- 1Center for Pharmacoeconomics and Outcomes Research, China Pharmaceutical University, Nanjing, China
- 2Centre for Health Management and Policy Research, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China
- 3Department of Pharmacotherapy, College of Pharmacy, University of Utah, Salt Lake City, UT, United States
- 4Department of Public Affairs Management, School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing, China
Background: This study aims to compare the potential short-term effects of non-pharmacological interventions (NPIs) on prehypertensive people, and provide evidence for intervention models with potential in future community-based management.
Methods: In this Bayesian network meta-analysis, Pubmed, Embase, and Web of science were screened up to 16 October 2021. Prehypertensive patients (systolic blood pressure, SBP 120–139 mmHg/diastolic blood pressure, DBP 80–89 mmHg) with a follow-up period longer than 4 weeks were targeted. Sixteen NPIs were identified during the scope review and categorized into five groups. Reduction in SBP and DBP was selected as outcome variables and the effect sizes were compared using consistency models among interventions and intervention groups. Grade approach was used to assess the certainty of evidence.
Results: Thirty-nine studies with 8,279 participants were included. For SBP, strengthen exercises were the most advantageous intervention group when compared with usual care (mean difference = −6.02 mmHg, 95% CI −8.16 to −3.87), and combination exercise, isometric exercise, and aerobic exercise were the three most effective specific interventions. For DBP, relaxation was the most advantageous intervention group when compared with usual care (mean difference = −4.99 mmHg, 95% CI −7.03 to −2.96), and acupuncture, meditation, and combination exercise were the three most effective specific interventions. No inconsistency was found between indirect and direct evidence. However, heterogeneity was detected in some studies.
Conclusion: NPIs can bring short-term BP reduction benefits for prehypertensive patients, especially exercise and relaxation. NPIs could potentially be included in community-based disease management for prehypertensive population once long-term real-world effectiveness and cost-effectiveness are proven.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=151518, identifier: CRD42020151518.
Introduction
Hypertension is one of the leading risk factors for morbidity and mortality around the world, which affect approximately one billion people (1–3). Elevated blood pressure, called prehypertension, defined as a blood pressure of 120–139/80–89 mmHg, is common and affects 25–50% of adults all over the world (4–6). Prehypertension confers a high risk of progression to hypertension and increases the risk of cardiovascular diseases with a 5-year progression rate of 40% (4, 7, 8). High prevalence and risk of prehypertension call for effective and cost-effective interventions. Current anti-hypertensive treatments include drug interventions and non-pharmacological interventions (NPIs) (9, 10), but previous research points out that there is a lack of evidence that early medication can bring benefits to prehypertensive people with no cardiovascular risk (5, 6), less to mention the unnecessary harm and economic loss brought by the resistance and side effects of anti-hypertensive drugs (11, 12). On the contrary, current available NPIs including exercises (e.g., aerobic exercise, resistance exercise), dietary intervention (e.g., salt reduction, alcohol reduction), relaxation (e.g., acupuncture, yoga), and so on Verma et al. (13), may be equally effective. Since NPIs focus more on changes in patient's behavior and lifestyle, they have no side effects and may be with potential cost-effectiveness (14). NPIs have been recommended by recent guidelines, which can be considered a priority in treating and managing prehypertensive people (6). Quite a few studies have proved that intensive lifestyle intervention as well as other NPIs can reduce the blood pressure (BP) of hypertensive and prehypertensive people in a short term (4, 15–18). However, the relative effects of NPIs are still unknown.
Although prehypertension has a large prevalence worldwide (6, 19), but patients are not managed in most countries currently. Simply using drug interventions may not be suitable to manage such a huge population since the economic burden can be large. NPIs are widely available and with low costs, can be good choices to be applied in management (20). However, implementing NPIs in current community-based chronic disease management still faced some barriers (21). NPIs require community-based chronic disease-management staff to have a medical background and additional professional training, and delivering NPIs is highly dependent on provider services (14, 22–24). However, these staffs are scarce, especially in many low- and middle-income countries (25). It is therefore necessary to explore the most effective and efficient type of service model which had the potential to be implemented. However, the evidence in this field is extremely lacking.
In this study, we aim to conduct a network meta-analysis to rank the short-term effects of NPIs among current available studies. The results will provide evidence for non-pharmacological treatment for prehypertensive patients from a global perspective, and hopefully lay a basis for the future inclusion of prehypertensive patients in community-based chronic disease management.
Methods
We used a network meta-analysis to evaluate the short-term efficacy of NPIs on prehypertension. This research was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (26). This research was registered with PROSPERO (registration number: CRD42020151518) and the protocol has been published (27).
Patient and public involvement
Patients were not involved in this study.
Literature search
We conducted a systematic search in PubMed, Web of Science, Embase, and the Cochrane Library up to 16 October 2021. We included randomized controlled trials and reasonably designed non-randomized controlled trials but excluded observational studies such as cross-sectional or cohort studies, systematic reviews and meta-analyses, and economic evaluations. We carried out a scope review and systematic review: the scope review was used to determine the NPIs included in the study, and the systematic review was then conducted to determine the studies included in the network meta-analysis. International guidelines were used to double-check the eligibility of interventions (6, 13, 28). The reference lists of relevant meta-analyses were scanned to identify other articles of interest. There was no limitation on the publication date of studies. The language of included studies was limited to English. Our search strategies and process are given in Supplementary material 1.
Inclusion and exclusion criteria
All retrieved articles were imported into Noteexpress (3.2.0.7535, China Pharmaceutical University). Two independent researchers (THS and LYL) screened the literature for inclusion. Disagreements were discussed and consensus was reached in all cases. Literature was included in the systematic review if it met the following criteria.
Population
Study targets of adults aged >18 years whose BP status met the following two criteria were included: (1) diagnosed with prehypertension; (2) baseline BP between 120–139/80–89 mmHg. Subjects were excluded when they: (1) received antihypertensive agents; (2) had cardiovascular diseases (e.g., stroke, myocardial infarction); (3) had pregnancy-induced hypertension or pulmonary hypertension.
Intervention and control
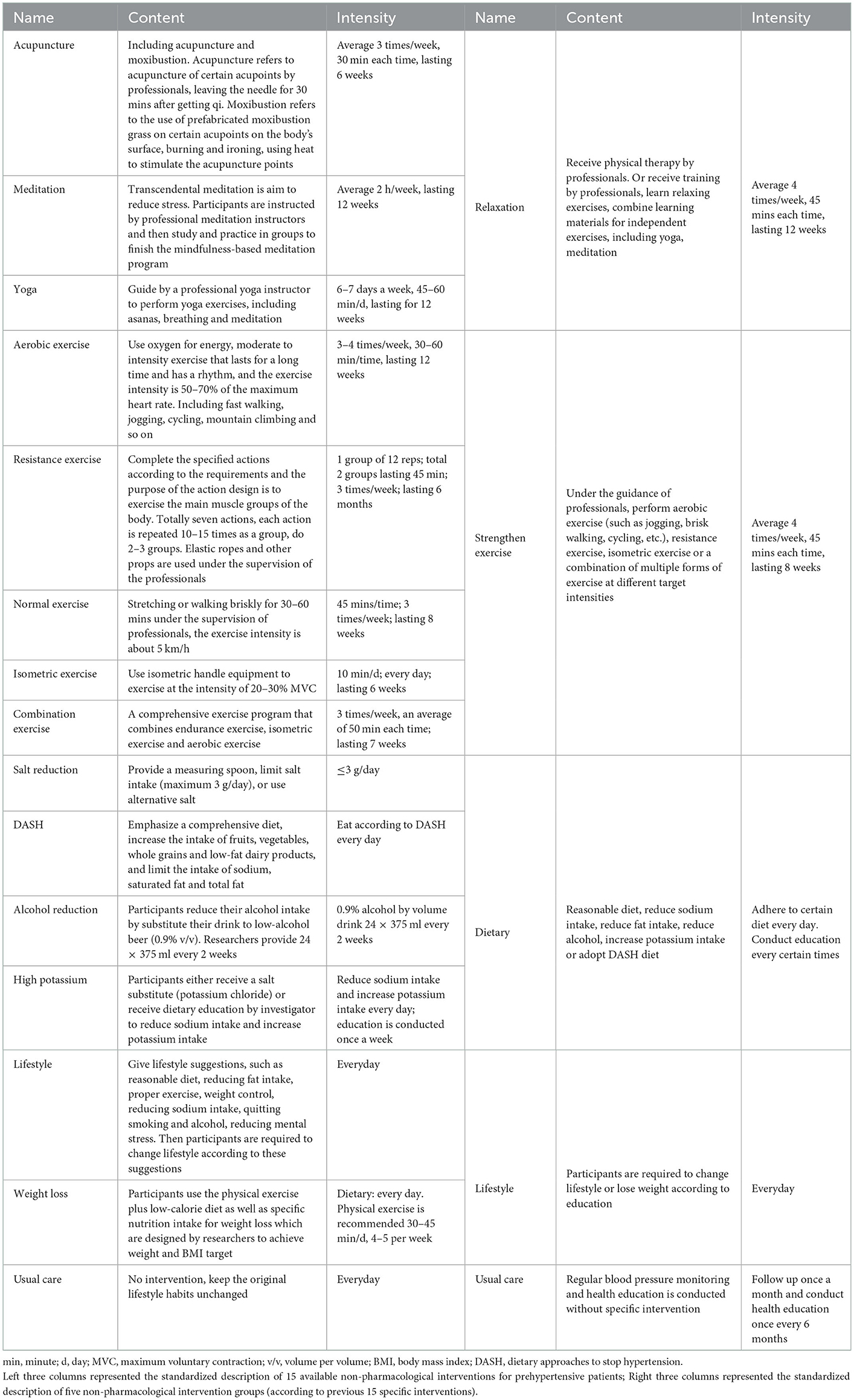
Studies with at least one study arm using the following 15 NPIs were included: acupuncture, aerobic exercise, combination exercise, Dietary Approaches to Stop Hypertension (DASH), high Potassium, isometric exercise, lifestyle, meditation, normal exercise, alcohol reduction, resistance exercise, salt restriction, weight loss, yoga, and usual care. We merged these 15 interventions into five groups: relaxation, dietary intervention, strengthen exercise, lifestyle modification, and usual care, based on a comprehensive consideration of the type of interventions (exercise or dietary) and the intensity of intervention (strengthen or relax). The standardized descriptions of the interventions are shown in Table 1.
Outcome indicators
The main outcome indicators were changes in SBP and DBP whose follow-up time was no more than a year. We used mean differences instead of median differences as the effect size. The follow-up period of included studies did not exceed 1 year, the risk of cardiovascular events was therefore not reported. In addition, adverse events were not reported in most studies.
Data extraction
Study characteristics extracted by four researchers (THS, LYL, YQT, and WQG) were as follows: title, first author, publication date, randomization, baseline characteristics (age, sex, country, number of participants, and lost to follow-up), details of interventions, follow-up time, baseline value as well as the changes of SBP and DBP after intervention.
Risk of bias and evidence quality assessment
Two investigators (YST and YY) used the Cochrane Risk of Bias Tool 1.0 to evaluate the following items: random sequence generation, allocation concealment, blinding of outcome assessors, completeness of outcome data, selective outcome reporting, and other potential biases (29, 30). All six aspects would be evaluated as (1) low risk of bias, (2) unknown risk of bias, and (3) high risk of bias; a high-quality study should include more than four aspects with low risk of bias. However, blinding and allocation concealment would be difficult to achieve in NPIs. Therefore, we would make particular note of articles that did not involve blinding and allocation concealment but had valuable data (27).
We assessed the certainty of evidence using the grading of recommendations assessment, development and evaluation (GRADE) approach for network meta-analysis (31–35). Two people (THS and LYL) with experience in using GRADE rated each domain for each comparison separately and resolved discrepancies by consensus. We rated the certainty for each comparison and outcome as high, moderate, low, or very low, based on considerations of risk of bias, inconsistency, indirectness, publication bias, intransitivity, incoherence (difference between direct and indirect effects), and imprecision. Judgments of imprecision were made using a minimally contextualized approach, with a null effect as the threshold of importance (36). A recommended four-step approach was used in this study (35). In the first step, the effect sizes and confidence intervals of the direct evidence, indirect evidence, and network meta-analysis evidence were presented separately. In the second step, the quality of the direct evidence for each comparison group was graded without considering the imprecision. If the direct evidence was graded “high” and the contribution to the network meta-analysis results was greater than or equal to indirect evidence, no indirect evidence quality grading was required. The network meta-analysis evidence quality was directly assessed based on the direct evidence quality. Otherwise, indirect evidence quality grading was required. In the third step, based on the quality of direct evidence in the first-order loop of indirect evidence, the quality of indirect evidence was determined. The intransitivity should also be considered. In the fourth step, based on the level of direct evidence and/or indirect evidence, and considering inconsistency and imprecision, the quality of evidence for network meta-analysis was finalized and presented.
Statistical analysis
We carried out a network meta-analysis using the Bayesian framework with the same priors for the variance and effect parameters. A plausible prior for the variance parameter and a uniform prior for the effect parameter suggested in a previous study based on empirical data were used in this network meta-analysis (37). We calculated the mean difference (MD) as the effect size using the reported means and standard deviations (SD) of changes in SBP and DBP. If the original study reported the standard error (SE), we would convert it to the SD through the sample size (n):
If the changes were not reported in the article but the BP at the start and end of the follow-up period were reported, we calculated the mean and SD using the following formula recommended in the Cochrane Handbook (29):
The two most widely used models in network meta-analysis were the fixed effect model and the random effects model (38). The fixed effect model was built under the assumption of existing no heterogeneity. But this assumption was recognized to be unrealistic. If the fixed effect model was applied when heterogeneity existed, uncertainty intervals become artificially narrow. Therefore, the random effects model was preferred since it assumed and accounted for unexplained heterogeneity. In this network meta-analysis, we used a random effects model as the most appropriate and conservative method to explain the heterogeneity among the included studies (38, 39). We used a Markov chain Monte Carlo simulation with four chains with scattered initial values, a total of 50,000 iterations, and annealed after 5,000 iterations. The convergence of the model was judged by the Brooks–Gelman–Rubin method (40).
A ranking probability curve of each treatment was provided by calculating the probability of each arm to achieve the best rank among all. We judged the inconsistency by comparing the deviance information criterion (DIC) between the consistency and inconsistency models (41). Evaluating local incoherence between the direct and indirect comparisons, and obtaining indirect estimation was done by the node-splitting models (42). We calculated the Bayesian P value to estimate the measure of the conflict between direct and indirect evidence (43). The heterogeneity between studies was determined by heterogeneity analysis (including unrelated study effects model, unrelated mean effects model, and consistency model), and Q test and I2 statistic were used to reflect the heterogeneity (I2higher than 75% was considered with high heterogeneity; smaller than 25% was considered with low heterogeneity) (44).
All statistical tests were conducted as two-sided, and a P < 0.05 was considered as being statistically significant. The network meta-analysis was performed using R software (https://www.r-project.org).
Results
Study selection
Our search identified a total of 4,951 references. After duplication, 3,412 studies underwent further analysis, of which 2,756 were excluded after reading the title or abstract and a further 617 were excluded after reading the full text. The remaining 39 studies involved 15 interventions and 8,279 patients were included in the analysis (45–83). The study flow chart is shown in Figure 1. The baseline patient characteristics are shown in the Supplementary material 2.
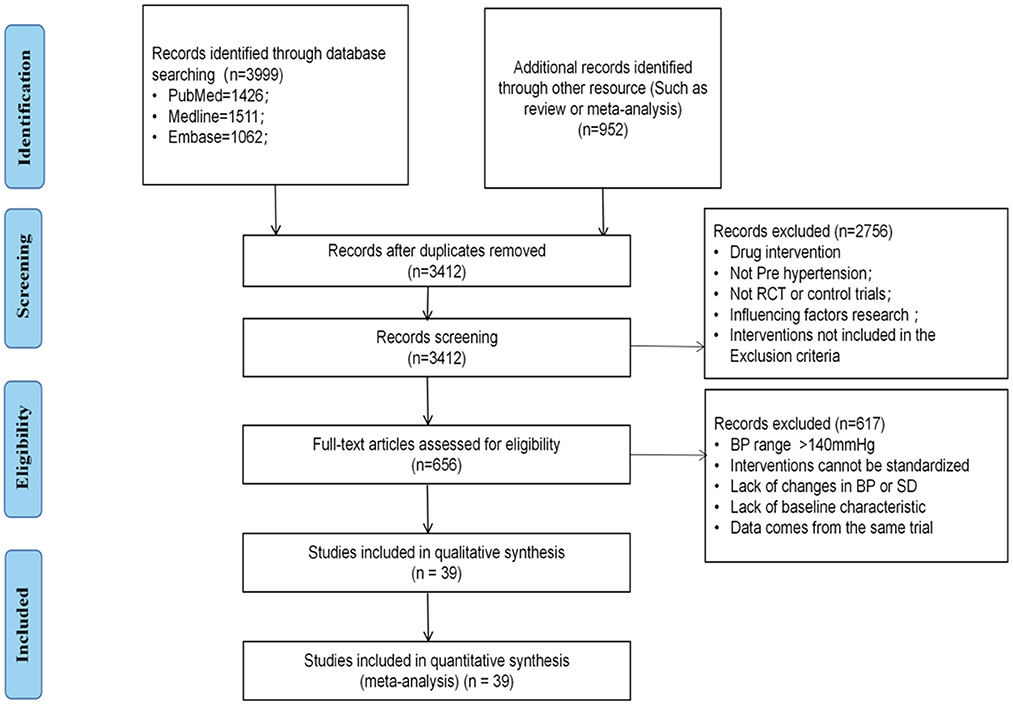
Figure 1. Flow chart of literature search and article inclusion. RCT, randomized controlled trials; BP, blood pressure; SD, standard difference.
Network meta-analysis
The network evidence plots for SBP and DBP were the same (as shown in Figure 2).
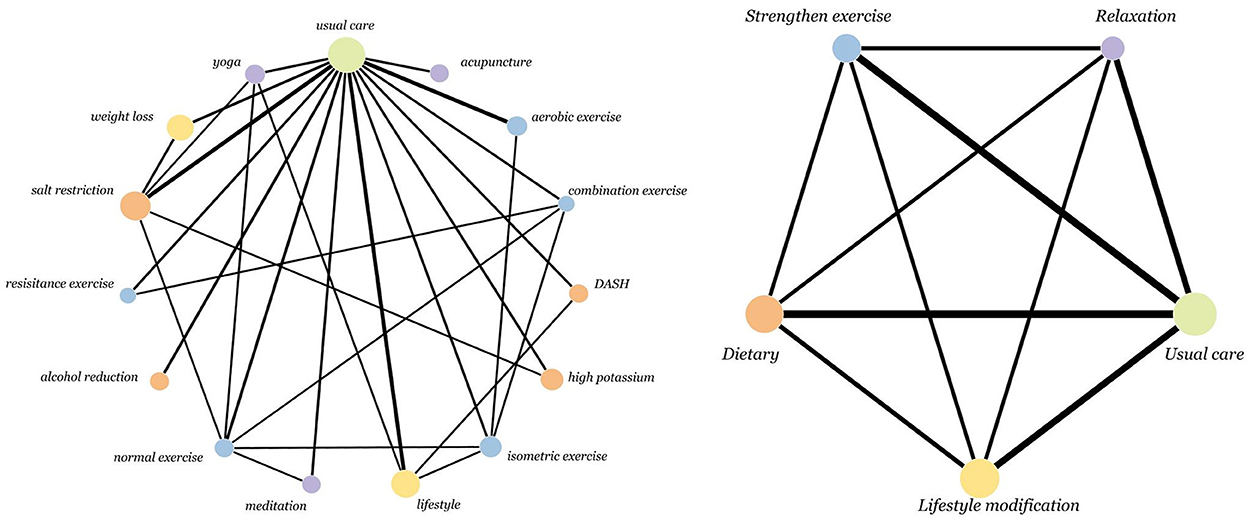
Figure 2. Network of intervention treatments included in meta-analysis. The size of the nodes represents the sample size. The thickness of the lines represents the number of studies included in the comparison.
Among the included intervention strategies, combination exercise (69.71%) ranked first in the reduction of SBP, followed by isometric exercise (33.30%), aerobic exercise (18.75%), yoga (11.90%) and normal exercise (12.08%). And acupuncture (46.09%) ranked first in the reduction of DBP, followed by meditation (27.67%), combination exercise (16.39%), isometric exercise (13.42%), yoga (11.86%) (as shown in Figure 3).
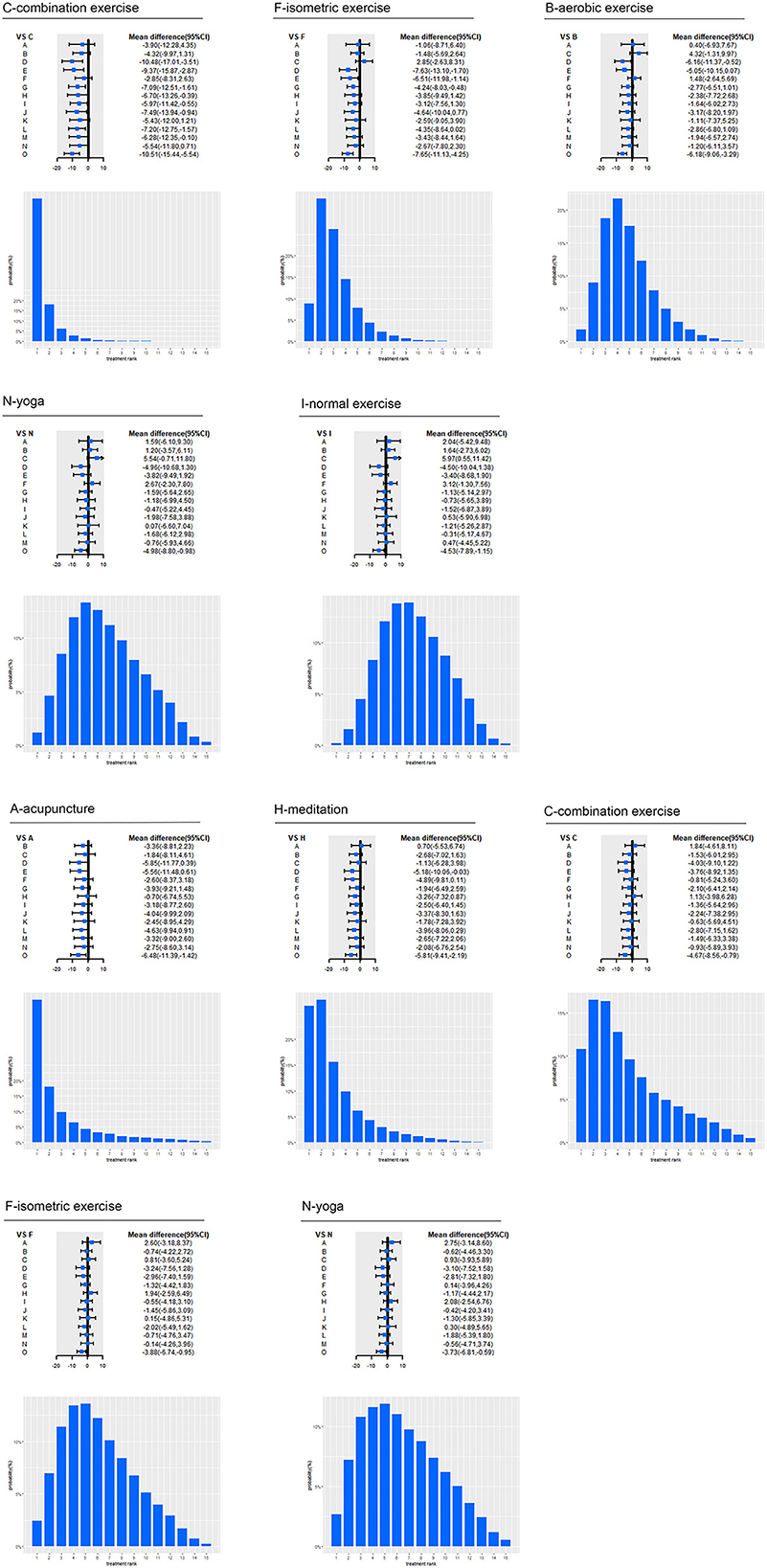
Figure 3. Effect of top five interventions on SBP and DBP. A, acupuncture; B, aerobic exercise; C, combination exercise; D, DASH; E, high Potassium; F, isometric exercise; G, lifestyle; H, meditation; I, normal exercise; J, reduced alcohol; K, resistance exercise; L, salt restriction; M, weight loss; N, yoga; O, usual care; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure. Forest plot represents the relative effects of the other interventions with one intervention as a reference (mean difference, 95% CI, mmHg); Bar plot represents the probability of ranking of the reference intervention.
Specifically, for SBP, aerobic exercise (−6.18 mmHg, 95% CI −9.06 to −3.29; moderate certainty), combination exercise (−10.51 mmHg, −15.44 to −5.54; moderate certainty), isometric exercise (−7.65 mmHg, −11.13 to −4.25; moderate certainty), lifestyle (−3.4 mmHg, −5.89 to −0.94; moderate certainty), normal exercise (−4.53 mmHg, −7.89 to −1.15; low certainty), salt restriction (−3.3 mmHg, −6.02 to −0.7; moderate certainty), weight loss (−4.23 mmHg, −7.97 to −0.58; moderate certainty) and yoga (−4.98 mmHg, −8.8 to −0.98; low certainty) significantly lowered SBP compared with usual care. Aerobic exercise (−6.16 mmHg, −11.37 to −0.52; moderate certainty) had a significant SBP reduction compared with DASH. Combination exercise had a significant SBP reduction compared with DASH (−10.48 mmHg, −17.01 to −3.51; moderate certainty), high potassium (−9.37 mmHg, −15.87 to −2.87; moderate certainty), lifestyle (−7.09 mmHg, −12.51 to −1.61; moderate certainty), meditation (−6.7 mmHg, −13.26 to −0.39; moderate certainty), normal exercise (−5.97 mmHg, −11.42 to −0.55; low certainty), alcohol reduction (−7.49 mmHg, −13.94 to −0.94; moderate certainty), salt restriction (−7.2 mmHg, −12.75 to −1.57; moderate certainty) and weight loss (−6.28 mmHg, −12.35 to −0.1; moderate certainty). Isometric exercise significantly lowered SBP compared with lifestyle (−4.24 mmHg, −8.03 to −0.48; very low certainty), DASH (−7.63 mmHg, −13.1 to −1.7; low certainty), and high Potassium (−6.51 mmHg, −11.98 to −1.14; low certainty). For DBP, acupuncture (−6.48 mmHg, −11.39 to −1.42; moderate certainty), aerobic exercise (−3.12 mmHg, −5.51 to −0.73; moderate certainty), combination exercise (−4.67 mmHg, −8.56 to −0.79; moderate certainty), isometric exercise (−3.88 mmHg, −6.74 to −0.95; low certainty), lifestyle (−2.56 mmHg, −4.56 to −0.58; moderate certainty), meditation (−5.81 mmHg, −9.41 to −2.19; moderate certainty), normal exercise (−3.32 mmHg, −6.05 to −0.59; low certainty), weight loss (−3.16 mmHg, −6.14 to −0.29; moderate certainty) and yoga (−3.73 mmHg, −6.81 to −0.59; moderate certainty) significantly lowered BP compared with usual care. Meditation (−5.18 mmHg, −10.06 to −0.03; moderate certainty) had a significant BP reduction than DASH.
Among the categorized intervention groups, Strengthen exercise (72.54%) ranked first in the reduction of SBP, followed by relaxation (54.56%), lifestyle modification (59.6%), dietary (71.62%), and usual care (99.08%). And relaxation (87.66%) ranked first in the reduction of DBP, followed by Strengthen Exercise (61.93%), lifestyle modification (60.55%), dietary (82.34%), and usual care (98.73%).
Specifically, for SBP (as shown in Figure 4), relaxation (−4.97 mmHg, −7.66 to −2.15; low certainty), lifestyle modification (−3.5 mmHg, −5.64 to −1.41; moderate certainty), dietary (−2.54 mmHg, −4.73 to −0.49; low certainty) and strengthen exercise (−6.02 mmHg, −8.16 to −3.87; low certainty) significantly reduced BP compared with usual care. Strengthen exercise (−3.47 mmHg, −6.36 to −0.53; moderate certainty) significantly reduced BP compared with dietary. For DBP, relaxation (−4.99 mmHg, −7.03 to −2.96; moderate certainty), lifestyle modification (−2.86 mmHg, −4.43 to −1.34; moderate certainty), dietary (−1.73 mmHg, −3.34 to −0.23; very low certainty) and strengthen exercise (−3.48 mmHg, −5.09 to −1.82; low certainty) significantly reduced BP compared with usual care. Relaxation (−3.25 mmHg, −5.68 to −0.73; moderate certainty) significantly reduced BP compared with dietary.
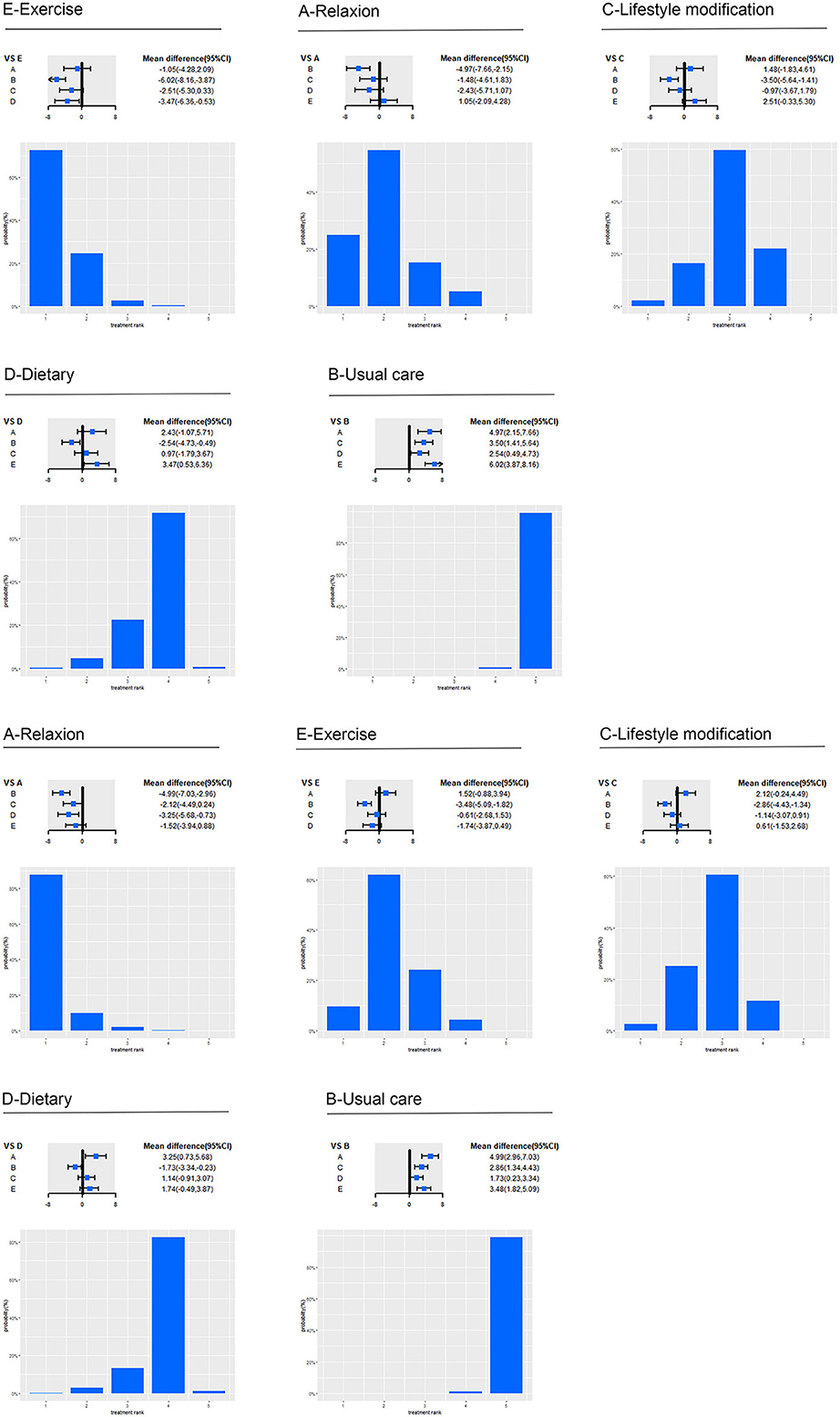
Figure 4. Effect of five intervention groups on SBP and DBP. A, relaxation; B, usual care; C, lifestyle; D, Dietary; E, strengthen exercise; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure. Forest plot represents the relative effects of the other intervention groups with one intervention group as a reference (mean difference, 95% CI, mmHg). Bar plot represents the probability of ranking of the reference intervention group.
Inconsistency and heterogeneity
Results of node-splitting and heterogeneity tests are shown in Supplementary material 3 for detail.
When compared among specific intervention programs, no inconsistency was found in SBP nor DBP, as indicated by the model parameters (consistency test for SBP and DBP were DIC = 162.70) and DIC = 164.43, respectively; inconsistency test for SBP and DBP were DIC = 166.97 and DIC = 167.49, respectively. The results of node-splitting analysis showed that there was no local incoherence in both of the models since every Bayesian P > 0.05.
When compared among intervention groups, no inconsistency was found in SBP nor DBP, as indicated by the model parameters (consistency test for SBP and DBP were DIC = 138.37 and DIC = 139.59, respectively; inconsistency test for SBP and DBP were DIC = 140.64 and DIC = 141.31, respectively). The results of node-splitting analysis showed that there was no local incoherence in both of the models since every Bayesian P > 0.05.
The heterogeneity test showed that there was high heterogeneity between some studies for both pair-wise pooled effects and consistency effects. Potential explanations are given in a later discussion.
Risk of bias
The specific literature quality assessment diagram and risk of bias summary are shown in Supplementary material 4. After assessing the quality of the literature by the Cochrane Handbook, we found that 27 studies reported the implementation of randomization, 8 studies reported the allocation concealment, and 15 studies reported on the implementation of blinding. There was no selective reporting bias or result bias in all studies. Because these studies were aimed at NPIs, some study designs could not apply the blinding. In summary, the quality of the articles included in this network meta-analysis was moderate. Detailed results of the certainty evaluation of evidence are shown in Supplementary material 5. Overall, most evidence was concentrated in the moderate and low grades since the existence of the risk of bias, inconsistency, indirectness, intransitivity, and imprecision.
Discussion
This study evaluated the short-term effects of 16 NPIs in patients with prehypertension. Considering the impact on community-based chronic disease-management staff and their need for professional cooperation (25), we merged 16 intervention items into five intervention groups. We evaluated the effects using a network meta-analysis with BP reduction as the outcome indicator and found that combination exercise, isometric exercise, aerobic exercise, yoga, and normal exercise were the top five in SBP reduction, and acupuncture, meditation, combination exercise, isometric exercise and yoga for DBP reduction. Also, according to our studies, sports intervention had more absolute SBP reduction and relaxation had more absolute DBP reduction than other interventions. Strengthen exercise and relaxation rank top two in both SBP and DBP reduction.
There were no inconsistencies but slight heterogeneity in this study. This was likely due to differences in the baseline characteristics (e.g., age, sex, and ethnicity) of the patients included in each study, as well as differences in the interventions adopted by each of the studies. Although the interventions share the same purpose, their contents were slightly different, given that current guidelines did not give standardized strategies for non-pharmacological intervention. Patient compliance and completion rates may also differ among studies. However, this type of heterogeneity was unavoidable.
Numerous studies have examined the short-term anti-hypertensive effects of NPIs. Williamson et al. (18) found that 3–6 months of exercise effectively reduced SBP (−4.40 mmHg, 95% CI −5.78 to −3.01) and DBP (−4.17 mmHg, 95% CI −5.42 to −2.93) in patients aged 18–40 years with both prehypertension and hypertension. Ndanuko et al. (84) conducted a meta-analysis on the BP-reducing effects of MBSR (Mindfulness-Based Stress Reduction) program in both prehypertensive and hypertensive patients and showed that MBSR reduced SBP by 6.64 mmHg and DBP by 2.47 mmHg. Khandekar et al. (85) conducted a meta-analysis of the anti-hypertensive effects of yoga and showed that both SBP (standard MD = −0.62, 95% CI −0.83 to −0.41) and DBP (standard MD = −0.81, 95% CI −1.39 to −0.22) were significantly reduced in the yoga group compared with the control group. According to Liao et al.'s study (86), massage significantly reduced SBP (−7.39 mmHg) and DBP (−5.04 mmHg) in people with both prehypertension and hypertension. Fu et al. (20) reported that the DASH diet was most effective for adults with prehypertension to established hypertension, followed by aerobic exercise and isometric training, which also had obvious effects on BP reduction. Population in current evidence are hypertensive patients or combined with prehypertensive people. Population in current evidence are hypertensive patients or combined with prehypertensive people. Pooled evidence studies target on the BP reduction effect of NPIs in prehypertensive people were lacking. In addition, current studies of the anti-hypertensive effects of NPIs have tended to target one specific intervention. So the current results filled a gap in proving and comparing the short-term effects of NPIs in people with prehypertension.
This was the first study to evaluate the short-term efficacy of NPIs in prehypertensive people. Our results not only supplemented existing evidence in this area but also had important implications for the management of chronic diseases in countries who had a high disease burden of hypertension but with limited medical resources and community-based chronic disease-management staff. Early prevention of hypertension through NPIs can be a potential way to reduce the disease burden. This meant that government administrators in these countries can start to initiate training programs that could reduce the BP of people with prehypertension effectively for community-based chronic disease-management staff to be prepared. For decision-makers, a comprehensive analysis of which types of interventions could be more effective will provide useful evidence to make the optimal health decision. In this study, strengthen exercise and relaxation, which could bring more short-term BP reduction than other interventions according to current evidence, may be considered the priority for government administrators and community-based chronic disease-management staff. Nevertheless, long-term effects of these NPIs with great short-term BP reduction benefits should be further examined (including the number of CVD events avoided), which can provide more evidence for decision-makers.
It is necessary to note that this study is not without shortcomings. First, the standardization of interventions in this study was carried out following the guidelines, still it may leave to subjectivity. Second, due to differences in population baseline of included studies, the heterogeneity could not be avoided. Therefore, in our certainty of the evidence analysis, most comparisons were downgraded in the indirectness of evidence due to the differences in population and intervention. Third, since individual patient data were not available, subgroup analyses were not conducted in this study. However, the heterogeneity caused by some key subgroups (e.g., baseline blood pressure, age) should not be ignored. Fourth, since long-term studies were lacking, only BP change could be selected as the outcome indicator in this study. Without outcome indicators like cardiovascular events or hypertension progression, long-term real-world effectiveness of NPIs could not be recognized. Even though we had proved that NPIs are effective for prehypertensive people, whether they are cost-effective was still unknown. For further research, more high-quality research with long-term outcome projections should be published to fill the gap in this field. Studies are needed to target people with different clinical characteristics. Empirical studies on the inputs and outputs of NPIs in prehypertensive people are also needed to explore the cost-effectiveness and feasibility of implementation in a specific region.
Conclusion
To date, there is no systematic study revealing the comparative effects of NPIs for prehypertensive patients. Our study indicates that strengthening exercise (including combination exercise, isometric exercise and aerobic exercise) and relaxation (including acupuncture, meditation, and yoga) have potential to be educated and applied in community-based chronic disease management. This will provide evidence for countries who have a high disease burden of hypertension but with limited medical resources and staff to prevent or delay the disease progression from prehypertension to hypertension. However, to have a decision on whether prehypertensive patients should be regularly managed and which strategy to be considered, further studies on cost-effectiveness and affordability are needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
WT and TS designed the study and interpreted findings. TS and LL wrote initial drafts of the manuscript, developed the model, performed all model analyses, and visualized the data. TS, LL, YTa, WG, YTu, and YY conducted the literature search, screen, and extract the data. TS, LL, CZ, WT, and DM revised and polished the initial manuscript drafts. All authors reviewed the manuscript. All authors had full access to all the data in the study and the corresponding authors had final responsibility for the decision to submit for publication.
Funding
This work was supported by General Program of National Natural Science Foundation of China (72174207).
Acknowledgments
We thank Susan Furness, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for basic language editing of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1051581/full#supplementary-material
Supplementary material 1. Search strategies of this study. Including reverse search and forward search.
Supplementary material 2. Main characteristics of included trials.
Supplementary material 3. Results of node-splitting and heterogeneity test.
Supplementary material 4. “Quality assessment diagram” and “Risk of bias summary.”
Supplementary material 5. Detailed results of the certainty of the evidence analysis.
References
1. Gakidou E, Afshin A, Abajobir AA, Abate KH, Abbafati C, Abbas KM, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1345–422. doi: 10.1016/S0140-6736(17)32366-8
2. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA J Am Med Assoc. (2017) 317:165–82. doi: 10.1001/jama.2016.19043
3. Rahimi K, Emdin CA, MacMahon S. The epidemiology of blood pressure and its worldwide management. Circ Res. (2015) 116:925–36. doi: 10.1161/CIRCRESAHA.116.304723
4. Egan BM, Stevens-Fabry S. Prehypertension–prevalence, health risks, and management strategies. Nat Rev Cardiol. (2015) 12:289–300. doi: 10.1038/nrcardio.2015.17
5. Izzo R, Mancusi C, de Simone G. Are we underestimating prehypertension? Hypertension. (2019) 73:541–2. doi: 10.1161/HYPERTENSIONAHA.118.12310
7. Kanegae H, Oikawa T, Kario K. Should pre-hypertension be treated? Curr Hypertens Rep. (2017) 19:91. doi: 10.1007/s11906-017-0789-z
8. Materson BJ, Garcia-Estrada M, Degraff SB, Preston RA. Prehypertension is real and can be associated with target organ damage. J Am Soc Hypertens. (2017) 11:704–8. doi: 10.1016/j.jash.2017.09.005
9. National Cardiovascular Disease Center Office. National basic hypertension prevention and management guidelines 2020 edition. China Recycling Magazine. (2021) 36:209–20. doi: 10.12037/YXQY.2021.04-06
10. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. (2018) 71:65. doi: 10.1161/HYP.0000000000000065
11. Hong D, Shan W. Improvement in hypertension management with pharmacological and non-pharmacological approaches: current perspectives. Curr Pharm Design. (2021) 27:548–55. doi: 10.2174/1381612826666200922153045
12. Intarakamhang U, Macaskill A, Prasittichok P. Mindfulness interventions reduce blood pressure in patients with non-communicable diseases: a systematic review and meta-analysis. Heliyon. (2020) 6:e3834. doi: 10.1016/j.heliyon.2020.e03834
13. Verma N, Rastogi S, Chia YC, Siddique S, Turana Y, Cheng HM, et al. Non-pharmacological management of hypertension. J Clin Hypertens. (2021) 23:1275–83. doi: 10.1111/jch.14236
14. Zhou YF, Liu N, Wang P, Jeong YJ, Song XY, Pan XF, et al. Cost-effectiveness of drug treatment for chinese patients with stage I hypertension according to the 2017 hypertension clinical practice guidelines. Hypertension. (2020) 76:750–8. doi: 10.1161/HYPERTENSIONAHA.119.14533
15. Herrod P, Doleman B, Blackwell J, O'Boyle F, Williams JP, Lund JN, et al. Exercise and other nonpharmacological strategies to reduce blood pressure in older adults: a systematic review and meta-analysis. J Am Soc Hypertens. (2018) 12:248–67. doi: 10.1016/j.jash.2018.01.008
16. Park S, Han KS. Blood pressure response to meditation and yoga: a systematic review and meta-analysis. J Altern Complement Med. (2017) 23:685–95. doi: 10.1089/acm.2016.0234
17. Patnode CD, Evans CV, Senger CA, Redmond N, Lin JS. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults without known cardiovascular disease risk factors. JAMA J Am Med Assoc. (2017) 318:175. doi: 10.1001/jama.2017.3303
18. Williamson W, Foster C, Reid H, Kelly P, Lewandowski AJ, Boardman H, et al. Will exercise advice be sufficient for treatment of young adults with prehypertension and hypertension? A systematic review and meta-analysis. Hypertension. (2016) 68:78–87. doi: 10.1161/HYPERTENSIONAHA.116.07431
19. Li W, Chen D, Liu S, Wang X, Chen X, Chen J, et al. The rates and the determinants of hypertension according to the 2017 definition of hypertension by ACC/AHA and 2014 evidence-based guidelines among population aged ≥40 years old. Glob Heart. (2021) 16:34. doi: 10.5334/gh.914
20. Fu J, Liu Y, Zhang L, Zhou L, Li D, Quan H, et al. Nonpharmacologic interventions for reducing blood pressure in adults with prehypertension to established hypertension. J Am Heart Assoc. (2020) 9:e16804. doi: 10.1161/JAHA.120.016804
21. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16:223–37. doi: 10.1038/s41581-019-0244-2
22. Dhungana RR, Pedisic Z, de Courten M. Implementation of non-pharmacological interventions for the treatment of hypertension in primary care: a narrative review of effectiveness, cost-effectiveness, barriers, and facilitators. BMC Prim Care. (2022) 23:298. doi: 10.1186/s12875-022-01884-8
23. Anderson P, Bendtsen P, Spak F, Reynolds J, Drummond C, Segura L, et al. Improving the delivery of brief interventions for heavy drinking in primary health care: outcome results of the Optimizing Delivery of Health Care Intervention (ODHIN) five-country cluster randomized factorial trial. Addiction. (2016) 111:1935–45. doi: 10.1111/add.13476
24. Morgan F, Battersby A, Weightman AL, Searchfield L, Turley R, Morgan H, et al. Adherence to exercise referral schemes by participants—what do providers and commissioners need to know? A systematic review of barriers and facilitators. BMC Public Health. (2016) 16:227. doi: 10.1186/s12889-016-2882-7
25. Anand TN, Joseph LM, Geetha AV, Prabhakaran D, Jeemon P. Task sharing with non-physician health-care workers for management of blood pressure in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Glob Health. (2019) 7:e761–71. doi: 10.1016/S2214-109X(19)30077-4
26. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev Lond. (2015) 4:1. doi: 10.1186/2046-4053-4-1
27. Shao T, Li X, Zhou C, Zang X, Malone DC, Zhang L, et al. Effectiveness and efficiency of non-drug therapy among community-dwelling adults with hypertension in China: a protocol for network meta-analysis and cost-effectiveness analysis. Front Med-Lausanne. (2021) 8:651559. doi: 10.3389/fmed.2021.651559
28. Mahmood S, Shah KU, Khan TM, Nawaz S, Rashid H, Baqar S, et al. Non-pharmacological management of hypertension: in the light of current research. Irish J Med Sci. (2019) 188:437–52. doi: 10.1007/s11845-018-1889-8
29. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: Wiley-Blackwell (2008).
30. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ Brit Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
31. Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. (2018) 93:36–44. doi: 10.1016/j.jclinepi.2017.10.005
32. Bonner A, Alexander PE, Brignardello-Petersen R, Furukawa TA, Siemieniuk RA, Zhang Y, et al. Applying GRADE to a network meta-analysis of antidepressants led to more conservative conclusions. J Clin Epidemiol. (2018) 102:87–98. doi: 10.1016/j.jclinepi.2018.05.009
33. Brignardello-Petersen R, Mustafa RA, Siemieniuk R, Murad MH, Agoritsas T, Izcovich A, et al. GRADE approach to rate the certainty from a network meta-analysis: addressing incoherence. J Clin Epidemiol. (2019) 108:77–85. doi: 10.1016/j.jclinepi.2018.11.025
34. Brignardello-Petersen R, Murad MH, Walter SD, McLeod S, Carrasco-Labra A, Rochwerg B, et al. GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol. (2019) 105:60–7. doi: 10.1016/j.jclinepi.2018.08.022
35. Puhan MA, Schunemann HJ, Murad MH Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ Brit Med J. (2014) 349:g5630. doi: 10.1136/bmj.g5630
36. Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. (2017) 87:4–13. doi: 10.1016/j.jclinepi.2017.05.006
37. Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JP. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat Med. (2015) 34:984–98. doi: 10.1002/sim.6381
38. Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ Brit Med J. (2013) 346:f2914. doi: 10.1136/bmj.f2914
39. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. (2013) 33:641–56. doi: 10.1177/0272989X12455847
40. Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. (1998) 7:434–55. doi: 10.2307/1390675
41. Mason A, Richardson S, Best N. Using DIC to Compare Selection Models With Non-Ignorable Missing Responses. Southampton: National Centre for Research Methods (2010).
42. van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. (2016) 7:80–93. doi: 10.1002/jrsm.1167
43. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29:932–44. doi: 10.1002/sim.3767
44. Van Valkenhoef G, Kuiper J., gemtc. (2021). Available online at: https://cran.r-project.org/web/packages/gemtc/gemtc.pdf (accessed December 3, 2022).
45. Seo B, Kwon O, Lee S, Kim H, Kang K, Seol IC, et al. Effects of acupuncture on lowering blood pressure in postmenopausal women with prehypertension or stage 1 hypertension: A propensity score-matched analysis. J Clin Med. (2021) 10:1426. doi: 10.3390/jcm10071426
46. Son W, Pekas EJ, Park S. Twelve weeks of resistance band exercise training improves age-associated hormonal decline, blood pressure, and body composition in postmenopausal women with stage 1 hypertension: a randomized clinical trial. Menopause. (2020) 27:199–207. doi: 10.1097/GME.0000000000001444
47. Tantiprasoplap S, Piaseu N, Kanungsukkasem V, Taneepanichskul S. A randomized controlled trial comparing the effects of an arm swing exercise and low sodium intake education program with low sodium intake education alone on cardiovascular outcomes in postmenopausal women with prehypertension. J Med Assoc Thailand. (2020) 103:22–31.
48. Ankolekar VH, Reddy GG, Sanju CSV, Mamatha H. Role of yoga intervention on quality of life and prehypertension. Indian J Tradit Know. (2019) 18:351–5.
49. Ogbutor GU, Nwangwa EK, Uyagu DD. Isometric handgrip exercise training attenuates blood pressure in prehypertensive subjects at 30% maximum voluntary contraction. Niger J Clin Pract. (2019) 22:1765. doi: 10.4103/njcp.njcp_240_18
50. Pengpid S, Peltzer K, Puckpinyo A, Chantarasongsuk IJ. Effectiveness of a cluster-randomized controlled trial community-based lifestyle intervention program to control prehypertension and/or prediabetes in Thailand. Int J Diabetes Dev C. (2019) 39:123–31. doi: 10.1007/s13410-018-0641-2
51. Shin K, Park J, Yook T, Kim J, Kwon O, Choi S. Moxibustion for prehypertension and stage I hypertension: a pilot randomized controlled trial. Integr Med Res. (2019) 8:1–7. doi: 10.1016/j.imr.2018.11.002
52. Taylor KA, Wiles JD, Coleman DA, Leeson P, Sharma R, O Driscoll JM. Neurohumoral and ambulatory haemodynamic adaptations following isometric exercise training in unmedicated hypertensive patients. J Hypertens. (2019) 37:827–36. doi: 10.1097/HJH.0000000000001922
53. Ponte Márquez PH, Feliu-Soler A, Solé-Villa MJ, Matas-Pericas L, Filella-Agullo D, Ruiz-Herrerias M, et al. Benefits of mindfulness meditation in reducing blood pressure and stress in patients with arterial hypertension. J Hum Hypertens. (2019) 33:237–47. doi: 10.1038/s41371-018-0130-6
54. Glodzik J, Rewiuk K, Adamiak J, Marchewka J, Salakowski A, Mazur M, et al. Controlled aerobic training improves endothelial function and modifies vascular remodeling in healthy adults with high normal blood pressure. J Physiol Pharmacol. (2018) 69:699–707. doi: 10.26402/jpp.2018.5.04
55. Goessler KF, Buys R, VanderTrappen D, Vanhumbeeck L, Cornelissen VA. A randomized controlled trial comparing home-based isometric handgrip exercise versus endurance training for blood pressure management. J Am Soc Hypertens. (2018) 12:285–93. doi: 10.1016/j.jash.2018.01.007
56. Juraschek SP, White K, Tang O, Yeh H, Cooper LA, Miller ER. Effects of a dietary approach to stop hypertension (DASH) diet intervention on serum uric acid in African Americans with hypertension. Arthrit Care Res. (2018) 70:1509–16. doi: 10.1002/acr.23515
57. Neupane D, McLachlan CS, Mishra SR, Olsen MH, Perry HB, Karki A, et al. Effectiveness of a lifestyle intervention led by female community health volunteers versus usual care in blood pressure reduction (COBIN): an open-label, cluster-randomised trial. Lancet Glob Health. (2018) 6:e66–73. doi: 10.1016/S2214-109X(17)30411-4
58. Staffileno BA, Tangney CC, Fogg L. Favorable outcomes using an ehealth approach to promote physical activity and nutrition among young African American women. J Cardiovasc Nurs. (2018) 33:62–71. doi: 10.1097/JCN.0000000000000409
59. Allaert F. Effect of NaCl + Chitosan 3% vs. NaCl on high blood pressure parameters of healthy volunteers with prehypertension. Minerva Cardiol Angi. (2017) 65:563–76. doi: 10.23736/S0026-4725.17.04451-6
60. Azadpour N, Tartibian B, Koşar SN. Effects of aerobic exercise training on ACE and ADRB2 gene expression, plasma angiotensin II level, and flow-mediated dilation: a study on obese postmenopausal women with prehypertension. Menopause. (2017) 24:269–77. doi: 10.1097/GME.0000000000000762
61. Baross AW, Hodgson DA, Padfield SL, Swaine IL. Reductions in resting blood pressure in young adults when isometric exercise is performed whilst walking. J Sports Med. (2017) 2017:1–6. doi: 10.1155/2017/7123834
62. Naseem S, Ghazanfar H, Assad S, Ghazanfar A. Role of sodium-restricted dietary approaches to control blood pressure in Pakistani hypertensive population. J Pak Med Assoc. (2016) 66:837–42.
63. Rubinstein A, Miranda JJ, Beratarrechea A, Diez-Canseco F, Kanter R, Gutierrez L, et al. Effectiveness of an mHealth intervention to improve the cardiometabolic profile of people with prehypertension in low-resource urban settings in Latin America: a randomised controlled trial. Lancet Diab Endocrinol. (2016) 4:52–63. doi: 10.1016/S2213-8587(15)00381-2
64. Thiyagarajan R, Pal P, Pal GK, Subramanian SK, Trakroo M, Bobby Z, et al. Additional benefit of yoga to standard lifestyle modification on blood pressure in prehypertensive subjects: a randomized controlled study. Hypertens Res. (2015) 38:48–55. doi: 10.1038/hr.2014.126
65. Lim S, Min S, Kwon Y, Park S, Park H. Effects of intermittent exercise on biomarkers of cardiovascular risk in night shift workers. Atherosclerosis. (2015) 242:186–90. doi: 10.1016/j.atherosclerosis.2015.06.017
66. Beck DT, Martin JS, Casey DP, Braith RW. Exercise training improves endothelial function in resistance arteries of young prehypertensives. J Hum Hypertens. (2014) 28:303–9. doi: 10.1038/jhh.2013.109
67. Zhao XS, Wang R, Bin LR, Wa SQG. Intervention for prehypertension and its cardiovascular risk factors in Inner Mongolia. Genet Mol Res. (2014) 13:4867–82. doi: 10.4238/2014.July.4.1
68. Kamalakkannan K, Kumar SM. Effect of land and shallow water aerobic exercises on selected physiological and biochemical variables of obese adult. J Phys Educ Sport. (2014) 14:532. doi: 10.7752/jpes.2014.04082
69. Hughes JW, Fresco DM, Myerscough RH, Van Dulmen M, Carlson LE, Josephson R. Randomized controlled trial of mindfulness-based stress reduction for prehypertension. Psychosom Med. (2013) 75:721–8. doi: 10.1097/PSY.0b013e3182a3e4e5
70. Kim J, Kim D. Effects of aerobic exercise training on serum sex hormone binding globulin, body fat index, and metabolic syndrome factors in obese postmenopausal women. Metab Syndr Relat D. (2012) 10:452–7. doi: 10.1089/met.2012.0036
71. Marquez-Celedonio FG, Texon-Fernandez O, Chavez-Negrete A, Hernandez-Lopez S, Marin-Rendon S, Berlin-Lascurain S. Clinical effect of lifestyle modification on cardiovascular risk in prehypertensives: PREHIPER I study. Rev Esp Cardiol. (2009) 62:86–90. doi: 10.1016/S0300-8932(09)70025-9
72. Saptharishi L, Soudarssanane M, Thiruselvakumar D, Navasakthi D, Mathanraj S, Karthigeyan M, et al. Community-based randomized controlled trial of non-pharmacological interventions in prevention and control of hypertension among young adults. Indian J Commun Med. (2009) 34:329–34. doi: 10.4103/0970-0218.58393
73. Zilkens RR, Rich L, Burke V, Beilin LJ, Watts GF, Puddey IB. Effects of alcohol intake on endothelial function in men: a randomized controlled trial. J Hypertens. (2003) 21:97–103. doi: 10.1097/00004872-200301000-00019
74. Rakic V, Puddey IB, Burke V, Dimmitt SB, Beilin LJ. Influence of pattern of alcohol intake on blood pressure in regular drinkers: a controlled trial. J Hypertens. (1998) 16:165–74. doi: 10.1097/00004872-199816020-00006
75. Jessup JV, Lowenthal DT, Pollock ML, Turner T. The effects of endurance exercise training on ambulatory blood pressure in normotensive older adults. Geriatr Nephrol Urol. (1998) 8:103–9. doi: 10.1023/a:1008287320868
76. Wenneberg SR, Schneider RH, Walton KG, Maclean CRK, Levitsky DK, Salerno JW, et al. A controlled study of the effects of the transcendental meditation® program on cardiovascular reactivity and ambulatory blood pressure. Int J Neurosci. (1997) 89:15–28. doi: 10.3109/00207459708988461
77. The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. Arch Intern Med. (1997) 157:657–67.
78. Anderssen S, Holme I, Urdal P, Hjermann I. Diet and exercise intervention have favourable effects on blood pressure in mild hypertensives: the Oslo Diet and Exercise Study (ODES). Blood Press. (1995) 4:343.
79. Whelton PK, Buring J, Borhani NO, Cohen JD, Cook N, Cutler JA, et al. The effect of potassium supplementation in persons with a high-normal blood pressure: results from phase I of the Trials of Hypertension Prevention (TOHP) Trials of Hypertension Prevention (TOHP) Collaborative Research Group. Ann Epidemiol. (1995) 5:85–95. doi: 10.1016/1047-2797(94)00053-v
80. Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E, Cook NR, Hebert P, et al. Weight loss intervention in phase 1 of the trials of hypertension prevention the TOHP Collaborative Research Group. Arch Intern Med. (1993) 153:849–58.
81. Puddey IB, Parker M, Beilin LJ, Vandongen R, Masarei JRL. Effects of alcohol and caloric restrictions on blood pressure and serum lipids in overweight men. Hypertension (Dallas, Tex. 1979). (1992) 20:533–41. doi: 10.1161/01.HYP.20.4.533
82. Hypertension Prevention Trial Research Group. The Hypertension Prevention Trial: three-year effects of dietary changes on blood pressure. Arch Intern Med. (1990) 150:153–62.
83. Puska P, Iacono JM, Nissinen A, Korhonen HJ, Vartianinen E, Pietinen P, et al. Controlled, randomised trial of the effect of dietary fat on blood pressure. Lancet. (1983) 1:1–5. doi: 10.1016/s0140-6736(83)91556-8
84. Lee E, Yeung N, Xu Z, Zhang D, Yu CP, Wong S. Effect and acceptability of mindfulness-based stress reduction program on patients with elevated blood pressure or hypertension: a meta-analysis of randomized controlled trials. Hypertension. (2020) 76:1992–2001. doi: 10.1161/HYPERTENSIONAHA.120.16160
85. Khandekar JS, Vasavi VL, Singh VP, Samuel SR, Sudhan SG, Khandelwal B. Effect of yoga on blood pressure in prehypertension: a systematic review and meta-analysis. ScientificWorldJournal. (2021) 2021:4039364. doi: 10.1155/2021/4039364
Keywords: Bayesian network meta-analysis, prehypertension, non-pharmacological intervention, chronic disease management, blood pressure reduction
Citation: Shao T, Liang L, Zhou C, Tang Y, Gao W, Tu Y, Yin Y, Malone DC and Tang W (2023) Short-term efficacy of non-pharmacological interventions for global population with elevated blood pressure: A network meta-analysis. Front. Public Health 10:1051581. doi: 10.3389/fpubh.2022.1051581
Received: 23 September 2022; Accepted: 30 December 2022;
Published: 13 January 2023.
Edited by:
Jing Yuan, Fudan University, ChinaReviewed by:
Natasa Krsto Rancic, University of Niš, SerbiaXiaomo Xiong, University of South Carolina, United States
Copyright © 2023 Shao, Liang, Zhou, Tang, Gao, Tu, Yin, Malone and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxi Tang,  tokammy@cpu.edu.cn; Daniel C. Malone,
tokammy@cpu.edu.cn; Daniel C. Malone,  Dan.Malone@utah.edu
Dan.Malone@utah.edu
†These authors have contributed equally to this work
 Taihang Shao
Taihang Shao Leyi Liang
Leyi Liang Chengchao Zhou
Chengchao Zhou Yaqian Tang1
Yaqian Tang1 Yue Yin
Yue Yin Wenxi Tang
Wenxi Tang