. Clinical Practice. Delayed Puberty. N. Engl. J. Med. 2012, 366, 443–453. [Google Scholar] [CrossRef]
- Hong, C.Y.; Park, J.H.; Ahn, R.S.; Im, S.Y.; Choi, H.-S.; Soh, J.; Mellon, S.H.; Lee, K. Molecular Mechanism of Suppression of Testicular Steroidogenesis by Proinflammatory Cytokine Tumor Necrosis Factor Alpha. Mol. Cell. Biol. 2004, 24, 2593–2604. [Google Scholar] [CrossRef] [Green Version]
- Rettori, V.; Belova, N.; Kamat, A.; Lyson, K.; Gimeno, M.; McCann, S.M. Blockade by Lnterleukin-1-Alpha of Nitricoxidergic Control of Luteinizing H-ormone-Releasing Hormone Release in Vivo and in Vitro. Neuroimmunomodulation 1994, 1, 86–91. [Google Scholar] [CrossRef]
- Elias, C.F.; Purohit, D. Leptin Signaling and Circuits in Puberty and Fertility. Cell. Mol. Life Sci. 2013, 70, 841–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantzoros, C.S.; Flier, J.S.; Rogol, A.D. A Longitudinal Assessment of Hormonal and Physical Alterations during Normal Puberty in Boys. V. Rising Leptin Levels May Signal the Onset of Puberty. J. Clin. Endocrinol. Metab. 1997, 82, 1066–1070. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Li, Y.; Cohn, S. Colitis Causes Delay in Puberty in Female Mice out of Proportion to Changes in Leptin and Corticosterone. J. Gastroenterol. 2010, 45, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Deboer, M.D.; Li, Y. Puberty Is Delayed in Male Mice with Dextran Sodium Sulfate Colitis out of Proportion to Changes in Food Intake, Body Weight, and Serum Levels of Leptin. Pediatr. Res. 2011, 69, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Deboer, M.D.; Denson, L.A. Delays in Puberty, Growth, and Accrual of Bone Mineral Density in Pediatric Crohn’s Disease: Despite Temporal Changes in Disease Severity, the Need for Monitoring Remains. J. Pediatr. 2013, 163, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef] [Green Version]
- Basmaison, O.; Ranchin, B.; Zouater, H.; Robertson, A.; Gomez, R.; Koppiker, N. Efficacy and Safety of Recombinant Growth Hormone Treatment in Children with Growth Retardation Related to Long-Term Glucocorticosteroid Therapy. Ann. Endocrinol. 2019, 80, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.B. Influence of Inhaled Corticosteroids on Growth: A Pediatric Endocrinologist’s Perspective. Acta Paediatr. 1998, 87, 123–129. [Google Scholar] [CrossRef]
- Schoon, E.J.; Bollani, S.; Mills, P.R.; Israeli, E.; Felsenberg, D.; Ljunghall, S.; Persson, T.; Haptén-White, L.; Graffner, H.; Porro, G.B.; et al. Bone Mineral Density in Relation to Efficacy and Side Effects of Budesonide and Prednisolone in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2005, 3, 113–121. [Google Scholar] [CrossRef]
- Odonnell, S.; Omorain, C.A. Therapeutic Benefits of Budesonide in Gastroenterology. Ther. Adv. Chronic Dis. 2010, 1, 177–186. [Google Scholar] [CrossRef]
- Kriel, M.; Sayers, A.; Fraser, W.D.; Williams, A.M.; Koch, A.; Zacharowski, K.; Probert, C.S.; Tobias, J.H. IL-6 May Modulate the Skeletal Response to Glucocorticoids during Exacerbations of Inflammatory Bowel Disease. Calcif. Tissue Int. 2010, 86, 375–381. [Google Scholar] [CrossRef]
- Vihinen, M.; Raivio, T.; Ashorn, M.; Verkasalo, M.; Kolho, K. Bone turnover in paediatric patients with inflammatory bowel disease treated with systemic glucocorticoids. Eur. J. Endocrinol. 2008, 159, 693–698. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, R.S.; Jilka, R.L.; Michael Parfitt, A.; Manolagas, S.C. Inhibition of Osteoblastogenesis and Promotion of Apoptosis of Osteoblasts End Osteocytes by Glucocorticoids Potential Mechanisms of Their Deleterious Effects on Bone. J. Clin. Investig. 1998, 102, 274–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, D.B. Growth Suppression by Glucocorticoid Therapy. Endocrinol. Metab. Clin. N. Am. 1996, 25, 699–717. [Google Scholar] [CrossRef]
- Jux, C.; Leiber, K.; Hügel, U.; Blum, W.; Ohlsson, C.; Klaus, G.; Mehls, O. Dexamethasone Impairs Growth Hormone (GH)-Stimulated Growth by Suppression of Local Insulin-like Growth Factor (IGF)-I Production and Expression of GH and IGF-I-Receptor in Cultured Rat Chondrocytes. Endocrinology 1998, 139, 3296–3305. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, N.; Rekers-Mombarg, L.T.M.; Mearin, M.L.; Wit, J.M.; Lamers, C.B.H.W.; Van Hogezand, R.A. Adult Height in Patients with Early Onset of Crohn’s Disease. Gut 2002, 51, 26–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousseaux, C.; Lefebvre, B.; Dubuquoy, L.; Lefebvre, P.; Romano, O.; Auwerx, J.; Metzger, D.; Wahli, W.; Desvergne, B.; Naccari, G.C.; et al. Intestinal Antiinflammatory Effect of 5-Aminosalicylic Acid Is Dependent on Peroxisome Proliferator-Activated Receptor-γ. J. Exp. Med. 2005, 201, 1205–1515. [Google Scholar] [CrossRef]
- Markowitz, J.; Grancher, K.; Kohn, N.; Lesser, M.; Daum, F. A Multicenter Trial of 6-Mercaptopurine and Prednisone in Children with Newly Diagnosed Crohn’s Disease. Gastroenterology 2000, 119, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Laharie, D.; Reffet, A.; Belleannée, G.; Chabrun, E.; Subtil, C.; Razaire, S.; Capdepont, M.; De Lédinghen, V. Mucosal Healing with Methotrexate in Crohns Disease: A Prospective Comparative Study with Azathioprine and Infliximab. Aliment. Pharmacol. Ther. 2011, 33, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Barabino, A.; Torrente, F.; Ventura, A.; Cucchiara, S.; Castro, M.; Barbera, C. Azathioprine in Paediatric Inflammatory Bowel Disease: An Italian Multicentre Survey. Aliment. Pharmacol. Ther. 2002, 16, 1125–1130. [Google Scholar] [CrossRef]
- Kirschner, B.S. Safety of Azathioprine and 6-Mercaptopurine in Pediatric Patients with Inflammatory Bowel Disease. Gastroenterology 1998, 115, 813–521. [Google Scholar] [CrossRef]
- Fuentes, D.; Torrente, F.; Keady, S.; Thirrupathy, K.; Thomson, M.A.; Walker-Smith, J.A.; Murch, S.H.; Heuschkel, R.B. High-Dose Azathioprine in Children with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2003, 17, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Herfarth, H.H.; Kappelman, M.D.; Long, M.D.; Isaacs, K.L. Use of Methotrexate in the Treatment of Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 224–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric Diet Alone Versus Corticosteroids in the Treatment of Active Pediatric Crohn’s Disease: A Randomized Controlled Open-Label Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Krantz, M.; Bodin, L.; Stenhammar, L.; Lindquist, B. Elemental versus Polymeric Enteral Nutrition in Paediatric Crohn’s Disease: A Multicentre Randomized Controlled Trial. Acta Paediatr. 2004, 93, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Pigneur, B.; Lepage, P.; Mondot, S.; Schmitz, J.; Goulet, O.; Doré, J.; Ruemmele, F.M. Mucosal Healing and Bacterial Composition in Response to Enteral Nutrition vs. Steroid-Based Induction Therapy—A Randomised Prospective Clinical Trial in Children with Crohn’s Disease. J. Crohns Colitis 2019, 13, 846–855. [Google Scholar] [CrossRef] [Green Version]
- Brückner, A.; Werkstetter, K.J.; Frivolt, K.; Shokry, E.; Ahmed, M.; Metwaly, A.; Marques, J.G.; Uhl, O.; Krohn, K.; Hajji, M.; et al. Partial Enteral Nutrition Has No Benefit on Bone Health but Improves Growth in Paediatric Patients with Quiescent or Mild Crohn’s Disease. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wilschanski, M.; Sherman, P.; Pencharz, P.; Davis, L.; Corey, M.; Griffiths, A. Supplementary Enteral Nutrition Maintains Remission in Paediatric Crohn’s Disease. Gut 1996, 38, 543–548. [Google Scholar] [CrossRef]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus Guidelines of ECCO/ESPGHAN on the Medical Management of Pediatric Crohn’s Disease. J. Crohns Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef] [Green Version]
- Hill, R.J. Update on Nutritional Status, Body Composition and Growth in Paediatric Inflammatory Bowel Disease. World J. Gastroenterol. 2014, 20, 3191–3197. [Google Scholar] [CrossRef]
- Hyams, J.; Crandall, W.; Kugathasan, S.; Griffiths, A.; Olson, A.; Johanns, J.; Liu, G.; Travers, S.; Heuschkel, R.; Markowitz, J.; et al. Induction and Maintenance Infliximab Therapy for the Treatment of Moderate-to-Severe Crohn’s Disease in Children. Gastroenterology 2007, 132, 863–1166. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.; Damaraju, L.; Blank, M.; Johanns, J.; Guzzo, C.; Winter, H.S.; Kugathasan, S.; Cohen, S.; Markowitz, J.; Escher, J.C.; et al. Induction and Maintenance Therapy with Infliximab for Children With Moderate to Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2012, 10, 391–399. [Google Scholar] [CrossRef]
- Hyams, J.S.; Griffiths, A.; Markowitz, J.; Baldassano, R.N.; Faubion, W.A.; Colletti, R.B.; Dubinsky, M.; Kierkus, J.; Rosh, J.; Wang, Y.; et al. Safety and Efficacy of Adalimumab for Moderate to Severe Crohn’s Disease in Children. Gastroenterology 2012, 143, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Afzal, N.A.; Ozzard, A.; Keady, S.; Thomson, M.; Murch, S.; Heuschkel, R. Infliximab Delays but Does Not Avoid the Need for Surgery in Treatment-Resistant Pediatric Crohn’ Disease. Dig. Dis. Sci. 2007, 52, 3329–3333. [Google Scholar] [CrossRef]
- Diamanti, A.; Basso, M.S.; Gambarara, M.; Papadatou, B.; Bracci, F.; Noto, C.; Castro, M. Positive Impact of Blocking Tumor Necrosis Factor α on the Nutritional Status in Pediatric Crohn’s Disease Patients. Int. J. Colorectal. Dis. 2009, 24, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sinitsky, D.M.; Lemberg, D.A.; Leach, S.T.; Bohane, T.D.; Jackson, R.; Day, A.S. Infliximab Improves Inflammation and Anthropometric Measures in Pediatric Crohn’s Disease. J. Gastroenterol. Hepatol. 2010, 25, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Ahmed, S.F.; Wilson, M.L.; Shah, N.; Loganathan, S.; Naik, S.; Bourke, B.; Thomas, A.; Akobeng, A.K.; Fagbemi, A.; et al. The Effects of Anti-TNF-α Treatment with Adalimumab on Growth in Children with Crohn’s Disease (CD). J. Crohns Colitis 2012, 6, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, S.; Wong, S.; Bishop, J.; Hassan, K.; McGrogan, P.; Ahmed, S.; Russell, R. Improvement in Growth of Children with Crohn Disease Following Anti-TNF-α Therapy Can Be Independent of Pubertal Progress and Glucocorticoid Reduction. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 31–37. [Google Scholar] [CrossRef]
- Walters, T.D.; Gilman, A.R.; Griffiths, A.M. Linear Growth Improves during Infliximab Therapy in Children with Chronically Active Severe Crohn’s Disease. Inflamm. Bowel Dis. 2007, 13, 424–430. [Google Scholar] [CrossRef]
- Cameron, F.L.; Altowati, M.A.; Rogers, P.; McGrogan, P.; Anderson, N.; Bisset, W.M.; Ahmed, S.F.; Wilson, D.C.; Russell, R.K. Disease Status and Pubertal Stage Predict Improved Growth in Antitumor Necrosis Factor Therapy for Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Hyams, J.S.; Lerer, T.; Griffiths, A.; Pfefferkorn, M.; Kugathasan, S.; Evans, J.; Otley, A.; Carvalho, R.; Mack, D.; Bousvaros, A.; et al. Long-Term Outcome of Maintenance Infliximab Therapy in Children with Crohn’s Disease. Inflamm. Bowel Dis. 2009, 15, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Ruemmele, F.M.; Lachaux, A.; Cézard, J.P.; Morali, A.; Maurage, C.; Giniès, J.L.; Viola, S.; Goulet, O.; Lamireau, T.; Scaillon, M.; et al. Efficacy of Infliximab in Pediatric Crohn’s Disease: A Randomized Multicenter Open-Label Trial Comparing Scheduled to on Demand Maintenance Therapy. Inflamm. Bowel Dis. 2009, 15, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, M.; Guariso, G. Crohn’s Disease and Growth Deficiency in Children and Adolescents. World J. Gastroenterol. 2014, 20, 13219–13233. [Google Scholar] [CrossRef]
- Wong, S.C.; Hassan, K.; McGrogan, P.; Weaver, L.T.; Ahmed, S.F. The Effects of Recombinant Human Growth Hormone on Linear Growth in Children with Crohn’s Disease and Short Stature. J. Pediatr. Endocrinol. Metab. 2007, 20, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Soendergaard, C.; Young, J.A.; Kopchick, J.J. Growth Hormone Resistance-Special Focus on Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 1019. [Google Scholar] [CrossRef] [Green Version]
- Ranger, G.S.; Lamparelli, M.J.; Aldridge, A.; Chong, S.K.; Mitton, S.G.; Albanese, A.; Kumar, D. Surgery Results in Significant Improvement in Growth in Children with Crohn’s Disease Refractory to Medical Therapy. Pediatr. Surg. Int. 2006, 22, 347–352. [Google Scholar] [CrossRef]
- Oliva, L.; Wyllie, R.; Alexander, F.; Caulfield, M.; Steffen, R.; Lavery, I.; Fazio, V. The Results of Strictureplasty in Pediatric Patients with Multifocal Crohn’s Disease. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 306–310. [Google Scholar] [CrossRef]
- Brain, C.E.; Savage, M.O. Growth and Puberty in Chronic Inflammatory Bowel Disease. Baillieres Clin. Gastroenterol. 1994, 8, 83–100. [Google Scholar] [CrossRef]
- Ferry, G.D.; BÜller, H.A. Mechanisms of Growth Retardation, Drug Therapy, and Nutritional Support in Pediatric Inflammatory Bowel Disease: A Workshop Sponsored by the North American and European Societies for Pediatric Gastroenterology and Nutrition. Inflamm. Bowel Dis. 1995, 1, 313–330. [Google Scholar] [CrossRef]


| Probable Risk Factor | Correlated Factors/Mechanisms of Action |
|---|---|
| Malnutrition state |
|
| GH-resistance |
|
| Cytokines increased levels |
|
| Susceptibility genes |
|
| Inflammation site (Jejunal disease) |
|
| Inhibition of the sex steroids production by cytokines |
|
| Therapy | Effects |
|---|---|
| Glucocorticoid therapy | Adverse effects on growth and bone metabolism
|
| Aminosalicylates | Further studies are needed to clarify their role |
| Immunosuppressive drugs |
|
| Exclusive enteral nutrition (EEN) |
|
| Biologics (infliximab-Adalimumab) |
|
| Growth hormone |
|
| Surgery option (Strictureplasty-Intestinal resection) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaro, F.; Chiarelli, F. Growth and Puberty in Children with Inflammatory Bowel Diseases. Biomedicines 2020, 8, 458. https://doi.org/10.3390/biomedicines8110458
Amaro F, Chiarelli F. Growth and Puberty in Children with Inflammatory Bowel Diseases. Biomedicines. 2020; 8(11):458. https://doi.org/10.3390/biomedicines8110458
Chicago/Turabian StyleAmaro, Flavia, and Francesco Chiarelli. 2020. "Growth and Puberty in Children with Inflammatory Bowel Diseases" Biomedicines 8, no. 11: 458. https://doi.org/10.3390/biomedicines8110458