- Department of Neurosurgery, Stanford University School of Medicine, Stanford, CA, United States
Solid tumors metastasizing to the brain are a frequent occurrence with an estimated incidence of approximately 30% of all cases. The longstanding conventional standard of care comprises surgical resection and whole-brain radiotherapy (WBRT); however, this approach is associated with limited long-term survival and local control outcomes. Consequently, stereotactic radiosurgery (SRS) has emerged as a potential alternative approach. The primary aim of SRS has been to improve long-term control rates. Nevertheless, rare observations of abscopal or out-of-field effects have sparked interest in the potential to elicit antitumor immunity via the administration of high-dose radiation. The blood-brain barrier (BBB) has traditionally posed a significant challenge to the efficacy of systemic therapy in managing intracranial metastasis. However, recent insights into the immune-brain interface and the development of immunotherapeutic agents have shown promise in preclinical and early-phase clinical trials. Researchers have investigated combining immunotherapy with SRS to enhance treatment outcomes in patients with brain metastasis. The combination approach aims to optimize long-term control and overall survival (OS) outcomes by leveraging the synergistic effects of both therapies. Initial findings have been encouraging in the management of various intracranial metastases, while further studies are required to determine the optimal order of administration, radiation doses, and fractionation regimens that have the potential for the best tumor response. Currently, several clinical trials are underway to assess the safety and efficacy of administering immunotherapeutic agents concurrently or consecutively with SRS. In this review, we conduct a comprehensive analysis of the advantages and drawbacks of integrating immunotherapy into conventional SRS protocols for the treatment of intracranial metastasis.
Introduction
Brain metastasis describes the dissemination of neoplastic cells from a primary malignancy to the brain tissue and is a common complication in adults with solid tumors (1, 2). The incidence rates vary and are commonly observed in patients with lung, melanoma, renal cell, and breast cancers (3). Various treatment modalities are available to manage brain metastasis, including chemotherapy (CT), surgical intervention, whole-brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), targeted therapies, and immunotherapy (4–6).
SRS has emerged as a popular choice among these modalities, primarily due to its superior efficacy and reduced toxicity compared to WBRT (7). WBRT triggers double-stranded DNA damage, leading to the generation of cytotoxic free radicals in the tumor cells due to oxygenation (8). In contrast, high-precision SRS elicits a local and systemic immune response against cancerous cells, resulting in better long-term control rates and a lower risk of neurocognitive decline when compared to conventional WBRT (9).
Animal studies have recently shown the occurrence of the abscopal effect (AE), a phenomenon in which the combination of radiation and dendritic cell growth factor leads to a reduction in distant metastases and improved disease-free survival compared to radiation alone (10). This effect occurs due to the ability to activate an immune response against cancer cells. The incorporation of immunotherapeutic agents that enhance the host immune response against cancer has expanded the range of therapeutic options available for neoplastic diseases (11). Anti–cytotoxic T-lymphocyte–associated antigen 4 (Anti-CTLA-4) and anti–programmed death 1/programmed death ligand 1 (anti-PD-1/PD-L1) antibodies have emerged as a key component of treatment for a range of tumors. However, the optimal combination and timing of these systemic agents with radiation therapy (RT) remains to be fully elucidated (12).
Recent advances in immunotherapy have led to the re-evaluation of the potential impact of RT, particularly through the use of hypo-fractionated ablative irradiation (13). The mechanism linking radiation dose and fractionation to antitumor immunity holds substantial implications for clinical translation and the potential synergistic effects with immunotherapy through the release of tumor-associated antigens (TAA), improved antigen presentation, and increased infiltration of immune cells into the tumor microenvironment (TME) (14). However, further investigation is crucial to optimize the combination of SRS and novel immunotherapies.
Methods
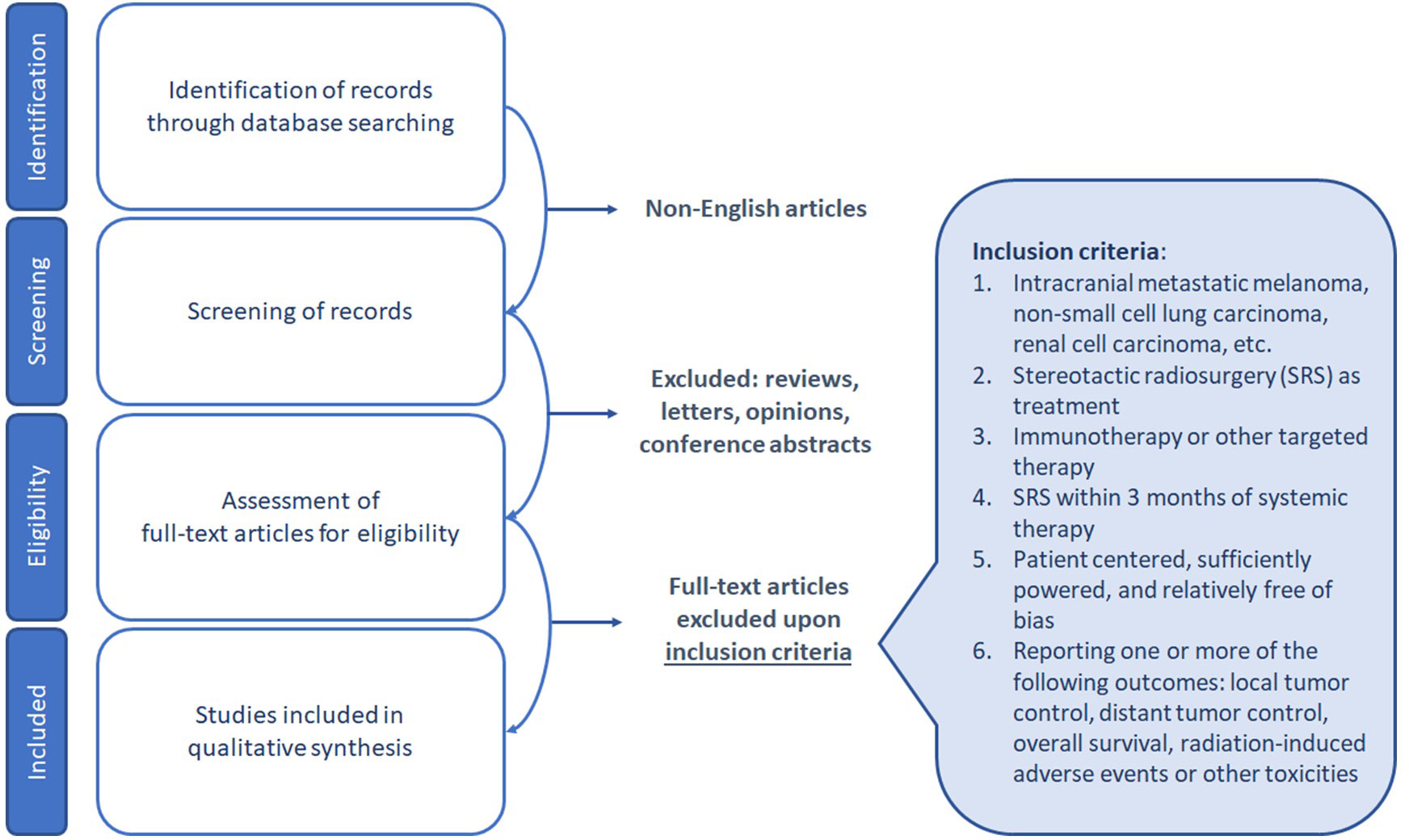
A systematic literature search was performed in the PubMed database, limited to articles published in the English language through April 28, 2023. The search strategy employed different keywords related to immunotherapy for the treatment of intracranial melanoma (M), non-small cell lung carcinoma (NSCLC), renal cell carcinoma (RCC), and other relevant malignancies. The identified publications were subjected to a screening process, during which non-English articles, as well as publications that fell under the categories of reviews, editorials, commentaries, case reports, opinion letters, and viewpoints, were excluded.
The eligibility criteria for inclusion in the qualitative synthesis were as follows: patient-centered studies with SRS as a treatment modality within three months of systemic therapy, and relatively unbiased reporting of one or more of the following outcomes: local tumor control, distant tumor control, overall survival, radiation-induced adverse events, or other toxicities. The selection of studies for qualitative synthesis was based on a rigorous evaluation of the study design, patient population, and quality of reporting (Figure 1).
Role of stereotactic radiosurgery in management of brain metastases
The therapeutic landscape for brain metastases has undergone significant evolution in recent years, with SRS emerging as the preferred treatment modality for patients with multiple intracranial metastases (15). The preference for SRS as a treatment option for multiple intracranial metastases has stemmed from the recognition that WBRT does not confer significant survival benefits and may adversely impact neurocognitive function, in contrast to SRS (9). SRS has consistently demonstrated high long-term control rates, with estimates of at least 70% at one year for SRS alone, and even higher rates for smaller metastases. Despite the favorable outcomes of SRS in treating brain metastases, studies estimate that a considerable proportion of patients (30-50%) may develop new distant brain metastases over the same period. As a strategy to minimize the risks of radiation-related toxicities and costs, and to defer or avoid the use of WBRT and its associated adverse effects, many patients undergo multiple rounds of SRS before considering the option of WBRT if necessary (16).
Immuno-modulation by stereotactic radiosurgery
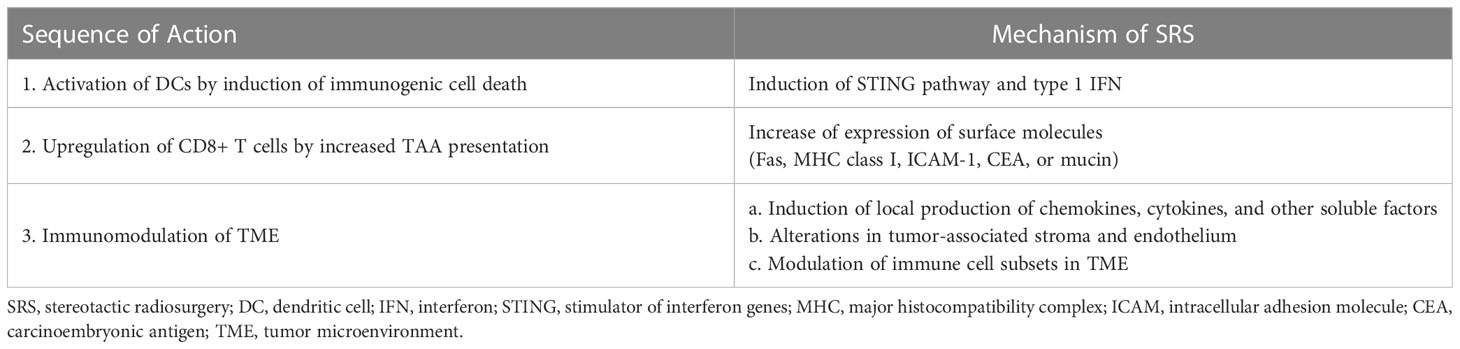
The process of stimulating the area undergoing SRS results in a complex set of physiological responses that can be broadly classified into two categories: (1) at the level of tumor cells, where it induces significant DNA damage leading to cell death, and (2) at the level of the TME, where it activates multiple signaling pathways, inducing a pro-inflammatory state within the TME, and potentially causing harm to the surrounding stromal and endothelial cells (Table 1) (17).
The interaction between radiation and the host’s immune response to brain metastases is multifaceted and influenced by numerous factors. Brain metastases can escape immune detection through several mechanisms, including the secretion of cytokines that suppress immune activity, decreased expression of TAA and major histocompatibility complex (MHC) class I, and the recruitment of regulatory T cells (Tregs) to the TME (Figure 2) (18). In the vicinity of the tumor, Tregs can increase in proportion to as high as 20-30% of CD4+ T cells. Furthermore, the suboptimal functioning of host dendritic cells (DCs) also contributes to the weakened immune response to tumor cells, even in the presence of radiation (19).
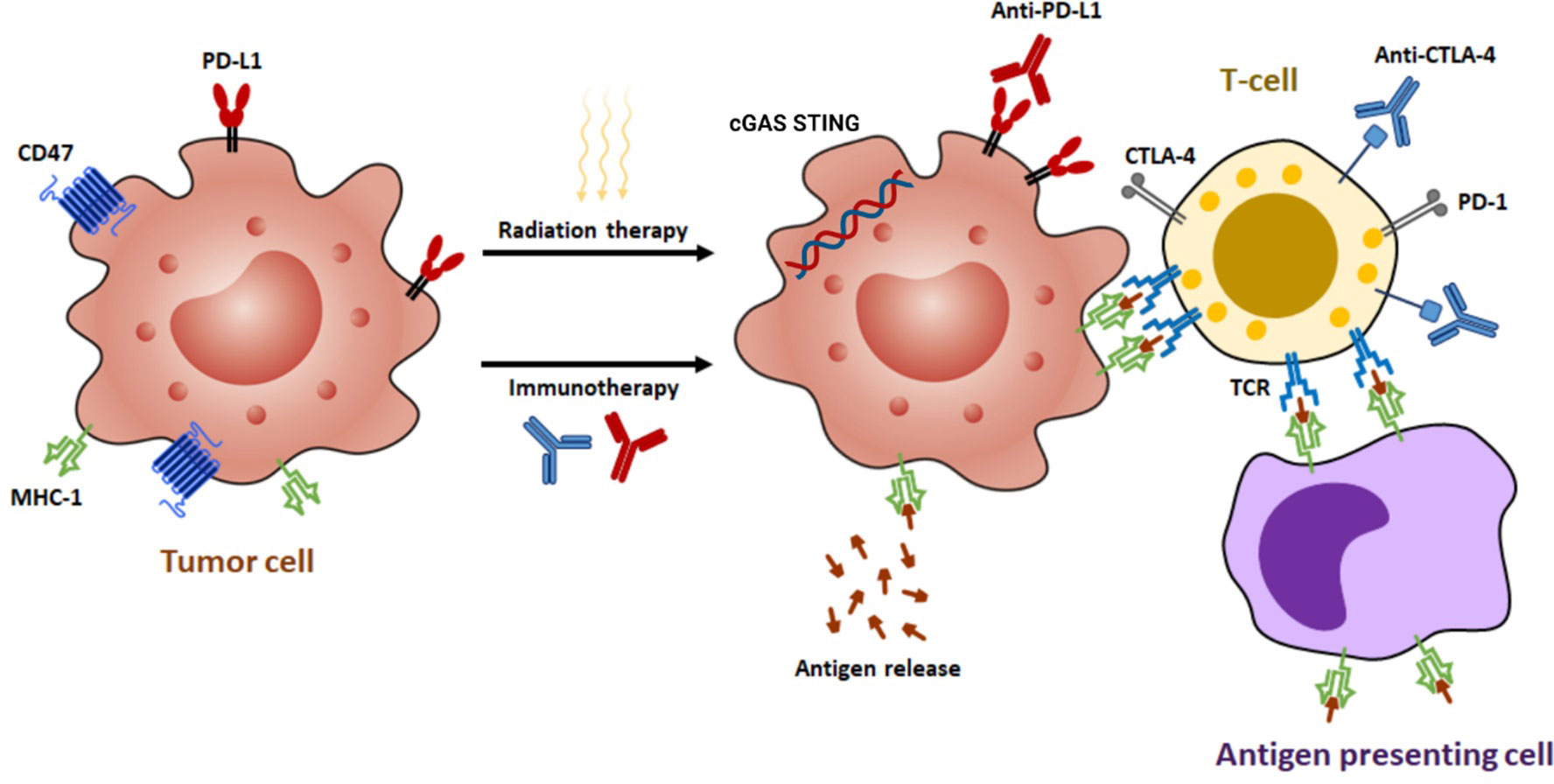
Figure 2 The synergistic effects of radiotherapy and immunotherapy mediated by various mechanisms. Radiation enhances the ability of antigen-presenting cells to present tumor antigens to naive T cells through the release of antigens, the stimulation of calreticulin, and the downregulation of CD47. This process leads to the expression of major histocompatibility complex class I (MHC-I) molecules and subsequent antigen presentation, which in turn results in the interaction between T-cell receptors (TCRs) and antigens. Moderate doses of radiation activate a type I interferon response by sensing cytoplasmic DNA via cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway. In addition, radiation can upregulate programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), making immunotherapy a potential strategy to augment radiation efficacy by targeting these pathways.
Radiation has been shown to augment the presentation of TAA by DCs to both CD4+ and CD8+ T cells, thereby reinforcing the ability of the immune system to recognize and target tumor cells (20). Furthermore, radiation has been observed to facilitate the maturation of antigen-presenting cells (APCs), enhance the assembly of antigen-MHC complexes, and induce the secretion of critical inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interferon gamma (IFN-β), and chemokine ligand 16 (CXCL16). These cytokines were found to attract immune cells to cross the blood-brain barrier (BBB) and infiltrate the TME (13).
In mouse models, single-fraction doses of 15 to 25 Gy have been demonstrated to elicit a CD8+ T-cell dependent immune response, leading to regression of the treated tumor. Depletion of CD8+ T cells has been associated with local tumor persistence, increased distant metastases, and decreased survival (21). Combining extracranial SRS with anti-PD-1 therapy has been shown to enhance the ratio of antigen-specific effector T cells to Tregs and increase T-cell infiltration into tumors, when compared to single-modality treatments, according to a study by Sharabi et al. (14).
Current insights into the immune response of the central nervous system
Recent discoveries have challenged the conventional idea that the brain is an “immunologically privileged” site due to the BBB and the absence of lymphatic drainage. Radiolabeled antigens have been found to travel through the subarachnoid space to cervical and retropharyngeal lymph nodes (22), and dendritic cells have been identified in the meninges and choroid plexus, involved in antigen presentation to T cells (23). The BBB can also be affected by brain cancer and RT, leading to increased permeability and lymphocyte accessibility (24).
Studies have shown that radiation exposure can increase MHC class I expression on glioma cells, leading to the infiltration of CD4+ and CD8+ T cells, while systemic administration of anti-CTLA-4 antibodies has been found to enhance the effector T cell response and decrease the number of Tregs (25). Combining SRS and immunotherapy has shown efficacy in the management of brain metastases, leading to an improved local tumor response, deferred progression, reduction in size of unirradiated brain lesions, and prevention of new brain and systemic metastases through the AE (26–28).
The AE is defined as the suppression of unirradiated distant tumors or metastases following RT to a target lesion. The underlying mechanism is thought to be immune-mediated, where RT activates the immune system by revealing tumor-specific antigens that are processed by dendritic cells to activate T cells in neighboring lymph nodes (29). Research into the AE is ongoing, and there is growing interest in exploring its potential as a treatment strategy, particularly with the use of immune checkpoint inhibitors (ICI) which display a higher degree of immunomodulatory activity compared to other therapeutic approaches. However, AE remains a topic of significant controversy within the field of RT. To date, only one study has reported a case of AE in SRS (30).
Immune checkpoint inhibitors
The human immune system plays a crucial role in defending against cancerous cells (31). Among the immunocompetent cells, T-cells have been identified as the most important ones in generating an antitumoral immune response (32). The potency of this response is determined by the modulation of stimulatory and inhibitory signals. The two immune checkpoints, CTLA-4 and PD-1, are crucial in regulating this response (33, 34). Antibodies such as ipilimumab, which targets CTLA-4, and nivolumab and pembrolizumab, which target PD-1, have shown promising results in cancer immunotherapy (Figure 2). These antibodies obstruct the inhibitory signals, amplifying the T-cell mediated immune response against cancer, and have been effectively used to manage various cancers (35).
Interactions between radiotherapy and the immune system
SRS is a highly effective method for treating tumors and achieving improved local tumor control (LTC). However, distant relapse remains a common issue after exclusive SRS due to the persistence of an immunosuppressive TME. To address this issue, the incorporation of immune evasion inhibitors alongside SRS may be beneficial, potentially enhancing the antitumor immune response and overall treatment outcomes.
The fundamental impact of ionizing radiation on biological systems is mainly attributed to the damage it inflicts on DNA molecules. Radiation’s immune-stimulating effects have been extensively studied over the past two decades. Studies have shown that local RT can enhance the systemic immune response by releasing TAAs from necrotic and apoptotic tumor cell debris. These antigens are then presented to CD8+ cytotoxic T cells by DCs, initiating an immune response that attacks tumor cells in other parts of the body where the antigens are recognized (36). Preclinical and clinical studies have further demonstrated that combining therapeutic radiation with ICIs can significantly enhance the systemic immune response, resulting in immunogenic tumor cell death (37, 38).
The optimal timing and dosage of radiation to maximize antitumoral immune stimulation have been elucidated through several research studies. For instance, Schaue et al. conducted a study on a mouse M model to examine the effects of total dose, dose per fraction, and number of fractions of RT on the RT-induced immune response and the outcomes (39). Tumor growth was effectively inhibited by single fraction doses of radiation. The LTC rates were positively correlated with radiation dose and quantity of tumor-reactive T cells (21).
The parallel between the sequence of SRS and ICIs and the potential benefits observed with neoadjuvant, concurrent, and adjuvant ICI utilization offers a valuable perspective for optimizing the administration of SRS and ICIs in the context of brain metastases (40). The timing of ICI administration emerges as a critical determinant of therapeutic outcomes. Studies have shown that administering ICI therapy 2 to 4 weeks before initiating SRS treatment during the first cycle yields the most favorable results in terms of long-term control and overall survival (OS). Concurrent use of both therapies demonstrated the highest effectiveness (41, 42). Nevertheless, the efficacy of different ICIs may be influenced by their specific sequence and timing in relation to SRS treatment (41).
Administering ICIs prior to SRS holds promise in priming the immune system and enhance its response to SRS, resulting in improved LTC while inducing systemic antitumor effects (43). Notably, encouraging results have been demonstrated in M patients receiving neoadjuvant ICI therapy, particularly when combined with agents like pembrolizumab or nivolumab in conjunction with SRS or local therapies. Nonetheless, the efficacy and safety of the neoadjuvant approach require further investigation, especially in the context of brain metastases (44). The adjuvant utilization of ICIs has demonstrated significant potential in reducing the risk of cancer recurrence and metastasis. Remarkably, adjuvant ICI treatment following SRS has led to improved progression-free survival (PFS) and OS in high-risk M patients (45).
The determination of the optimal sequencing and timing of SRS and ICIs remains an active area of investigation through ongoing research and clinical trials. The effectiveness and safety of these combined treatment approaches may vary based on factors such as tumor type, patient characteristics, and treatment regimens (46). As the field of oncology continues to evolve, we anticipate that further data and evidence will emerge regarding the neoadjuvant and adjuvant administration of ICIs in combination with SRS across different cancer types. To inform treatment decisions effectively, it is of utmost importance to stay up-to-date with the latest literature and clinical trial results (Tables 2, 3).
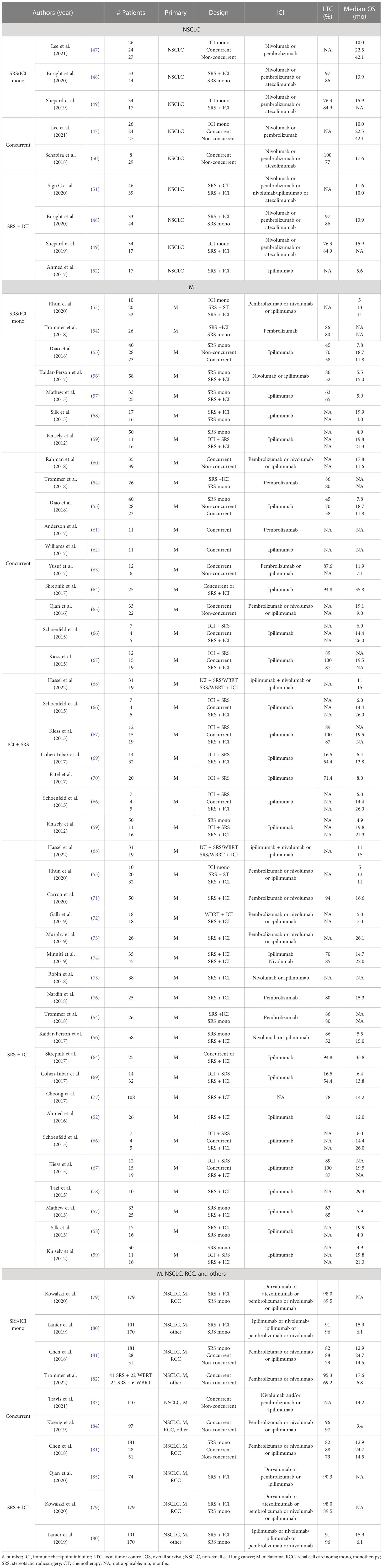
Table 2 Clinical trials of combination therapy in NSCLC, M, RCC, and other patients with brain metastasis.
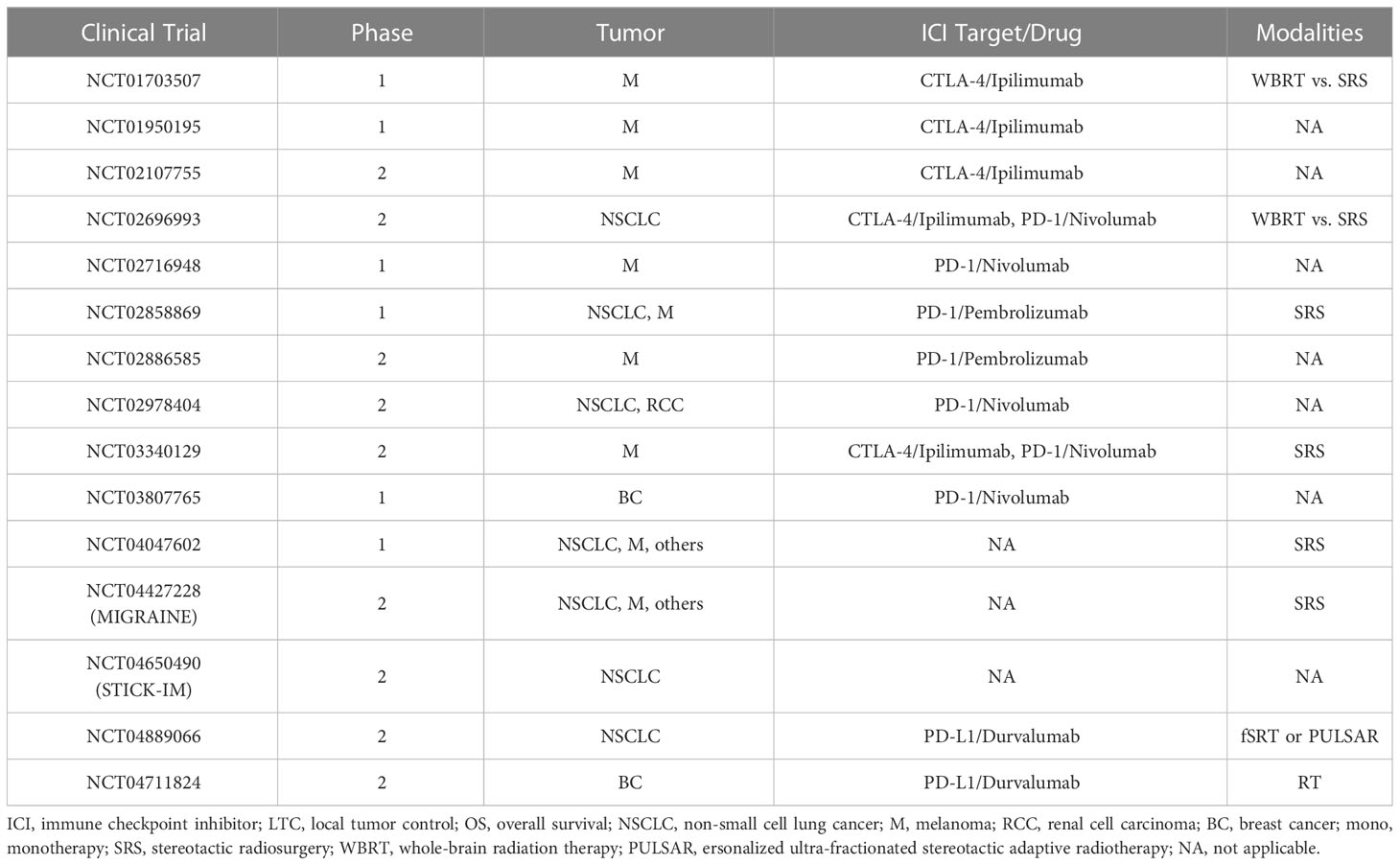
Table 3 Completed or ongoing clinical trials of stereotactic radiosurgery and immune checkpoint inhibitors in brain metastases treatment.
Clinical evidence of combination therapy
Ionizing radiation is known to cause DNA damage and subsequent cell death and its impact on the immune system has been extensively studied in recent years (86). Local RT can stimulate an antitumoral immune response by releasing TAAs from necrotic and apoptotic tumor cells, which are presented to CD8+ cytotoxic T cells by DCs (87). This activates the immune system to attack tumor cells throughout the body (88). Numerous preclinical and clinical studies have demonstrated that combining RT with ICIs can significantly enhance the systemic immune response, resulting in immunogenic tumor cell death. This approach has shown promising results in improving treatment outcomes and may be a valuable strategy for cancer management.
Clinical standpoint
From a clinical perspective, the application of SRS and ICIs for the treatment of brain metastasis has garnered significant interest, driven by preclinical and theoretical evidence. A number of studies have examined the optimal treatment sequence, SRS fractionation regimen and dosing, appropriate selection of ICIs, therapeutic efficacy, and potential adverse effects, generating a debate among experts in the field. The complexities surrounding optimal treatment strategies for brain metastasis are further compounded by the reliance on retrospective cohort analyses with small sample sizes, despite reporting improved LTC and OS rates with acceptable toxicity profiles. Notably, these studies primarily involve patients with M and the utilization of ipilimumab as the most frequently administered ICI. To address the current challenges, we present a comprehensive overview of studies examining brain metastasis in various cancer types, including NSCLC (47–52), M (53–78, 89, 90), and RCC among others (68, 79, 80, 82–85) (Table 2).
The definition of concomitant administration of ICI and SRS exhibits significant variability among studies. A notable portion of the literature defines concomitance as the simultaneous delivery of SRS and ICI within a timeframe of four weeks before or after the initiation of ICI (55, 69, 71, 73, 84, 91). Although some studies employ a narrower definition of concomitance with a window of less than two weeks (74, 81), others define it as extending up to over 2 months (61, 92).
Numerous studies have conducted comparisons between exclusive SRS monotherapy and combined ICI-SRS treatment with the overall consensus indicating improved LTC rates and patient outcomes with the concomitant administration of SRS and ICI (54, 93). Moreover, the combined SRS-ICI treatment resulted in a significant decrease in local failure compared to SRS monotherapy in M brain metastases (94). In addition, the combination of ICI and SRS may also provide benefit for PFS compared to SRS alone (55, 91).
Furthermore, a trend towards improved patient outcomes and LTC rates was observed in the patients who received concomitant SRS-ICI treatment, compared to those who received sequential treatment with no discernible difference in toxicity (81). The impact of combining SRS with ICI on treatment-related toxicity remains a subject of debate. While some studies have reported grade 3 or higher toxicity rates ranging from 5% to 24%, other investigations have identified a higher incidence of symptomatic radionecrosis after SRS-ICI treatment (hazard ratio 2.56, 95% confidence interval: 1.35–4.86, p = 0.004). Therefore, the true extent of toxicity resulting from this treatment modality remains inconclusive (95).
To provide a more comprehensive evaluation of the efficacy and safety of the combination of SRS and ICIs for brain metastasis, meta-analyses are warranted, given the limited patient population and the diversity of SRS and ICI treatment regimens across studies (96). One such meta-analysis found that concurrent administration of ICIs and SRS led to a statistically significant improvement in 1-year OS compared to non-concurrent ICI administration (96, 97).
While previous studies have yielded valuable insights into the combination of SRS and ICIs, it is imperative to recognize that their conclusions are based on retrospective observational studies, which may not fully reflect the extent of their effects. The true therapeutic value of this modality can only be established through well-designed prospective clinical trials, which aim to minimize biases and confounding variables that may influence the results of retrospective observational studies. Therefore, it is crucial to await the results of these trials to gain a more robust understanding of the impact of combining SRS and ICIs on patient outcomes and to provide healthcare professionals with a clear guidance to make informed treatment decisions.
Optimal dose and fractionation
Achieving the optimal dose and fractionation of radiation in SRS for treating spontaneous brain metastases poses a complex clinical challenge due to the need to balance effective tumor cell cytotoxicity against suppression of radiosensitive lymphocyte (98). Advancements in technology have enabled delivery of high radiation doses in a fractionated manner over multiple days or as a single fraction, thereby addressing this challenge (98). Preclinical studies provide limited insight into the complex interactions that occur within the CNS, but still furnish valuable information. For instance, in a murine M model, single-fraction doses ranging from 7.5 to 15 Gy optimized LTC, with a dose of 7.5 Gy providing a balance between tumor control and minimal suppression of Tregs. Fractionating the 15 Gy dose into two or three smaller doses improved LTC, reduced Tregs, and elicited an immune response (39).
The appropriate timing for administering immunotherapy in conjunction with RT remains a topic of ongoing research. Administering immunotherapy prior to SRS could enhance the antitumor response by allowing APCs and effector cells to be present when tumor cells are destroyed. However, this sequence could result in a reduced response if the circulating lymphocytes are recruited and then damaged by subsequent radiation. On the other hand, administering RT prior to immunotherapy could enhance expression of TAAs and increase BBB permeability, potentially improving drug delivery and immune cell infiltration. The optimal sequence of immunotherapy and RT depends on various factors, including radiation delivery parameters, the mode of action of immunotherapy, tumor histology, and overall mutational profile (99).
It is widely accepted that ICI therapy should be administered either in conjunction with or prior to SRS or RT. A preclinical study of colorectal cancer treatment with a combination of 2 Gy x 5 fractions of radiation and a PD-L1 inhibitor has demonstrated that simultaneous administration of the inhibitor on either the first or fifth day is the most efficacious approach. However, administering the inhibitor seven days after radiation completion failed to improve survival compared to RT alone (100). The optimal scheduling of SRS or RT in combination with immunotherapy remains a topic of ongoing investigation and research.
In a series of experiments evaluating the effect of the timing of administration of anti-CTLA-4 and anti-OX40 antibodies in relation to a single dose of 20 Gy radiation, the optimal timing of anti-CTLA-4 administration in relation to RT was found to depend on the dose and timing of the antibody (101). Administering anti-CTLA-4 prior to RT cleared all tumors, whereas administering it after RT resulted in only 50% of tumors being eliminated. The optimal timing of administering anti-OX40 was only after radiation completion, and mice that received anti-OX40 1 day after radiation showed improved tumor clearance and a doubling of median survival time. These results suggest that anti-CTLA-4 therapy in combination with RT improves outcomes, regardless of when the therapy is administered (101).
The order in which anti-CTLA-4 antibodies are administered has a significant effect on treatment efficacy. Prior studies have indicated that administering the drug either two days before or concurrent with radiation completion leads to improved treatment outcomes compared to administration two days after radiation (102). Administering the CTLA-4 inhibitor ipilimumab during or after SRS has demonstrated better survival rates compared to pretreatment administration in clinical practices. This outcome may occur because radiation releases antigens and prepares the immune system prior to the administration of ipilimumab (67). Optimal results were obtained when both local and systemic modalities were delivered within a four-week window for patients with M brain metastases who underwent SRS and received CTLA-4 or PD-1 inhibitors (65).
Conclusion
Recent evidence highlights a paradigm shift in our knowledge of the BBB and its role in brain metastases treatment. While traditionally considered a protective barrier against immune cells, emerging data suggests that certain immune cells and treatments can penetrate the BBB, opening new avenues for therapeutic interventions. As a result, the combination of SRS and immunotherapy has gained significant interest as a potential synergistic approach to combat brain tumors.
Radiation has been found to enhance the immune response, and T-cell-mediated responses are crucial in controlling tumors post-radiation locally and systemically. The potential benefit of combining of SRS and ICIs are promising, including improved OS, reduced local failure rates, and decreased risk of local recurrence, surpassing the outcomes of either treatment as a monotherapy. However, it is essential to acknowledge that existing data on this combination primarily stems from single-center retrospective cohort studies, warranting further investigation.
The optimal administration sequence of immunotherapy with SRS remains uncertain and may vary depending on the specific systemic immunotherapy agent utilized. As we delve deeper into intricacies of this treatment combination, the possibility of increased incidence of severe adverse events compared to monotherapies still requires definitive determination.
In light of these advancements, we need to consider whether SRS might lose its necessity in the treatment of brain metastases due to the efficacy of immunotherapy. This question challenges us to identify predictive factors that enable better patient selection for initial immunotherapy, while reserving SRS for cases of intracranial progression. Recognizing the importance of patient selection, we must develop strategies to tailor treatment plans for individual cases, optimizing therapeutic outcomes and reducing treatment-related toxicities. For instance, recent findings supporting the efficacy of immunotherapy in treating brain metastases underscores its clinical relevance (103). Nonetheless, it is crucial to remain cautious and focused on refining our treatment approaches to strike a delicate balance between efficacy and safety.
In conclusion, the synergistic integration of SRS and immunotherapy holds immense potential in revolutionizing brain metastases treatment. To fully harness the potential of this combination, there is an imperative need to conduct additional well-designed prospective studies that elucidate the intricate interplay between SRS and ICIs. These studies hold the key to establishing robust clinical guidelines and tailored treatment plans, optimizing therapeutic outcomes for patients while mitigating the risk of treatment-related toxicities.
As we pursue advancement in this dynamic field, we aspire to propel the frontiers of neuro-oncology in patients facing the challenges of brain metastases.
Author contributions
DP and KY have made a substantial contribution to the concept and design of the article. KY drafted the article. SC and DP revised it critically for important intellectual content. ML approved the version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro-oncology (2015) 17:iv1–iv62. doi: 10.1093/neuonc/nov189
2. Nabors LB, Portnow J, Ammirati M, Baehring J, Brem H, Butowski N, et al. NCCN guidelines insights: central nervous system cancers, version 1.2017. J Natl Compr Cancer Netw J Natl Compr Canc Netw (2017) 15:1331–45. doi: 10.6004/jnccn.2017.0166
3. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep (2012) 14:48–54. doi: 10.1007/s11912-011-0203-y
4. Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro-oncology (2017) 19:162–74. doi: 10.1093/neuonc/now241
5. Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol (2021) 40:492–516. doi: 10.1200/JCO.21.02314
6. Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol (2021) 32:1332–47. doi: 10.1016/j.annonc.2021.07.016
7. Rusthoven CG, Camidge DR, Robin TP, Brown PD. Radiosurgery for small-cell brain metastases: challenging the last bastion of preferential whole-brain radiotherapy delivery. J Clin Oncol (2020) 38:3587–91. doi: 10.1200/JCO.20.01823
8. Ashrafizadeh M, Farhood B, Eleojo Musa A, Taeb S, Rezaeyan A, Najafi M. Abscopal effect in radioimmunotherapy. Int Immunopharmacol (2020) 85:106663. doi: 10.1016/j.intimp.2020.106663
9. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA (2016) 316:401–9. doi: 10.1001/jama.2016.9839
10. Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Physics (2004) 58:862–70. doi: 10.1016/j.ijrobp.2003.09.012
11. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. New Engl J Med (2015) 372:2018–28. doi: 10.1056/NEJMoa1501824
12. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol (2018) 8:86. doi: 10.3389/fonc.2018.00086
13. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol (2005) 174:7516–23. doi: 10.4049/jimmunol.174.12.7516
14. Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res (2015) 3:345–55. doi: 10.1158/2326-6066.CIR-14-0196
15. Arvold ND, Lee EQ, Mehta MP, Margolin K, Alexander BM, Lin NU, et al. Updates in the management of brain metastases. Neuro-oncology (2016) 18:1043–65. doi: 10.1093/neuonc/now127
16. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA (2006) 295:2483–91. doi: 10.1001/jama.295.21.2483
17. Qiu B, Aili A, Xue L, Jiang P, Wang J. Advances in radiobiology of stereotactic ablative radiotherapy. Front Oncol (2020) 10:1165. doi: 10.3389/fonc.2020.01165
18. Oleinika K, Nibbs RJ, Graham GJ, Fraser AR. Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin Exp Immunol (2013) 171:36–45. doi: 10.1111/j.1365-2249.2012.04657.x
19. Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol (2001) 2:293–9. doi: 10.1038/86297
20. Fonteneau JF, Larsson M, Bhardwaj N. Interactions between dead cells and dendritic cells in the induction of antiviral CTL responses. Curr Opin Immunol (2002) 14:471–7. doi: 10.1016/s0952-7915(02)00358-8
21. Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood (2009) 114:589–95. doi: 10.1182/blood-2009-02-206870
22. Laman JD, Weller RO. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J Neuroimmune Pharmacol (2013) 8:840–56. doi: 10.1007/s11481-013-9470-8
23. Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med (2011) 208:1695–705. doi: 10.1084/jem.20102657
24. Holman DW, Klein RS, Ransohoff RM. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta (2011) 1812:220–30. doi: 10.1016/j.bbadis.2010.07.019
25. Newcomb EW, Demaria S, Lukyanov Y, Shao Y, Schnee T, Kawashima N, et al. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res (2006) 12:4730–7. doi: 10.1158/1078-0432.CCR-06-0593
26. Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Physics (2013) 86:343–9. doi: 10.1016/j.ijrobp.2012.12.025
27. Lehrer EJ, McGee HM, Sheehan JP, Trifiletti DM. Integration of immuno-oncology with stereotactic radiosurgery in the management of brain metastases. J Neuro-Oncol (2021) 151:75–84. doi: 10.1007/s11060-020-03427-6
28. Janopaul-Naylor JR, Shen Y, Qian DC, Buchwald ZS. The abscopal effect: A review of pre-clinical and clinical advances. Int J Mol Sci (2021) 22(20). doi: 10.3390/ijms222011061
29. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer (2018) 18:313–22. doi: 10.1038/nrc.2018.6
30. Mowery YM, Patel K, Chowdhary M, Rushing CN, Roy Choudhury K, Lowe JR, et al. Retrospective analysis of safety and efficacy of anti-PD-1 therapy and radiation therapy in advanced melanoma: A bi-institutional study. Radiother Oncol (2019) 138:114–20. doi: 10.1016/j.radonc.2019.06.013
31. Greenwald RJ, Freeman GJ, Sharpe AH. THE B7 FAMILY REVISITED. Annu Rev Immunol (2004) 23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611
32. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol (2008) 8:467–77. doi: 10.1038/nri2326
33. Wong SK, Beckermann KE, Johnson DB, Das S. Combining anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and -programmed cell death protein 1 (PD-1) agents for cancer immunotherapy. Expert Opin Biol Ther (2021) 21:1623–34. doi: 10.1080/14712598.2021.1921140
34. Odorizzi PM, Wherry EJ. Inhibitory receptors on lymphocytes: insights from infections. J Immunol (Baltimore Md.: 1950) (2012) 188:2957–65. doi: 10.4049/jimmunol.1100038
35. Sobhani N, Tardiel-Cyril DR, Davtyan A, Generali D, Roudi R, Li Y. CTLA-4 in regulatory T cells for cancer immunotherapy. Cancers (2021) 13(6):1440. doi: 10.3390/cancers13061440
36. Chow MT, Möller A, Smyth MJ. Inflammation and immune surveillance in cancer. Semin Cancer Biol (2012) 22:23–32. doi: 10.1016/j.semcancer.2011.12.004
37. Salama AKS, Postow MA, Salama JK. Irradiation and immunotherapy: From concept to the clinic. Cancer (2016) 122:1659–71. doi: 10.1002/cncr.29889
38. Yoo HJ, Liu Y, Wang L, Schubert M-L, Hoffmann J-M, Wang S, et al. Tumor-specific reactive oxygen species accelerators improve chimeric antigen receptor T cell therapy in B cell Malignancies. Int J Mol Sci (2019) 20. doi: 10.3390/ijms20102469
39. Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Physics (2012) 83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049
40. Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med (2019) 25:477–86. doi: 10.1038/s41591-018-0337-7
41. ElJalby M, Pannullo SC, Schwartz TH, Parashar B, Wernicke AG. Optimal timing and sequence of immunotherapy when combined with stereotactic radiosurgery in the treatment of brain metastases. World Neurosurg (2019) 127:397–404. doi: 10.1016/j.wneu.2019.04.093
42. Wong P, Masucci L, Florescu M, Plourde M-E, Panet-Raymond V, Pavic M, et al. Phase II multicenter trial combining nivolumab and radiosurgery for NSCLC and RCC brain metastases. Neuro-oncol Adv (2023) 5:vdad018. doi: 10.1093/noajnl/vdad018
43. Lehrer EJ, McGee HM, Peterson JL, Vallow L, Ruiz-Garcia H, Zaorsky NG, et al. Stereotactic radiosurgery and immune checkpoint inhibitors in the management of brain metastases. Int J Mol Sci (2018) 19. doi: 10.3390/ijms19103054
44. Guan H, Zhou Z, Hou X, Zhang F, Zhao J, Hu K. Safety and potential increased risk of toxicity of radiotherapy combined immunotherapy strategy. Asia-Pacific J Clin Oncol (2023) 19:35–50. doi: 10.1111/ajco.13688
45. Randall Patrinely, Funck-Brentano E, Nguyen K, Rapisuwon S, Salem J-E, Gibney GT, et al. A multicenter analysis of immune checkpoint inhibitors as adjuvant therapy following treatment of isolated brain metastasis. Oncol (2021) 26:e505–7. doi: 10.1002/onco.13608
46. Lehrer EJ, Jones BM, Sindhu KK, Dickstein DR, Cohen M, Lazarev S, et al. A review of the role of stereotactic radiosurgery and immunotherapy in the management of primary central nervous system tumors. Biomedicines (2022) 10. doi: 10.3390/biomedicines10112977
47. Lee MH, Cho K-R, Choi JW, Kong D-S, Seol HJ, Nam D-H, et al. Immune checkpoint inhibitors for non-small-cell lung cancer with brain metastasis : the role of gamma knife radiosurgery. J Korean Neurosurg Soc (2020) 64:271–81. doi: 10.3340/jkns.2020.0135
48. Enright TL, Witt JS, Burr AR, Yadav P, Leal T, Baschnagel AM. Combined immunotherapy and stereotactic radiotherapy improves neurologic outcomes in patients with non–small-cell lung cancer brain metastases. Clin Lung Cancer (2021) 22:110–9. doi: 10.1016/j.cllc.2020.10.014
49. Shepard M, Xu Z, Donahue J, Muttikkal TE, Codeiro D, Hansen L, et al. Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: A matched cohort study. J Neurosurgery (2019) 26:1–8. doi: 10.1093/neuros/nyz310_217
50. Schapira E, Hubbeling H, Yeap BY, Mehan WA, Shaw AT, Oh K, et al. Improved overall survival and locoregional disease control with concurrent PD-1 pathway inhibitors and stereotactic radiosurgery for lung cancer patients with brain metastases. Int J Radiat Oncol Biol Physics (2018) 101:624–9. doi: 10.1016/j.ijrobp.2018.02.175
51. Singh C, Qian JM, Yu JB, Chiang VL. Local tumor response and survival outcomes after combined stereotactic radiosurgery and immunotherapy in non–small cell lung cancer with brain metastases. J Neurosurg JNS (2020) 132:512–7. doi: 10.3171/2018.10.JNS181371
52. Ahmed KA, Kim S, Arrington J, Naghavi AO, Dilling TJ, Creelan BC, et al. Outcomes targeting the PD-1/PD-L1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J Neuro-Oncol (2017) 133:331–8. doi: 10.1007/s11060-017-2437-5
53. Le Rhun E, Wolpert F, Fialek M, Devos P, Andratschke N, Reyns N, et al. Response assessment and outcome of combining immunotherapy and radiosurgery for brain metastasis from Malignant melanoma. ESMO Open (2020) 5(4). doi: 10.1136/esmoopen-2020-000763
54. Trommer-Nestler M, Marnitz S, Kocher M, Rueß D, Schlaak M, Theurich S, et al. Robotic stereotactic radiosurgery in melanoma patients with brain metastases under simultaneous anti-PD-1 treatment. Int J Mol Sci (2018) 19(9). doi: 10.3390/ijms19092653
55. Diao K, Bian SX, Routman DM, Yu C, Ye JC, Wagle NA, et al. Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: clinical outcomes and toxicity. J Neuro-Oncol (2018) 139:421–9. doi: 10.1007/s11060-018-2880-y
56. Kaidar-Person O, Zagar TM, Deal A, Moschos SJ, Ewend MG, Sasaki-Adams D, et al. The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: the potential impact of immunotherapy. Anti-Cancer Drugs (2017) 28:669–675. doi: 10.1097/CAD.0000000000000497
57. Mathew M, Tam M, Ott PA, Pavlick AC, Rush SC, Donahue BR, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res (2013) 23:191–5. doi: 10.1097/CMR.0b013e32835f3d90
58. Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med (2013) 2:899–906. doi: 10.1002/cam4.140
59. Knisely JPS, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VLS. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival: Clinical article. J Neurosurg JNS (2012) 117:227–33. doi: 10.3171/2012.5.JNS111929
60. Rahman R, Cortes A, Niemierko A, Oh KS, Flaherty KT, Lawrence DP, et al. The impact of timing of immunotherapy with cranial irradiation in melanoma patients with brain metastases: intracranial progression, survival and toxicity. J Neuro-Oncol (2018) 138:299–306. doi: 10.1007/s11060-018-2795-7
61. Anderson ES, Postow MA, Wolchok JD, Young RJ, Ballangrud Å., Chan TA, et al. Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J ImmunoTher Cancer (2017) 5:76. doi: 10.1186/s40425-017-0282-x
62. Williams NL, Wuthrick EJ, Kim H, Palmer JD, Garg S, Eldredge-Hindy H, et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int J Radiat Oncol Biol Physics (2017) 99:22–30. doi: 10.1016/j.ijrobp.2017.05.028
63. Yusuf MB, Amsbaugh MJ, Burton E, Chesney J, Woo S. Peri-SRS administration of immune checkpoint therapy for melanoma metastatic to the brain: investigating efficacy and the effects of relative treatment timing on lesion response. World Neurosurg (2017) 100:632–640.e4. doi: 10.1016/j.wneu.2017.01.101
64. Skrepnik T, Sundararajan S, Cui H, Stea B. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. OncoImmunology (2017) 6:e1283461. doi: 10.1080/2162402X.2017.1283461
65. Qian JM, Yu JB, Kluger HM, Chiang VLS. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer (2016) 122:3051–8. doi: 10.1002/cncr.30138
66. Schoenfeld JD, Mahadevan A, Floyd SR, Dyer MA, Catalano PJ, Alexander BM, et al. Ipilmumab and cranial radiation in metastatic melanoma patients: a case series and review. J ImmunoTher Cancer (2015) 3:50. doi: 10.1186/s40425-015-0095-8
67. Kiess AP, Wolchok JD, Barker CA, Postow MA, Tabar V, Huse JT, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Physics (2015) 92:368–75. doi: 10.1016/j.ijrobp.2015.01.004
68. Hassel JC, Schank TE, Smetak H, Mühlbauer J, Salzmann M, Machiraju D, et al. Evaluation of radio-immunotherapy sequence on immunological responses and clinical outcomes in patients with melanoma brain metastases (ELEKTRA). OncoImmunology (2022) 11:2066609. doi: 10.1080/2162402X.2022.2066609
69. Cohen-Inbar O, Lee C-C, Mousavi SH, Kano H, Mathieu D, Meola A, et al. Stereotactic radiosurgery for intracranial hemangiopericytomas: a multicenter study. J Neurosurg (2017) 126:744–54. doi: 10.3171/2016.1.JNS152860
70. Patel KR, Shoukat S, Oliver DE, Chowdhary M, Rizzo M, Lawson DH, et al. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol (2017) 40:440–50. doi: 10.1097/COC.0000000000000199
71. Carron R, Gaudy-Marqueste C, Amatore F, Padovani L, Malissen N, Balossier A, et al. Stereotactic radiosurgery combined with anti-PD1 for the management of melanoma brain metastases: A retrospective study of safety and efficacy. Eur J Cancer (2020) 135:52–61. doi: 10.1016/j.ejca.2020.04.028
72. Galli G, Cavalieri S, Di Guardo L, Cimminiello C, Nichetti F, Corti F, et al. Combination of immunotherapy and brain radiotherapy in metastatic melanoma: A retrospective analysis. Oncol Res Treat (2019) 42:182–9. doi: 10.1159/000497211
73. Murphy B, Walker J, Bassale S, Monaco D, Jaboin J, Ciporen J, et al. Concurrent radiosurgery and immune checkpoint inhibition: improving regional intracranial control for patients with metastatic melanoma. Am J Clin Oncol (2019) 42:253–7. doi: 10.1097/COC.0000000000000509
74. Minniti G, Anzellini D, Reverberi C, Cappellini GCA, Marchetti L, Bianciardi F, et al. Stereotactic radiosurgery combined with nivolumab or Ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J ImmunoTher Cancer (2019) 7:102. doi: 10.1186/s40425-019-0588-y
75. Robin TP, Breeze RE, Smith DE, Rusthoven CG, Lewis KD, Gonzalez R, et al. Immune checkpoint inhibitors and radiosurgery for newly diagnosed melanoma brain metastases. J Neuro-Oncol (2018) 140:55–62. doi: 10.1007/s11060-018-2930-5
76. Nardin C, Mateus C, Texier M, Lanoy E, Hibat-Allah S, Ammari S, et al. Tolerance and outcomes of stereotactic radiosurgery combined with anti-programmed cell death-1 (pembrolizumab) for melanoma brain metastases. Melanoma Res (2018) 28(2):111–9. doi: 10.1097/CMR.0000000000000413
77. Choong ES, Lo S, Drummond M, Fogarty GB, Menzies AM, Guminski A, et al. Survival of patients with melanoma brain metastasis treated with stereotactic radiosurgery and active systemic drug therapies. Eur J Cancer (2017) 75:169–78. doi: 10.1016/j.ejca.2017.01.007
78. Tazi K, Hathaway A, Chiuzan C, Shirai K. Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med (2015) 4:1–6. doi: 10.1002/cam4.315
79. Kowalski ES, Remick JS, Sun K, Alexander GS, Khairnar R, Morse E, et al. Immune checkpoint inhibition in patients treated with stereotactic radiation for brain metastases. Radiat Oncol (2020) 15:245. doi: 10.1186/s13014-020-01644-x
80. Lanier CM, Hughes R, Ahmed T, LeCompte M, Masters AH, Petty WJ, et al. Immunotherapy is associated with improved survival and decreased neurologic death after SRS for brain metastases from lung and melanoma primaries. Neuro-Oncol Pract (2019) 6:402–9. doi: 10.1093/nop/npz004
81. Chen L, Douglass J, Kleinberg L, Ye X, Marciscano AE, Forde PM, et al. Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non-small cell lung cancer, melanoma, and renal cell carcinoma. Int J Radiat Oncol Biol Physics (2018) 100:916–25. doi: 10.1016/j.ijrobp.2017.11.041
82. Trommer M, Adams A, Celik E, Fan J, Funken D, Herter JM, et al. Oncologic outcome and immune responses of radiotherapy with anti-PD-1 treatment for brain metastases regarding timing and benefiting subgroups. Cancers (2022) 14(5). doi: 10.3390/cancers14051240
83. Travis RL, Marcrom SR, Brown MH, Patel MP, Markert JM, Riley KO, et al. Control and toxicity in melanoma versus other brain metastases in response to combined radiosurgery and PD-(L)1 immune checkpoint inhibition. Adv Radiat Oncol (2021) 6(1):100561. doi: 10.1016/j.adro.2020.08.017
84. Koenig JL, Shi S, Sborov K, Gensheimer MF, Li G, Nagpal S, et al. Adverse radiation effect and disease control in patients undergoing stereotactic radiosurgery and immune checkpoint inhibitor therapy for brain metastases. World Neurosurg (2019) 126:e1399–411. doi: 10.1016/j.wneu.2019.03.110
85. Qian JM, Martin AM, Martin K, Hammoudeh L, Catalano PJ, Hodi FS, et al. Response rate and local recurrence after concurrent immune checkpoint therapy and radiotherapy for non–small cell lung cancer and melanoma brain metastases. Cancer (2020) 126:5274–82. doi: 10.1002/cncr.33196
86. Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules–mechanisms of damage and emerging methods of detection. Antioxid Redox Signaling (2014) 21:260–92. doi: 10.1089/ars.2013.5489
87. Nastasi C, Mannarino L, D'Incalci M. DNA damage response and immune defense. Int J Mol Sci (2020) 21(20). doi: 10.3390/ijms21207504
88. Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J Cell Mol Med (2019) 23:4854–65. doi: 10.1111/jcmm.14356
89. Ahmed KA, Stallworth DG, Kim Y, Johnstone P, Harrison LB, Caudell JJ, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol (2016) 27:434–41. doi: 10.1093/annonc/mdv622
90. Cohen-Inbar O, Shih H-H, Xu Z, Schlesinger D, Sheehan JP. The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J Neurosurg (2017) 127:1007–14. doi: 10.3171/2016.9.JNS161585
91. Stera S, Balermpas P, Blanck O, Wolff R, Wurster S, Baumann R, et al. Stereotactic radiosurgery combined with immune checkpoint inhibitors or kinase inhibitors for patients with multiple brain metastases of Malignant melanoma. Melanoma Res (2019) 29:187–95. doi: 10.1097/CMR.0000000000000542
92. An Y, Jiang W, Kim BYS, Qian JM, Tang C, Fang P, et al. Stereotactic radiosurgery of early melanoma brain metastases after initiation of anti-CTLA-4 treatment is associated with improved intracranial control. Radiother Oncol (2017) 125:80–8. doi: 10.1016/j.radonc.2017.08.009
93. Hadi I, Roengvoraphoj O, Bodensohn R, Hofmaier J, Niyazi M, Belka C, et al. Stereotactic radiosurgery combined with targeted/ immunotherapy in patients with melanoma brain metastasis. Radiat Oncol (2020) 15:37. doi: 10.1186/s13014-020-1485-8
94. Acharya S, Mahmood M, Mullen D, Yang D, Tsien CI, Huang J, et al. Distant intracranial failure in melanoma brain metastases treated with stereotactic radiosurgery in the era of immunotherapy and targeted agents. Adv Radiat Oncol (2017) 2:572–80. doi: 10.1016/j.adro.2017.07.003
95. Martin AM, Cagney DN, Catalano PJ, Alexander BM, Redig AJ, Schoenfeld JD, et al. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol (2018) 4:1123–4. doi: 10.1001/jamaoncol.2017.3993
96. Lehrer EJ, Peterson J, Brown PD, Sheehan JP, Quiñones-Hinojosa A, Zaorsky NG, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother Oncol (2019) 130:104–12. doi: 10.1016/j.radonc.2018.08.025
97. Lu VM, Goyal A, Rovin RA, Lee A, McDonald KL. Concurrent versus non-concurrent immune checkpoint inhibition with stereotactic radiosurgery for metastatic brain disease: a systematic review and meta-analysis. J Neuro-Oncol (2019) 141:1–12. doi: 10.1007/s11060-018-03020-y
98. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with Malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest (2013) 31:140–4. doi: 10.3109/07357907.2012.762780
99. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med (2017) 9:34. doi: 10.1186/s13073-017-0424-2
100. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res (2014) 74:5458–68. doi: 10.1158/0008-5472.CAN-14-1258
101. Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PloS One (2016) 11:e0157164. doi: 10.1371/journal.pone.0157164
102. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res (2009) 15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265
103. Paz-Ares LG, Ciuleanu T-E, Cobo M, Bennouna J, Schenker M, Cheng Y, et al. First-line nivolumab plus ipilimumab with chemotherapy versus chemotherapy alone for metastatic NSCLC in checkMate 9LA: 3-year clinical update and outcomes in patients with brain metastases or select somatic mutations. J Thorac Oncol (2023) 18:204–22. doi: 10.1016/j.jtho.2022.10.014
Keywords: stereotactic radiosurgery, immunotherapy, immune checkpoint inhibitor, brain metastasis, combination therapy
Citation: Yoo KH, Park DJ, Choi JH, Marianayagam NJ, Lim M, Meola A and Chang SD (2023) Optimizing the synergy between stereotactic radiosurgery and immunotherapy for brain metastases. Front. Oncol. 13:1223599. doi: 10.3389/fonc.2023.1223599
Received: 16 May 2023; Accepted: 24 July 2023;
Published: 11 August 2023.
Edited by:
Yi Shi, Shanghai Jiao Tong University, ChinaReviewed by:
Stefania Canova, San Gerardo Hospital, Monza, ItalyDavid M. Peereboom, Case Western Reserve University, United States
Copyright © 2023 Yoo, Park, Choi, Marianayagam, Lim, Meola and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven D. Chang, sdchang@stanford.edu
 Kelly H. Yoo
Kelly H. Yoo David J. Park
David J. Park John H. Choi
John H. Choi Michael Lim
Michael Lim Antonio Meola
Antonio Meola Steven D. Chang
Steven D. Chang