Taxonomic and Feeding Trait-Based Analysis of Macroinvertebrates in the Antisana River Basin (Ecuadorian Andean Region)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
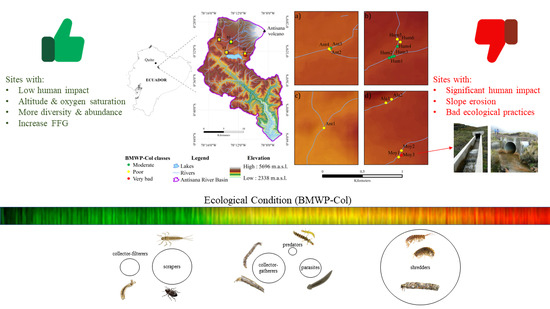
2.1. Study Area
2.2. Physico-Chemical Analysis
2.3. Macroinvertebrate Data Collection
2.4. Calculation of Ecological Condition Indices for River Assessment
2.5. Feeding Habit Trait Allocation
2.6. Data Analysis
2.6.1. Environmental Variables and Functional Feeding Group Assemblages Compared between Sites Based on Ecological Condition Indices
2.6.2. Macroinvertebrate Community and Functional Feeding Groups
2.6.3. Other Ecological Quality and Trait Indices
3. Results
3.1. Physico-Chemical Analysis
3.2. Ecological Condition Indices for River Assessment
3.3. Ecological Conditions and Functional Feeding Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Order | Family | Total Abundance | Number of Sampling Sites | BMWP-Col Tolerance Score |
|---|---|---|---|---|
| Acari | Acari | 4 | 2 | - |
| Amphipoda | Hyalellidae | 494 | 14 | 7 |
| Arhynchobdellida | Erpobdellidae | 1 | 1 | - |
| Coleoptera | Elmidae | 53 | 12 | 7 |
| Scirtidae | 60 | 10 | 6 | |
| Diptera | Ceratopogonidae | 5 | 4 | 5 |
| Chironomidae | 34 | 10 | 2 | |
| Limoniidae | 1 | 1 | - | |
| Muscidae | 3 | 3 | 5 | |
| Simuliidae | 87 | 10 | 6 | |
| Ephemeroptera | Baetidae | 458 | 15 | 5 |
| Haplotaxida | Tubificidae | 6 | 4 | 1 |
| Hirudinida | Glossiphoniidae | 6 | 5 | 3 |
| Opisthopora | Lumbricidae | 14 | 3 | - |
| Plecoptera | Gripopterygidae | 11 | 5 | 10 |
| Trichoptera | Hydrobiosidae | 14 | 7 | 9 |
| Hydroptilidae | 1 | 1 | 8 | |
| Leptoceridae | 227 | 9 | 8 | |
| Limnephilidae | 169 | 11 | 8 | |
| Tricladida | Dugesiidae | 97 | 13 | 6 |
| Sampling Code | BMWP-Col | ABI | AAMBI | RAO | Index | Value | Class |
| BMWP-Col | >100 | Good | |||||
| A1 | 58 | 47 | 47 | 0.48 | 61–100 | Moderate | |
| AL1 | 53 | 36 | 36 | 0.43 | 36–60 | Poor | |
| AL2 | 50 | 33 | 37 | 0.32 | 16–35 | Bad | |
| AL3 | 9 | 6 | 6 | 0.14 | 0–15 | Very bad | |
| ALB1 | 41 | 36 | 40 | 0.35 | |||
| ALB2 | 45 | 33 | 33 | 0.24 | ABI | >96 | Very good |
| H1 | 75 | 60 | 60 | 0.38 | 59–96 | Good | |
| H2 | 82 | 66 | 66 | 0.30 | 35–58 | Regular | |
| H3 | 69 | 50 | 50 | 0.40 | <35 | Bad | |
| H4 | 61 | 45 | 45 | 0.31 | |||
| H5 | 57 | 36 | 36 | 0.45 | AAMBI | >121 | Excellent |
| H6 | 51 | 39 | 39 | 0.47 | 90–120 | Very good | |
| J1A | 47 | 35 | 35 | 0.22 | 50–89 | Good | |
| J1B | 74 | 52 | 52 | 0.33 | 36–49 | Regular | |
| J1C | 56 | 37 | 37 | 0.32 | <35 | Bad | |



References
- Andino, P.; Espinosa, R.; Crespo-Pérez, V.; Cauvy-Frauníe, S.; Dangles, O.; Jacobsen, D. Functional Feeding Groups of Macrofauna and Detritus Decomposition along a Gradient of Glacial Meltwater Influence in Tropical High-Andean Streams. Water 2021, 13, 3303. [Google Scholar] [CrossRef]
- Jacobsen, D. Tropical High-Altitude Streams. In Tropical Stream Ecology; Dudgeon, D., Ed.; Elservier Inc.: Amsterdam, The Netherlands, 2008; Volume 46, ISBN 978-0-12-088449-0. [Google Scholar]
- Jacobsen, D.; Dangles, O.; Andino, P.; Espinosa, R.; Hamerlík, L.; Cadier, E. Longitudinal Zonation of Macroinvertebrates in an Ecuadorian Glacier-Fed Stream: Do Tropical Glacial Systems Fit the Temperate Model? Freshw. Biol. 2010, 55, 1234–1248. [Google Scholar] [CrossRef]
- Favier, V.; Coudrain, A.; Cadier, E.; Francou, B.; Ayabaca, E.; Maisincho, L.; Praderio, E.; Villacis, M.; Wagnon, P. Evidence of Groundwater Flow on Antizana Ice-Covered Volcano, Ecuador. Hydrol. Sci. J. 2008, 53, 278–291. [Google Scholar] [CrossRef]
- Rosero-López, D.; Knighton, J.; Lloret, P.; Encalada, A.C. Invertebrate Response to Impacts of Water Diversion and Flow Regulation in High-Altitude Tropical Streams. River Res. Appl. 2020, 36, 223–233. [Google Scholar] [CrossRef]
- Hofstede, R.G.M.; Llambí, L.D. Plant Diversity in Páramo-Neotropical High Mountain Humid Grasslands. Encycl. World’s Biomes 2020, 1–5, 362–372. [Google Scholar] [CrossRef]
- Kaser, G. Glacier-Climate Interaction at Low Latitudes. J. Glaciol. 2001, 47, 195–204. [Google Scholar] [CrossRef]
- Samaniego, P.; Barba, D.; Robin, C.; Fornari, M.; Bernard, B. Eruptive History of Chimborazo Volcano (Ecuador): A Large, Ice-Capped and Hazardous Compound Volcano in the Northern Andes. J. Volcanol. Geotherm. Res. 2012, 221–222, 33–51. [Google Scholar] [CrossRef]
- Young, K.R. Ecosystem Change in High Tropical Mountains. In The High-Mountain Cryosphere: Environmental Changes and Human Risks; Cambridge University Press: Cambridge, UK, 2015; pp. 227–246. [Google Scholar] [CrossRef]
- Joslin, A. Dividing “Above” and “Below”: Constructing Territory for Ecosystem Service Conservation in the Ecuadorian Highlands. Ann. Am. Assoc. Geogr. 2020, 110, 1874–1890. [Google Scholar] [CrossRef]
- Lazo, P.X.; Mosquera, G.M.; McDonnell, J.J.; Crespo, P. The Role of Vegetation, Soils, and Precipitation on Water Storage and Hydrological Services in Andean Páramo Catchments. J. Hydrol. 2019, 572, 805–819. [Google Scholar] [CrossRef]
- Boyero, L.; Pearson, R.G.; Gessner, M.O.; Barmuta, L.A.; Ferreira, V.; Graça, M.A.S.; Dudgeon, D.; Boulton, A.J.; Callisto, M.; Chauvet, E.; et al. A Global Experiment Suggests Climate Warming Will Not Accelerate Litter Decomposition in Streams but Might Reduce Carbon Sequestration. Ecol. Lett. 2011, 14, 289–294. [Google Scholar] [CrossRef]
- Ragettli, S.; Immerzeel, W.W.; Pellicciotti, F. Contrasting Climate Change Impact on River Flows from High-Altitude Catchments in the Himalayan and Andes Mountains. Proc. Natl. Acad. Sci. USA 2016, 113, 9222–9227. [Google Scholar] [CrossRef]
- Tomanova, S.; Usseglio-Polatera, P. Patterns of Benthic Community Traits in Neotropical Streams: Relationship to Mesoscale Spatial Variability. Fundam. Appl. Limnol. 2007, 170, 243–255. [Google Scholar] [CrossRef]
- Eriksen, T.E.; Brittain, J.E.; Søli, G.; Jacobsen, D.; Goethals, P.; Friberg, N. A Global Perspective on the Application of Riverine Macroinvertebrates as Biological Indicators in Africa, South-Central America, Mexico and Southern Asia. Ecol. Indic. 2021, 126, 107609. [Google Scholar] [CrossRef]
- Castella, E.; Adalsteinsson, H.; Brittain, J.E.; Gislason, G.M.; Lehmann, A.; Lencioni, V.; Lods-Crozet, B.; Maiolini, B.; Milner, A.M.; Olafsson, J.S.; et al. Macrobenthic Invertebrate Richness and Composition along a Latitudinal Gradient of European Glacier-Fed Streams. Freshw. Biol. 2001, 46, 1811–1831. [Google Scholar] [CrossRef]
- Di Cugno, N.; Robinson, C.T. Trophic Structure of Macroinvertebrates in Alpine Non-Glacial Streams. Fundam. Appl. Limnol. 2017, 190, 319–330. [Google Scholar] [CrossRef]
- Villamarín, C.; Rieradevall, M.; Prat, N. Macroinvertebrate Diversity Patterns in Tropical Highland Andean Rivers. Limnetica 2020, 39, 677–691. [Google Scholar] [CrossRef]
- Jacobsen, D.; Marín, R. Bolivian Altiplano Streams with Low Richness of Macroinvertebrates and Large Diel Fluctuations in Temperature and Dissolved Oxygen. Aquat. Ecol. 2008, 42, 643–656. [Google Scholar] [CrossRef]
- Madsen, P.B.; Morabowen, A.; Andino, P.; Espinosa, R.; Cauvy-Fraunié, S.; Dangles, O.; Jacobsen, D. Altitudinal Distribution Limits of Aquatic Macroinvertebrates: An Experimental Test in a Tropical Alpine Stream. Ecol. Entomol. 2015, 40, 629–638. [Google Scholar] [CrossRef]
- Crespo-Pérez, V.; Andino, P.; Espinosa, R.; Dangles, O.; Jacobsen, D. The Altitudinal Limit of Leptohyphes Eaton, 1882 and Lachlania Hagen, 1868 (Ephemeroptera: Leptohyphidae, Oligoneuriidae) in Ecuadorian Andes Streams: Searching for Mechanisms. Aquat. Insects 2016, 37, 69–86. [Google Scholar] [CrossRef]
- Chará-Serna, A.M.; Chará, J.D.; Zúñiga, M.D.C.; Pearson, R.G.; Boyero, L. Diets of Leaf Litter-Associated Invertebrates in Three Tropical Streams. Ann. Limnol. 2012, 48, 139–144. [Google Scholar] [CrossRef]
- Espinosa, R.; Andino, P.; Cauvy-Fraunié, S.; Dangles, O.; Jacobsen, D.; Crespo-Pérez, V. Diversity Patterns of Aquatic Macroinvertebrates in a Tropical High-Andean Catchment. Rev. Biol. Trop. 2020, 68, S39–S53. [Google Scholar] [CrossRef]
- Niedrist, G.H.; Füreder, L. When the Going Gets Tough, the Tough Get Going: The Enigma of Survival Strategies in Harsh Glacial Stream Environments. Freshw. Biol. 2018, 63, 1260–1272. [Google Scholar] [CrossRef]
- Rios-Touma, B.; Acosta, R.; Prat, N. The Andean Biotic Index (ABI): Revised Tolerance to Pollution Values for Macroinvertebrate Families and Index Performance Evaluation. Rev. Biol. Trop. 2014, 62, 249. [Google Scholar] [CrossRef]
- Encalada, A.C.; Suarez, E.; Mena, C.; Lessmann, J.; Guayasamin, J.M.; Sanpedro, C.; Martinez, P.; Ochoa-Herrera, V.; Swing, K.; Vieira, J.P. Los Ríos de Las Cuencas Andino-Amazónicas: Herramientas y Guía de Invertebrados Para El Diseño Efectivo de Programas de Monitoreo; Encalada, A.C., Ed.; Trama: Quito, Ecuador, 2019; ISBN 978-9942-808-03-5. [Google Scholar]
- Encalada, A.C.; Rieradevall, M.; Ríos-Touma, B.L.; García, N.; Prat, N. Protocolo Simplificado y Guía de Evaluación de La Calidad Ecológica de Ríos Andinos (CERA-S); Usfq, U.B., Fonag, A., Eds.; Trama: Quito, Ecuador, 2011; ISBN 978-9942-03-734-3. [Google Scholar]
- Encalada, A.C.; Flecker, A.S.; Poff, N.L.R.; Suárez, E.; Herrera, G.A.; Ríos-Touma, B.; Jumani, S.; Larson, E.I.; Anderson, E.P. A Global Perspective on Tropical Montane Rivers. Science 2019, 365, 1124–1129. [Google Scholar] [CrossRef]
- Álvarez, L.F. Metodología Para La Utilización de Los Macroinvertebrados Acuáticos Como Indicadores de La Calidad Del Agua; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2005. [Google Scholar]
- Roldán-Pérez, G. Bioindicación de la Calidad del Agua en Colombia. Uso del Método BMWP/Col; Universidad de Antioquia: Antioquia, Colombia, 2003; 170p. [Google Scholar]
- Zilli, F.L.; Montalto, L.; Marchese, M.R. Benthic Invertebrate Assemblages and Functional Feeding Groups in the Paraná River Floodplain (Argentina). Limnologica 2008, 38, 159–171. [Google Scholar] [CrossRef]
- Amoros, C.; Bornette, G. Connectivity and Biocomplexity in Waterbodies of Riverine Floodplains. Freshw. Biol. 2002, 47, 761–776. [Google Scholar] [CrossRef]
- Cummins, K.W.; Merritt, R.W.; Andrade, P.C.N. The Use of Invertebrate Functional Groups to Characterize Ecosystem Attributes in Selected Streams and Rivers in South Brazil. Stud. Neotrop. Fauna Environ. 2005, 40, 69–89. [Google Scholar] [CrossRef]
- Tomanova, S.; Goitia, E.; Helešic, J. Trophic Levels and Functional Feeding Groups of Macroinvertebrates in Neotropical Streams. Hydrobiologia 2006, 556, 251–264. [Google Scholar] [CrossRef]
- Townsend, C.; Hildrew, A. Species Traits in Relation to a Habitat Templet for River Systems. Freshw. Biol. 1994, 31, 265–275. [Google Scholar] [CrossRef]
- Archaimbault, V.; Usseglio-Polatera, P.; Vanden Bossche, J.P. Functional Differences among Benthic Macroinvertebrate Communities in Reference Streams of Same Order in a given Biogeographic Area. Hydrobiologia 2005, 551, 171–182. [Google Scholar] [CrossRef]
- Piscart, C.; Usseglio-Polatera, P.; Moreteau, J.C.; Beisel, J.N. The Role of Salinity in the Selection of Biological Traits of Freshwater Invertebrates. Arch. Hydrobiol. 2006, 166, 185–198. [Google Scholar] [CrossRef]
- Cabrera, S.; Eurie Forio, M.A.; Lock, K.; Vandenbroucke, M.; Oña, T.; Gualoto, M.; Goethals, P.L.M.; Der Heyden, C. Van Variations in Benthic Macroinvertebrate Communities and Biological Quality in the Aguarico and Coca River Basins in the Ecuadorian Amazon. Water 2021, 13, 1692. [Google Scholar] [CrossRef]
- Kerans, A.B.L.; Karr, J.R. A Benthic Index of Biotic Integrity (B-IBI) for Rivers of the Tennessee Valley. Ecol. Appl. 2016, 4, 768–785. [Google Scholar] [CrossRef]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999; EPA/841-B-99-002. [Google Scholar]
- Covich, A.P. Geographical and Historical Comparisons of Neotropical Streams: Biotic Diversity and Detrital Processing in Highly Variable Habitats. J. N. Am. Benthol. Soc. 1988, 7, 361–386. [Google Scholar] [CrossRef]
- Damanik-Ambarita, M.N.; Lock, K.; Boets, P.; Everaert, G.; Nguyen, T.H.T.; Forio, M.A.E.; Musonge, P.L.S.; Suhareva, N.; Bennetsen, E.; Landuyt, D.; et al. Ecological Water Quality Analysis of the Guayas River Basin (Ecuador) Based on Macroinvertebrates Indices. Limnologica 2016, 57, 27–59. [Google Scholar] [CrossRef]
- Dudgeon, D.; Gao, B.W. Biodiversity and Ecosystem Functioning in a Species-Poor Guild: A Test Using Tropical Stream Detritivores. Hydrobiologia 2010, 652, 329–336. [Google Scholar] [CrossRef]
- Niu, S.Q.; Dudgeon, D. The Influence of Flow and Season upon Leaf-Litter Breakdown in Monsoonal Hong Kong Streams. Hydrobiologia 2011, 663, 205–215. [Google Scholar] [CrossRef]
- Ramírez, A.; Gutiérrez-Fonseca, P.E. Functional Feeding Groups of Aquatic Insect Families in Latin America: A Critical Analysis and Review of Existing Literature. Rev. Biol. Trop. 2014, 62, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Tomanova, A.; Moya, N.; Oberdorff, T. Using Macroinvertebrate Biological Traits for Assessing Biotic Integrity of Neotropical Streams. River Res. Appl. 2008, 24, 1230–1239. [Google Scholar] [CrossRef]
- Wehrtmann, I.S.; Hernández-Díaz, D.; Cumberlidge, N. Freshwater Crabs as Predators and Prey: The Case of Ptychophallus Uncinatus Campos & Lemaitre, 1999 (Brachyura, Pseudothelphusidae) from Costa Rica, Central America. Lat. Am. J. Aquat. Res. 2019, 47, 18–26. [Google Scholar] [CrossRef]
- Yang, C.; Wehrtmann, I.S.; Wenger, S.J.; Rugenski, A.T. Neotropical Freshwater Crabs (Decapoda: Pseudothelphusidae) Shred Leaves. Nauplius 2020, 28. [Google Scholar] [CrossRef]
- Nessimian, J.L.; Dorvillé, L.F.M.; Sanseverino, A.M.; Baptista, D.F. Relation between Flood Pulse and Functional Composition of the Macroinvertebrate Benthic Fauna in the Lower Rio Negro, Amazonas, Brazil. Amazoniana 1998, 15, 35–50. [Google Scholar]
- Berger, E.; Haase, P.; Schäfer, R.B.; Sundermann, A. Towards Stressor-Specific Macroinvertebrate Indices: Which Traits and Taxonomic Groups Are Associated with Vulnerable and Tolerant Taxa? Sci. Total Environ. 2018, 619–620, 144–154. [Google Scholar] [CrossRef] [PubMed]
- EPMAPS; FONAG. Actualización del Plan de Manejo del Área de Conservación Hídrica Antisana; Ministerio del Ambiente: Quito, Ecuador, 2018. [Google Scholar]
- ECOLAP; MAE. Guía del Patrimonio de Áreas Naturales Protegidas del Ecuador; ECOFUND, FAN, DarwinNet, IGM: Quito, Ecuador, 2007. [Google Scholar]
- EPMAPS. Estudio Geológico Geotécnico a Nivel de Prefactibilidad del Proyecto de Agua Potable Ríos Orientales; Empresa Pública Metropolitana de Agua Potable y Saneamiento: Quito, Ecuador, 2006. [Google Scholar]
- Gabriels, W.; Lock, K.; De Pauw, N.; Goethals, P.L.M. Limnologica Multimetric Macroinvertebrate Index Flanders (MMIF) for Biological Assessment of Rivers and Lakes in Flanders (Belgium). Limnologica 2010, 40, 199–207. [Google Scholar] [CrossRef]
- Domínguez, E.; Fernández, H.R. Macroinvertebrados Bentónicos Sudamericanos; Fundación Miguel Lillo: Tucumán, Argentina, 2009; ISBN 9789506680152. [Google Scholar]
- Dominguez-Granda, L.; Lock, K.; Goethals, P.L.M. Using Multi-Target Clustering Trees as a Tool to Predict Biological Water Quality Indices Based on Benthic Macroinvertebrates and Environmental Parameters in the Chaguana Watershed (Ecuador). Ecol. Inform. 2011, 6, 303–308. [Google Scholar] [CrossRef]
- Everaert, G.; De Neve, J.; Boets, P.; Dominguez-granda, L.; Mereta, S.T.; Ambelu, A.; Hoang, T.H.; Goethals, P.L.M.; Thas, O. Comparison of the Abiotic Preferences of Macroinvertebrates in Tropical River Basins. PLoS ONE 2014, 9, e108898. [Google Scholar] [CrossRef]
- Mereta, S.T.; Boets, P.; De Meester, L.; Goethals, P.L.M. Development of a Multimetric Index Based on Benthic Macroinvertebrates for the Assessment of Natural Wetlands in Southwest Ethiopia. Ecol. Indic. 2013, 29, 510–521. [Google Scholar] [CrossRef]
- Ho, L.; Pham, D.T.; Id, W.V.E.; Muchene, L.; Shkedy, Z.; Goethals, P. A Closer Look on Spatiotemporal Variations of Dissolved Oxygen in Waste Stabilization Ponds. Water 2018, 10, 201. [Google Scholar] [CrossRef]
- Schmera, D.; Heino, J.; Podani, J.; Erős, T.; Dolédec, S. Functional Diversity: A Review of Methodology and Current Knowledge in Freshwater Macroinvertebrate Research. Hydrobiologia 2017, 787, 27–44. [Google Scholar] [CrossRef]
- Pearson, T.; Rosenberg, R. Macrobenthic Succession in Relation to Organic Enrichment and Pollution of the Marine Environment. Oceanogr. Mar. Biol. Annu. Rev. 1978, 16, 229–331. [Google Scholar]
- Clarke, K.R. Non-parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. J. Veg. Sci. 2019, 14, 927–930. [Google Scholar]
- Martinez-Arbizu, P. PairwiseAdonis: Pairwise Multilevel Comparison Using Adonis 2020, R package version 0.4 1. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 30 September 2023).
- Rao, C.R. Quadratic Entropy and Analysis of Diversity. Sankhya Indian J. Stat. 2010, 72, 70–80. [Google Scholar] [CrossRef]
- Crespo-Pérez, V.; Dangles, O.; Ibarra, C.; Espinosa, R.; Andino, P.; Jacobsen, D.; Cauvy-Fraunié, S. Functional Structure and Diversity of Invertebrate Communities in a Glacierised Catchment of the Tropical Andes. Freshw. Biol. 2020, 65, 1348–1362. [Google Scholar] [CrossRef]
- Iñiguez-Armijos, C.; Hampel, H.; Breuer, L. Land-Use Effects on Structural and Functional Composition of Benthic and Leaf-Associated Macroinvertebrates in Four Andean Streams. Aquat. Ecol. 2018, 52, 77–92. [Google Scholar] [CrossRef]
- Joao, C.; Perez-Bilbao, A.; Garrido, J. Macroinvertebrates as Indicators of Water Quality in Running Waters: 10 Years of Research in Rivers with Different Degrees of Anthropogenic Impacts. In Ecological Water Quality—Water Treatment and Reuse; InTech: Thessaloniki, Greece, 2012. [Google Scholar] [CrossRef]
- Ward, J.V. Ecology of Alpine Streams. Freshw. Biol. 1994, 32, 277–294. [Google Scholar] [CrossRef]
- Montgomery, D.R.; Buffington, J.M. Channel-Reach Morphology in Mountain Drainage Basins. Bull. Geol. Soc. Am. 1997, 109, 596–611. [Google Scholar] [CrossRef]
- Monaghan, K.A.; Peck, M.R.; Brewin, P.A.; Masiero, M.; Zarate, E.; Turcotte, P.; Ormerod, S.J. Macroinvertebrate Distribution in Ecuadorian Hill Streams: The Effects of Altitude and Land Use. Arch. Hydrobiol. 2000, 149, 421–440. [Google Scholar] [CrossRef]
- Hynes, H. The Ecology of Running Waters; Liverpool University Press: Liverpool, UK, 1970; ISBN 978-1930665330. [Google Scholar]
- Ward, J.V.; Stanford, J.A. Evolutionary Ecology of Aquatic Insects. Annu. Rev. Entomol. 1982, 27, 97–117. [Google Scholar] [CrossRef]
- Jacobsen, D.; Rostgaard, S.; Vásconez, J.J. Are Macroinvertebrates in High Altitude Streams Affected by Oxygen Deficiency? Freshw. Biol. 2003, 48, 2025–2032. [Google Scholar] [CrossRef]
- Spicer, J.; Gaston, K. Amphipod Gigantism Dictated by Oxygen Availability? Ecol. Lett. 1999, 2, 397–403. [Google Scholar] [CrossRef]
- Rostgaard, S.; Jacobsen, D. Respiration Rate of Stream Insects Measured in Situ along a Large Altitude Range. Hydrobiologia 2005, 549, 79–98. [Google Scholar] [CrossRef]
- Nguyen, T.; Boets, P.; Lock, K.; Anne, M.; Forio, E.; Van Echelpoel, W.; Van Butsel, J.; Dueñas, J.A.; Everaert, G.; Elvin, L.; et al. Water Quality Related Macroinvertebrate Community Responses to Environmental Gradients in the Portoviejo River (Ecuador). Ann. Limnol.-Int. J. Limnol. 2017, 53, 203–219. [Google Scholar] [CrossRef]
- Van Echelpoel, W.; Forio, E.; VanButsel, J.; Lock, K.; Dueñas, J.; Dominguez-granda, L.; Goethals, P.L.M. Macroinvertebrate Functional Feeding Group Structure along an Impacted Tropical River: The Portoviejo River (Ecuador). Limnologica 2018, 73, 12–19. [Google Scholar] [CrossRef]
- Damanik-Ambarita, M.N.; Boets, P.; Tien, H.; Thi, N.; Anne, M.; Forio, E.; Everaert, G.; Lock, K.; Liz, P.; Musonge, S.; et al. Impact Assessment of Local Land Use on Ecological Water Quality of the Guayas River Basin (Ecuador). Ecol. Inform. 2018, 48, 226–237. [Google Scholar] [CrossRef]
- Mathers, K.L.; Kowarik, C.; Rachelly, C.; Robinson, C.T.; Weber, C. The Effects of Sediment Traps on Instream Habitat and Macroinvertebrates of Mountain Streams. J. Environ. Manag. 2021, 295, 113066. [Google Scholar] [CrossRef]
- Mellado-Díaz, A.; Sánchez-González, J.R.; Guareschi, S.; Magdaleno, F.; Toro Velasco, M. Exploring Longitudinal Trends and Recovery Gradients in Macroinvertebrate Communities and Biomonitoring Tools along Regulated Rivers. Sci. Total Environ. 2019, 695, 133774. [Google Scholar] [CrossRef]
- Han, M.; Yu, H.; Zhou, B.; Zhang, Y.; Wang, B. The Impact of Run-of Stream Dams on Benthic Macroinvertebrate Assemblages in Urban Streams. Shengtai Xuebao/Acta Ecol. Sin. 2012, 32, 0380–0385. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Q.; Jiang, W.; Qu, X. Assessing Impacts of a Dam Construction on Benthic Macroinvertebrate Communities in a Mountain Stream. Fresenius Environ. Bull. 2013, 22, 103–110. [Google Scholar]
- Mbaka, J.G.; Wanjiru Mwaniki, M. A Global Review of the Downstream Effects of Small Impoundments on Stream Habitat Conditions and Macroinvertebrates. Environ. Rev. 2015, 23, 257–262. [Google Scholar] [CrossRef]
- Fierro, P.; Bertrán, C.; Mercado, M.; Peña-Cortés, F.; Tapia, J.; Hauenstein, E.; Caputo, L.; Vargas-Chacoff, L. Landscape Composition as a Determinant of Diversity and Functional Feeding Groups of Aquatic Macroinvertebrates in Southern Rivers of the Araucanía, Chile. Lat. Am. J. Aquat. Res. 2015, 43, 186–200. [Google Scholar] [CrossRef]
- Sotomayor, G.; Hampel, H.; Vázquez, R.F.; Forio, M.A.E.; Goethals, P.L.M. Implications of Macroinvertebrate Taxonomic Resolution for Freshwater Assessments Using Functional Traits: The Paute River Basin (Ecuador) Case. Divers. Distrib. 2022, 28, 1735–1747. [Google Scholar] [CrossRef]
- Jacobsen, D. Altitudinal Changes in Diversity of Macroinvertebrates from Small Streams in the Ecuadorian Andes. Arch. Hydrobiol. 2003, 158, 145–167. [Google Scholar] [CrossRef]
- Jacobsen, D. Contrasting Patterns in Local and Zonal Family Richness of Stream Invertebrates along an Andean Altitudinal Gradient. Freshw. Biol. 2004, 49, 1293–1305. [Google Scholar] [CrossRef]
- Marques, M.M.; Barbosa, F. Biological Quality of Waters from an Impacted Tropical Watershed (Middle Rio Doce Basin, Southeast Brazil), Using Benthic Macroinvertebrate Communities as an Indicator. Hydrobiologia 2001, 457, 69–76. [Google Scholar] [CrossRef]
- Leite-Rossi, L.A.; Saito, V.S.; Cunha-Santino, M.B.; Trivinho-Strixino, S. How Does Leaf Litter Chemistry Influence Its Decomposition and Colonization by Shredder Chironomidae (Diptera) Larvae in a Tropical Stream? Hydrobiologia 2016, 771, 119–130. [Google Scholar] [CrossRef]
- Mesa, L.M. Influence of Riparian Quality on Macroinvertebrate Assemblages in Subtropical Mountain Streams. J. Nat. Hist. 2014, 48, 1153–1167. [Google Scholar] [CrossRef]
- Buss, D.F.; Carlisle, D.M.; Chon, T.S.; Culp, J.; Harding, J.S.; Keizer-Vlek, H.E.; Robinson, W.A.; Strachan, S.; Thirion, C.; Hughes, R.M. Stream Biomonitoring Using Macroinvertebrates around the Globe: A Comparison of Large-Scale Programs. Environ. Monit. Assess. 2015, 187, 4132. [Google Scholar] [CrossRef]
- Brewin, P.A.; Buckton, S.T.; Ormerod, S.J. The Seasonal Dynamics and Persistence of Stream Macroinvertebrates in Nepal: Do Monsoon Floods Represent Disturbance? Freshw. Biol. 2000, 44, 581–594. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Fossati, O.; Wasson, J.G.; Hery, C.; Marin, R.; Salinas, G. Impact of Sediment Releases on Water Chemistry and Macroinvertebrate Communities in Clear Water Andean Streams (Bolivia). Arch. Hydrobiol. 2001, 151, 33–50. [Google Scholar] [CrossRef]
- Lock, M.A.; Wallace, R.R.; Costerton, J.W.; Ventullo, R.M.; Charlton, S.E. River Epilithon: Toward a Structural-Functional Model. Oikos 1984, 42, 10. [Google Scholar] [CrossRef]
- Ilg, C.; Castella, E. Patterns of Macroinvertebrate Traits along Three Glacial Stream Continuums. Freshw. Biol. 2006, 51, 840–853. [Google Scholar] [CrossRef]
- Forio, M.; Goethals, P.L.M.; Lock, K.; Asio, V.; Bande, M.; Thas, O. Model-Based Analysis of the Relationship between Macroinvertebrate Traits and Environmental River Conditions. Environ. Model. Softw. 2018, 106, 57–67. [Google Scholar] [CrossRef]
- Buss, D.F.; Vitorino, A.S. Rapid Bioassessment Protocols Using Benthic Macroinvertebrates in Brazil: Evaluation of Taxonomic Sufficiency. J. N. Am. Benthol. Soc. 2010, 29, 562–571. [Google Scholar] [CrossRef]
- Feminella, J.; Hawkins, C. Interactions between Stream Herbivores and Periphyton: A Quantitative Analysis of Past Experiments. J. N. Am. Benthol. Soc. 1995, 14, 465–509. [Google Scholar] [CrossRef]
- Ding, N.; Yang, W.; Zhou, Y.; González-Bergonzoni, I.; Zhang, J.; Chen, K.; Vidal, N.; Jeppesen, E.; Liu, Z.; Wang, B. Different Responses of Functional Traits and Diversity of Stream Macroinvertebrates to Environmental and Spatial Factors in the Xishuangbanna Watershed of the Upper Mekong River Basin, China. Sci. Total Environ. 2017, 574, 288–299. [Google Scholar] [CrossRef]
- Jacobsen, D.; Encalada, A. The Macroinvertebrate Fauna of Ecuadorian Highland Streams in the Wet and Dry Season. Arch. Hydrobiol. 1998, 142, 53–70. [Google Scholar] [CrossRef]
- Boonsoong, B.; Sangpradub, N.; Barbour, M.T.; Simachaya, W. An Implementation Plan for Using Biological Indicators to Improve Assessment of Water Quality in Thailand. Environ. Monit. Assess. 2010, 165, 205–215. [Google Scholar] [CrossRef]
- Buss, D.; Salles, F. Using Baetidae Species as Biological Indicators of Environmental Degradation in a Brazilian River Basin. Environ. Monit. Assess. 2007, 130, 365–372. [Google Scholar] [CrossRef]
- Sotomayor, G.; Hampel, H.; Vázquez, R.F.; Goethals, P.L.M. Multivariate-Statistics Based Selection of a Benthic Macroinvertebrate Index for Assessing Water Quality in the Paute River Basin (Ecuador). Ecol. Indic. 2020, 111, 106037. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Oglęcki, P.; Keller, A.; Kardel, I.; Piniewski, M.; Mirosław-światek, D. Effect of a Summer Flood on Benthic Macroinvertebrates in a Medium-Sized, Temperate, Lowland River. Water 2021, 13, 885. [Google Scholar] [CrossRef]
- Bibi, H.; Rraffaelli, D.; Sharif, M. The Variation of Size Distributions of Benthic Communities Across a Range of Irrigating Ponds and Canals of North Yorkshire, UK. Sarhad J. Agric. 2021, 38, 137–148. [Google Scholar] [CrossRef]
- Ertaş, A.; Yorulmaz, B.; Sukatar, A. Comparative Analysis of Biotic Indices for Assessment of Water Quality of Balaban Stream in West Anatolia, Turkey. Biologia 2022, 77, 721–730. [Google Scholar] [CrossRef]
- Baryshev, I.A. Evaluating Water Quality in a Large River System in the Northern European Russia by Macrozoobenthos. Water Resour. 2021, 48, 774–781. [Google Scholar] [CrossRef]
- Armitage, P.D.; Moss, D.; Wright, J.F.; Furse, M.T. The Performance of a New Biological Water Quality Score System Based on Macroinvertebrates over a Wide Range of Unpolluted Running-Water Sites. Water Res. 1983, 17, 333–347. [Google Scholar] [CrossRef]
- Damanik-Ambarita, M.N.; Everaert, G.; Anne, M.; Forio, E.; Hanh, T.; Nguyen, T.; Lock, K.; Liz, P.; Musonge, S.; Suhareva, N.; et al. Generalized Linear Models to Identify Key Hydromorphological and Chemical Variables Determining the Occurrence of Macroinvertebrates in the Guayas River Basin (Ecuador). Water 2016, 8, 297. [Google Scholar] [CrossRef]
- Tampo, L.; Kaboré, I.; Alhassan, E.H.; Ouéda, A.; Bawa, L.M.; Djaneye-Boundjou, G. Benthic Macroinvertebrates as Ecological Indicators: Their Sensitivity to the Water Quality and Human Disturbances in a Tropical River. Front. Water 2021, 3, 662765. [Google Scholar] [CrossRef]
- Voulvoulis, N.; Arpon, K.D.; Giakoumis, T.; Barcelo, D. The EU Water Framework Directive: From Great Expectations to Problems with Implementation. Sci. Total Environ. 2016, 575, 358–366. [Google Scholar] [CrossRef]
- Martini, S.; Larras, F.; Boyé, A.; Faure, E.; Aberle, N.; Archambault, P.; Bacouillard, L.; Beisner, B.E.; Bittner, L.; Castella, E.; et al. Functional Trait-Based Approaches as a Common Framework for Aquatic Ecologists. Limnol. Oceanogr. 2021, 66, 965–994. [Google Scholar] [CrossRef]
- Zakharova, L.; Meyer, K.M.; Seifan, M. Trait-Based Modelling in Ecology: A Review of Two Decades of Research. Ecol. Model. 2019, 407, 108703. [Google Scholar] [CrossRef]
- Menezes, S.; Baird, D.J.; Soares, A.M.V.M. Beyond Taxonomy: A Review of Macroinvertebrate Trait-Based Community Descriptors as Tools for Freshwater Biomonitoring. J. Appl. Ecol. 2010, 47, 711–719. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding Community Ecology from Functional Traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]






| Variable | Mean ± SD | Minimum | Maximum |
|---|---|---|---|
| Dissolved oxygen (mg O2·L−1) | 7.6 ± 0.55 | 6.9 | 8.8 |
| Dissolved oxygen saturation (%) | 103 ± 2.2 | 96 | 105 |
| pH | 7.5 ± 0.64 | 6.1 | 8.8 |
| Conductivity (µS·cm−1) | 121 ± 55.6 | 34 | 217 |
| (mg·L−1) | 0.33 ± 0.214 | 0.07 | 0.76 |
| COD (mg·L−1) | 12 ± 4.1 | 10 | 23 |
| (mg·L−1) | 0.81 ± 0.853 | 0.05 | 2.80 |
| (mg·L−1) | 0.02 ± 0.023 | 0.01 | 0.10 |
| Total nitrogen (mg·L−1) | 1.04 ± 0.825 | 0.22 | 3.60 |
| (mg·L−1) | 0.29 ± 0.258 | 0.05 | 0.93 |
| Total phosphorus (mg·L−1) | 0.13 ± 0.104 | 0.05 | 0.40 |
| Elevation (m.a.s.l.) | 4009 ± 31.1 | 3932 | 4037 |
| Velocity (m·s−1) | 0.7 ± 0.31 | 0.2 | 1.4 |
| Width (m) | 1.63 ± 0.918 | 0.75 | 4.00 |
| Depth (m) | 0.34 ± 0.097 | 0.15 | 0.5 |
| Site | Al3 | A1 | Al1 | Al2 | Alb1 | Alb2 | H5 | H6 | J1A | J1C | H1 | H2 | H3 | H4 | J1B | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa (FFG) | Ecological Condition | Very Bad | Poor | Moderate | |||||||||||||
| Sensitivity Score | |||||||||||||||||
| Acari (PR) | - | p | p | ||||||||||||||
| Erpobdellidae (PR) | - | p | |||||||||||||||
| Lumbricidae (CG) | - | p | p | p | |||||||||||||
| Tubificidae (CG) | 1 | p | p | p | p | ||||||||||||
| Chironomidae (CG) | 2 | p | p | p | p | p | p | p | p | p | p | ||||||
| Limoniidae (CG) | 3 | p | |||||||||||||||
| Muscidae (PR) | 4 | p | p | p | |||||||||||||
| Scirtidae (CG) | 4 | p | p | p | p | p | p | p | p | p | p | ||||||
| Ceratopogonidae (PR) | 5 | p | p | p | p | ||||||||||||
| Glossiphoniidae (PR) | 5 | p | p | p | p | p | |||||||||||
| Dugesiidae (PA) | 6 | p | p | p | p | p | p | p | p | p | p | p | p | p | |||
| Elmidae (SC) | 6 | p | p | p | p | p | p | p | p | p | p | p | p | ||||
| Hyallelidae (SH) | 7 | p | p | p | p | p | p | p | p | p | p | p | p | p | p | ||
| Baetidae (SC) | 7 | p | p | p | p | p | p | p | p | p | p | p | p | p | p | p | |
| Simuliidae (CF) | 7 | p | p | p | p | p | p | p | p | p | p | ||||||
| Hydroptilidae (SC) | 8 | p | |||||||||||||||
| Leptoceridae (CG) | 8 | p | p | p | p | p | p | p | p | p | |||||||
| Limnephilidae (SH) | 8 | p | p | p | p | p | p | p | p | p | p | p | |||||
| Hydrobioscidae (PR) | 9 | p | p | p | p | p | p | p | |||||||||
| Gripopterygidae (SC) | 10 | p | p | p | p | p | |||||||||||
| Functional Feeding Groups | |||||||
|---|---|---|---|---|---|---|---|
| Source | df | SS | MS | F | N. Perm | p | |
| BMWP-Col index | |||||||
| BMWP-Col classes | 2 | 0.40049 | 0.200245 | 2.8834 | 9999 | 0.0148 * | |
| Residuals | 12 | 0.83337 | 0.0694475 | ||||
| Total | 14 | 1.23387 | |||||
| ABI index | |||||||
| ABI classes | 2 | 0.24140 | 0.1207 | 1.4594 | 9999 | 0.1626 | |
| Residuals | 12 | 0.99246 | 0.082705 | ||||
| Total | 14 | 1.23387 | |||||
| AAMBI index | |||||||
| AAMBI classes | 2 | 0.18213 | 0.091065 | 1.039 | 9999 | 0.4163 | |
| Residuals | 12 | 1.05174 | 0.087645 | ||||
| Total | 14 | 1.23387 | |||||
| Environmental Variables | |||||||
| BMWP-Col index | |||||||
| BMWP-Col classes | 2 | 0.0029954 | 0.0014977 | 2.5571 | 9999 | 0.0563 | |
| Residuals | 12 | 0.0070285 | 0.0005857083 | ||||
| Total | 14 | 0.0100239 | |||||
| ABI index | |||||||
| ABI classes | 2 | 0.0025451 | 0.00127255 | 2.0418 | 9999 | 0.1259 | |
| Residuals | 12 | 0.0074788 | 0.0006232333 | ||||
| Total | 14 | 0.0100239 | |||||
| AAMBI index | |||||||
| AAMBI classes | 2 | 0.0020292 | 0.0010146 | 1.5229 | 9999 | 0.2172 | |
| Residuals | 12 | 0.0079946 | 0.0006662167 | ||||
| Total | 14 | 0.0100239 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-García, S.; Goethals, P.L.M.; Lock, K.; Domínguez-Granda, L.; Villacís, M.; Galárraga-Sánchez, R.; Van der heyden, C.; Eurie Forio, M.A. Taxonomic and Feeding Trait-Based Analysis of Macroinvertebrates in the Antisana River Basin (Ecuadorian Andean Region). Biology 2023, 12, 1386. https://doi.org/10.3390/biology12111386
Cabrera-García S, Goethals PLM, Lock K, Domínguez-Granda L, Villacís M, Galárraga-Sánchez R, Van der heyden C, Eurie Forio MA. Taxonomic and Feeding Trait-Based Analysis of Macroinvertebrates in the Antisana River Basin (Ecuadorian Andean Region). Biology. 2023; 12(11):1386. https://doi.org/10.3390/biology12111386
Chicago/Turabian StyleCabrera-García, Santiago, Peter L. M. Goethals, Koen Lock, Luis Domínguez-Granda, Marcos Villacís, Remigio Galárraga-Sánchez, Christine Van der heyden, and Marie Anne Eurie Forio. 2023. "Taxonomic and Feeding Trait-Based Analysis of Macroinvertebrates in the Antisana River Basin (Ecuadorian Andean Region)" Biology 12, no. 11: 1386. https://doi.org/10.3390/biology12111386