Introduction
In recent years, the rate of habitat change due to agricultural expansion has been highest in the tropics (Laurance et al. Reference Laurance, Sayer and Cassman2014), where production pressures often threaten areas of forest with high biodiversity. One of the crops whose production has expanded most rapidly is oil palm (Elaeis guineensis). Production of palm oil has increased by 35 times from 1970 to 2018, with 85% of current global supply produced in Indonesia and Malaysia (Meijaard et al. Reference Meijaard, Garcia-Ulloa, Sheil, Carlson, Wich, Juffe-Bignoli and Brooks2018). In 2019, palm oil accounted for nearly 40% of the vegetable oil used in food, cosmetics, and biofuels (FAO, 2019). With a yield 5–9 times higher per unit area than alternative oilseed crops, oil palm is currently the most efficient option for meeting growing demand for vegetable oil (Meijaard et al. Reference Meijaard, Garcia-Ulloa, Sheil, Carlson, Wich, Juffe-Bignoli and Brooks2018). However, oil palm expansion has driven 47% of deforestation in Indonesia and Malaysia from 1973 to 2015, causing losses of forest species and declines in biodiversity (Vijay et al. Reference Vijay, Pimm, Jenkins and Smith2016). Generally, oil palm plantations support lower numbers of bird (Koh and Wilcove, Reference Koh and Wilcove2008), arthropod (Turner and Foster, Reference Turner and Foster2009), and mammal (Mendes-Oliveira et al. Reference Mendes-Oliveira, Peres, Maués, Oliveira, Mineiro, de Maria and Lima2017) species and have altered rates of ecosystem functioning compared to forest habitats (Dislich et al. Reference Dislich, Keyel, Salecker, Kisel, Meyer, Auliya, Barnes, Corre, Darras, Faust, Hess, Klasen, Knohl, Kreft, Meijide, Nurdiansyah, Otten, Pe’er, Steinebach, Tarigan, Tölle, Tscharntke and Wiegand2017). To maximise conservation impact, it is critical that remaining intact habitats are protected and that existing oil palm plantations are managed as sustainably as possible to maximise the biodiversity they can support, whilst maintaining high levels of production. Supporting biodiversity in agricultural areas is not only vital for conservation but can also support agricultural production through the ecosystem services provided by biotic communities (Dislich et al. Reference Dislich, Keyel, Salecker, Kisel, Meyer, Auliya, Barnes, Corre, Darras, Faust, Hess, Klasen, Knohl, Kreft, Meijide, Nurdiansyah, Otten, Pe’er, Steinebach, Tarigan, Tölle, Tscharntke and Wiegand2017).
The reduction in biodiversity in oil palm, and its knock-on effects on ecosystem functioning, is thought to be largely due to reductions in habitat complexity, heterogeneity, and changes in microclimate compared to natural habitat (Benton et al. Reference Benton, Vickery and Wilson2003, Foster et al. Reference Foster, Snaddon, Turner, Fayle, Cockerill, Ellwood, Broad, Chung, Eggleton, Khen and Yusah2011, Meijide et al. Reference Meijide, Badu, Moyano, Tiralla, Gunawan and Knohl2018). Habitat complexity and heterogeneity describe the diversity and structural complexity of environmental factors found in an area, including vegetation composition, variation in microclimatic conditions, and substrate variability (Maskell et al. Reference Maskell, Botham, Henrys, Jarvis, Maxwell, Robinson, Rowland, Siriwardena, Smart, Skates, Tebbs, Tordoff and Emmett2019). Across land-use types, these aspects are influenced by abiotic factors such as topography, wind, water, and sun exposure, which result in distinct habitats (Dobrowski, Reference Dobrowski2011, Hardwick et al. Reference Hardwick, Toumi, Pfeifer, Turner, Nilus and Ewers2015). In agricultural systems such as oil palm, they are also impacted by management practices and plantation inputs. By creating more structurally and floristically complex habitats, plantation managers may be able to support greater biodiversity than that found in more simple landscapes (Tscharntke et al. Reference Tscharntke, Tylianakis, Rand, Didham, Fahrig, Batáry, Bengtsson, Clough, Crist, Dormann, Ewers, Fründ, Holt, Holzschuh, Klein, Kleijn, Kremen, Landis, Laurance, Lindenmayer, Scherber, Sodhi, Steffan-Dewenter, Thies, van der Putten and Westphal2012). Furthermore, as a perennial crop with a 20- to 30-year life cycle (Corley and Tinker, Reference Corley and Tinker2008), and mature palms reaching up to 18 metres in height (Barcelos et al. Reference Barcelos, Rios, Cunha, Lopes, Motoike, Babiychuk, Skirycz and Kushnir2015), oil palm may be able to foster more complex habitats than annual or shorter-lived agricultural crops. This can create microhabitats and microclimates for a wide range of species (Pashkevich et al. Reference Pashkevich, Aryawan, Luke, Dupérré, Waters, Caliman, Naim and Turner2021).
The complexity and biodiversity that oil palm plantations can support is dependent on how the crop is established and managed. More complex habitats can be developed at a landscape scale through preservation of forest patches or inclusion of diverse features such as wetland ponds (Meijaard et al. Reference Meijaard, Ancrenaz and van Balen2020) or riparian strips (Gray et al. Reference Gray, Slade, Mann and Lewis2017). At a local scale, they can be developed by allowing greater understory growth or planting non-crop plant species (Ghazali et al. Reference Ghazali, Asmah, Syafiq, Yahya, Aziz, Tan, Norhisham, Puan, Turner and Azhar2016, Teuscher et al. Reference Teuscher, Gérard, Brose, Buchori, Clough, Ehbrecht, Hölscher, Irawan, Sundawati, Wollni and Kreft2016). In both large and small-scale oil palm plantations, less-intensive understory management, preservation of forest margins around rivers, and intercropping of crops have all been reported to have a positive effect on biodiversity (Ashton-Butt et al. Reference Ashton-Butt, Aryawan, Hood, Naim, Purnomo, Suhardi, Willcock, Poppy, Caliman, Turner, Foster, Peh and Snaddon2018, Williamson et al. Reference Williamson, Slade, Luke, Swinfield, Chung, Coomes, Heroin, Jucker, Lewis, Vairappan, Rossiter and Struebig2021, Yahya et al. Reference Yahya, Syafiq, Ashton-Butt, Ghazali, Asmah and Azhar2017). These management practices can support biodiversity without compromising yield or profitability and have therefore been incorporated into sustainability initiatives such as the Roundtable on Sustainable Palm Oil (2018).
Some of the most ecologically important groups affected by habitat structure are within the arthropods (Crowder and Jabbour, Reference Crowder and Jabbour2014). Arthropods carry out a range of ecological roles that can benefit crop productivity, including decomposition, soil processing, and food web support (Crowder and Jabbour, Reference Crowder and Jabbour2014, Dislich et al. Reference Dislich, Keyel, Salecker, Kisel, Meyer, Auliya, Barnes, Corre, Darras, Faust, Hess, Klasen, Knohl, Kreft, Meijide, Nurdiansyah, Otten, Pe’er, Steinebach, Tarigan, Tölle, Tscharntke and Wiegand2017). In agricultural areas, arthropods can benefit crop yield as pollinators, pest control agents, and soil processors, or can decrease crop yields as pests and vectors of disease transmission (Ashraf et al. Reference Ashraf, Zulkifli, Sanusi, Tohiran, Terhem, Moslim, Norhisham, Ashton-Butt and Azhar2018). Of all arthropods, the order Lepidoptera are perhaps the best recorded taxonomically and ecologically (Thomas, Reference Thomas2005). Butterflies and moths have been described as indicators of evolutionary history and ecosystem health, have been widely used in biological assessment (Bates, Reference Bates1865, Fisher et al. Reference Fisher, Corbet and Williams1943, Raffles, Reference Raffles2001) and contribute to ecosystem functioning as pollinators, prey, and herbivores (Hahn and Brühl, Reference Hahn and Brühl2016). Lepidoptera are abundant in oil palm systems (Panjaitan et al. Reference Panjaitan, Drescher, Buchori, Peggie, Harahap, Scheu and Hidayat2020), with some species being yield-damaging pests (Saravanan et al. Reference Saravanan, Kalidas, Phanikumar, Dwarakakumar, Gupta and Arunkumar2020). Lepidoptera are an ideal taxon with which to study the effects of management practices, as they are highly affected by vegetation structure and microclimate (Öckinger and Smith, Reference Öckinger and Smith2006), and are responsive to environmental change in oil palm systems (Koh and Wilcove, Reference Koh and Wilcove2008). Lepidoptera resource and habitat requirements vary at both the species and family level and influence behavioural responses to environmental change (Slade et al. Reference Slade, Merckx, Riutta, Bebber, Redhead, Riordan and Macdonald2013). The behaviour of butterflies and moths is perhaps the least understood facet of their ecology (Merckx et al. Reference Merckx, Van Dyck, Karlsson and Leimar2003), although it can directly affect the stability of populations through mating, foraging, and predator avoidance behaviours. Behaviour has been found to differ significantly between habitat types (Evans et al. Reference Evans, Sibly, Thorbek, Sims, Oliver and Walters2020) and between species (Mair et al. Reference Mair, Thomas, Franco and Hill2015), with land managers manipulating vegetation through actions such as planting nectar sources to encourage butterfly biodiversity and activity (Kamarudin et al. Reference Kamarudin, Seman and Madri2019).
While reductions in Lepidoptera have been recorded after conversion from forest to oil palm (Koh and Wilcove, Reference Koh and Wilcove2008, Kwatrina et al. Reference Kwatrina, Santosa, Bismark and Santoso2018), and communities in oil palm have been compared to other crops (Panjaitan et al. Reference Panjaitan, Drescher, Buchori, Peggie, Harahap, Scheu and Hidayat2020), less research attention has been given to the influence of environmental conditions and management practices within existing plantations on Lepidopteran communities. The taxonomic diversity of Indonesia is highly understudied (Hughes, Reference Hughes2017); however, recent surveys have shown that Indonesia is home to more than 2,500 species of butterfly (Murwitaningsih and Dharma, Reference Murwitaningsih and Dharma2015), nearly 900 of which are found in Sumatra (Panjaitan et al. Reference Panjaitan, Drescher, Buchori, Peggie, Harahap, Scheu and Hidayat2020). Although Indonesia could therefore be considered a hotspot of butterfly biodiversity and is home to over twelve-million hectares of oil palm plantations (Estate Crops Statistics, 2018), no studies have yet investigated variation in Lepidoptera community and behaviour in oil palm plantations of differing habitat structure. In this paper, we investigate whether butterfly and day-flying moth density and behaviour at a whole-community level and individual species level vary between different habitats within existing oil palm plantations. We surveyed Lepidoptera communities in two distinct and commonly occurring oil palm plantation microhabitats, areas bordered by a roadside (hereafter ‘Edge’), and within-plantation areas surrounded on all sides by oil palm (‘Core’), in Riau, Indonesia across two seasons (March to April, hereafter ‘March’, and September) to answer the following questions:
-
1) How does overall Lepidoptera density and individual density of common species vary across two frequently occurring plantation microhabitats, and between seasons?
-
2) How does overall Lepidoptera behaviour and individual behaviour of common species differ across two frequently occurring plantation microhabitats, and between seasons?
Methods
Sites
Data were collected in oil palm plantations in Riau Province, central Sumatra, Indonesia (Supplementary Information 1). Until ∼1970, the area was covered by lowland dipterocarp forest, but was largely deforested for agriculture from the 1970s onwards. The area has a tropical climate, with average annual rainfall of 2350 mm (Tao et al. Reference Tao, Slade, Willis, Caliman and Snaddon2016). The plantations are owned and managed by PT Ivo Mas Tunggal (a subsidiary company of Golden Agri-Resources, GAR), with technical input from Sinar Mas Agro Resources and Technology Research Institute (SMARTRI) (the research and development centre of GAR). As of 2019, the closest forest was approximately 30 km from any part of our site, and no forested riparian buffers or forest fragments had been planted at the time of survey.
The sites surveyed were part of the Biodiversity and Ecosystem Function in Tropical Agriculture (BEFTA) Understory Vegetation Project (Luke et al. Reference Luke, Advento, Aryawan, Adhy, Ashton-Butt, Barclay, Dewi, Drewer, Dumbrell, Edi, Eycott, Harianja, Hinsch, Hood, Kurniawan, Kurz, Mann, Matthews Nicholass, Naim, Pashkevich, Prescott, Ps, Pujianto, Purnomo, Purwoko, Putra, Rambe, Soeprapto, Spear, Suhardi, Tan, Tao, Tarigan, Wahyuningsih, Waters, Widodo, Whendy, Woodham, Caliman, Slade, Snaddon, Foster and Turner2020), which is testing the effects of varying understory vegetation management practices on the oil palm ecosystem. However, our investigation took place before any experimental manipulations were implemented. They therefore represent typical understory vegetation management within an industrial plantation. This involves clearance of herbaceous understory vegetation with herbicide spraying (using glyphosate (Rollup 480 SL), metsulfuron-methyl (Erkafuron 20 WG), fluroxypyr (Starane 290 EC), paraquat dichloride (Rolixone 276 SL) along harvesting paths and within 1.5 m circles around palms, and manual removal of woody vegetation (using a machete), with understory vegetation otherwise being undisturbed (Luke et al. Reference Luke, Advento, Aryawan, Adhy, Ashton-Butt, Barclay, Dewi, Drewer, Dumbrell, Edi, Eycott, Harianja, Hinsch, Hood, Kurniawan, Kurz, Mann, Matthews Nicholass, Naim, Pashkevich, Prescott, Ps, Pujianto, Purnomo, Purwoko, Putra, Rambe, Soeprapto, Spear, Suhardi, Tan, Tao, Tarigan, Wahyuningsih, Waters, Widodo, Whendy, Woodham, Caliman, Slade, Snaddon, Foster and Turner2020). The study sites are sprayed three times a year at a standardised time and were not sprayed in the immediate months before surveys were conducted.
The sample plots were located within two neighbouring plantation estates (Ujung Tanjung and Kandista) that had comparable management practices, with mature oil palm divided into 1000 × 300 m plantation blocks across both areas (blocks all edged by plantation roads). We surveyed eighteen square plots within these blocks, distributed over ∼50 km2, with sites arranged in triplets (Supplementary Information 2). All plots were on flat ground, containing mature oil palms (>20 years since planting; planting dates across plots 1987–1993). Each plot was bordered on one side by a drainage ditch, an unpaved road, and by neighbouring oil palm on the other sides. Therefore, each plot contained a habitat that is within the plantation (at least 50 m from a road and surrounded by oil palm on all four sides, hereafter referred to as ‘Core’ habitat), and a habitat which is bordered by a road on one side (hereafter referred to as ‘Edge’ habitat). The term ‘Edge’ is used throughout the BEFTA Programme and refers to the areas bordered by plantation unmade roads and ditches, rather than describing where two different habitats abut. The ‘Core’ and ‘Edge’ habitats were at least 50 m apart from each other within each plot, and there was at least 150 m between neighbouring plots. ‘Edge’ and ‘Core’ habitats had different vegetation structure, with Core habitats containing a higher percentage of bare ground, empty fruit bunches (fruit bunches that have had their fruits removed and that are applied to plantations as a mulch), and dead palm fronds, and Edge habitats containing a higher canopy openness, average vegetation height, plant species richness and biomass, and number of herb species (for a full description of vegetation characteristics, see Luke et al. Reference Luke, Advento, Aryawan, Adhy, Ashton-Butt, Barclay, Dewi, Drewer, Dumbrell, Edi, Eycott, Harianja, Hinsch, Hood, Kurniawan, Kurz, Mann, Matthews Nicholass, Naim, Pashkevich, Prescott, Ps, Pujianto, Purnomo, Purwoko, Putra, Rambe, Soeprapto, Spear, Suhardi, Tan, Tao, Tarigan, Wahyuningsih, Waters, Widodo, Whendy, Woodham, Caliman, Slade, Snaddon, Foster and Turner2020). The most common non-crop species in both Edge and Core habitats were the ferns Nephrolepis biserrata and Asplenium longissimum, and the herb Asystasia gangetica subsp. micrantha. A stream ran through two of the sites. Refer to Luke et al. (Reference Luke, Advento, Aryawan, Adhy, Ashton-Butt, Barclay, Dewi, Drewer, Dumbrell, Edi, Eycott, Harianja, Hinsch, Hood, Kurniawan, Kurz, Mann, Matthews Nicholass, Naim, Pashkevich, Prescott, Ps, Pujianto, Purnomo, Purwoko, Putra, Rambe, Soeprapto, Spear, Suhardi, Tan, Tao, Tarigan, Wahyuningsih, Waters, Widodo, Whendy, Woodham, Caliman, Slade, Snaddon, Foster and Turner2020) for further plot details.
Lepidoptera surveys
We surveyed adult Lepidoptera along transects in both Core and Edge habitats in two seasons in 2013: between March and April, hereafter referred to as the ‘March’ season, and in September, hereafter referred to as the ‘September’ season. Monthly rainfall in the two estates was 163 mm in March and 239 mm in September 2013, consistent with historically high variation in monthly and annual rainfall in the area. While 2013 experienced higher average rainfall than following two years (2013–2015 monthly average: 152 mm), it was not an El Niño year (see Luke et al. Reference Luke, Advento, Aryawan, Adhy, Ashton-Butt, Barclay, Dewi, Drewer, Dumbrell, Edi, Eycott, Harianja, Hinsch, Hood, Kurniawan, Kurz, Mann, Matthews Nicholass, Naim, Pashkevich, Prescott, Ps, Pujianto, Purnomo, Purwoko, Putra, Rambe, Soeprapto, Spear, Suhardi, Tan, Tao, Tarigan, Wahyuningsih, Waters, Widodo, Whendy, Woodham, Caliman, Slade, Snaddon, Foster and Turner2020 for full analysis of rainfall patterns at SMARTRI sites). Due to relatively low numbers for each visit, two surveys of each plot were carried out on separate days for both March and September seasons, with total counts being summed for each transect.
Core transect walks were carried out in the centre of the plot, at least 50 m from a road at all points (Supplementary Information 2). Edge transect walks were carried out immediately alongside a roadside edge, adjacent to the Core transect and in the same plot (Supplementary Information 2). We followed standard transect methods to survey Lepidoptera (Pollard and Yates, Reference Pollard and Yates1993). In brief, a recorder walked slowly along the transect and recorded any butterflies and day-flying moths seen within a 5 m-sided cube in front of them. As the transects were originally designed to compare changes over time within habitats rather than between Core and Edge, the transect shape and volume differed between Core and Edge sites (Supplementary Information 2), with the roadside Edge transects represented as a straight line of 150 m (survey volume 3750 m3) and Core transects represented in the shape of a picture frame of 200 m (survey volume 4969 m3, with 0.75 m at the beginning and end of the transect only being surveyed once to avoid double counting. See Supplementary Information 3 for full calculations). We acknowledge that this difference means the distance between furthest points within transects is higher in the Edge than Core areas and that there could be a higher chance of resurveying individual butterflies in the Core than in the Edge transects. However, this is unlikely to have a large impact, as the plantations we surveyed were highly homogenous, with most obvious variation being related to distance from palms (planted at eight-metre distances), rather than across transects. To control for this variation as far as possible, we standardised abundance counts to density of individuals within 150 m transects by multiplying Core values by 0.75 and by analysing density of all butterflies and individual butterfly species, rather than composition or richness, which should be robust to the impacts of resampling. We focused our analyses on the total density and behaviour of all butterflies, and the six most common species individually, each of which independently represented more than 10% of the population in either Core or Edge habitats. When combined, these six species represented over 70% of the total count.
Transects were walked between 9:00 and 17:00 and only when it was not raining. When the recorder was not able to identify a species by eye, the individual was caught and photographed for later identification. Identification followed Corbet et al. (Reference Corbet, Pendlebury, van der Poorten and van der Poorten1978) as well as cross-referencing with region-specific photographs and information at the end of the survey period (using iNaturalist, 2020). Identification was done to family and species level, where possible, but morphospecies when this was not possible, all hereafter referred to simply as ‘species’. There were six cases where we were only able to accurately identify to genus level (Mycalesis spp., Amathusia spp., Erionota spp., Graphium spp., Telicota spp., Macroglossum spp., and Ypthima spp.). There were also three species (comprising seven individuals) that were not identified nor caught in the field, which we have therefore been unable to reliably identify to species, genus, or family level, and were therefore excluded (Supplementary Information 4).
The behaviour of individuals was recorded in the same transect walk. Behaviours were classified as follows: Flying (in flight when first observed); perching (perching and defending territory when first seen); resting (wings open or closed but individual not moving when first seen); nectaring (feeding on flowers or fruit when first seen); interacting (chasing another butterfly or mating when first seen); mudpuddling (feeding on minerals or water when first seen); or unknown (behaviour not recorded or unsure). For analysis, these behaviours were lumped into active (flying), mating (perching and interacting), inactive (resting), and foraging (nectaring and mudpuddling), and unknown. For nectaring individuals, we also recorded the plant species that butterflies were using, when known. Due to low numbers, we do not formally analyse plant-nectaring data, but present qualitative findings.
Statistical methods
All statistical analyses were performed in R version 4.0.2 (R Core Team, 2020) within R Studio version 1.4.456 (R Core Team, 2020). We used tidyverse (Wickham et al. Reference Wickham, Averick, Bryan, Chang, McGowan, François, Grolemund, Hayes, Henry, Hester, Kuhn, Pedersen, Miller, Bache, Müller, Ooms, Robinson, Seidel, Spinu, Takahashi, Vaughan, Wilke, Woo and Yutani2019), data.table (Dowle and Srinivasan, Reference Dowle and Srinivasan2021), plyr (Wickham, Reference Wickham2011), dplyr (Wickham et al. Reference Wickham and François2015), and reshape2 (Wickham, Reference Wickham2007) for data wrangling and visualisation.
To determine the effects of habitat and season on density, we fitted generalised linear mixed-effect models (GLMMs) using mvabund (Wang et al. Reference Wang, Naumann, Wright and Warton2012) and lme4 (Bates et al. Reference Bates, Mächler, Bolker and Walker2015). Seven separate GLMMs were run: one for the total density of all species combined, and one for each of the six most common species. All linear models were fitted to negative binomial distributions using log links, with habitat (levels: Core, Edge), season (levels: March, September), and their interaction as fixed effects and triplet as a random intercept effect. We validated GLMM models by plotting Pearson residuals against fitted values and covariates and verifying that no patterns were present. We further validated models by simulating eight datasets for frequency, residuals, and residuals against variables using identical effects and were unable to detect the observed data from the simulated sets, indicating that there were no issues in model fit.
Differences in Lepidoptera behaviour across habitat (Core or Edge) and season (March or September) were visualised using Non-metric Multidimensional Scaling (NMDS) with package ‘vegan’ (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O’Hara, Simpson, Solymos, Stevens, Szöcs and Wagner2020) for the population as a whole, and separately for the six most common species. We tested for significant differences using ANOSIM with a Bray-Curtis dissimilarity matrix with 999 permutations, again using ‘vegan’. All figures were plotted using ggplot2 (Wickham, Reference Wickham2016).
Results
We observed a total of 1464 individual butterflies and moths, from 41 different species within eight different families (Callidulidae, Erebidae, Hesperiidae, Lycaenidae, Nymphalidae, Papilionidae, Pieridae, and Sphingidae) (Supplementary Information 3). The most common species were Ypthima spp. (20% of total observed), Elymnias hypermnestra (nearly 15% of total observed), and Acraea terpsicore (13% of total observed). We did not find any species endemic to Sumatra, or species classified as forest specialists from the literature (See Supplementary Information 3 for full species list, and Supplementary Information 4 for figure of species counts).
Lepidoptera density across habitats and seasons
Of the 41 species recorded, only five were found more than 100 times (Ypthima spp., Elymnias hypermnestra, Acraea terpsicore, Leptosia nina, and Amathusia spp.). Nineteen species were represented by fewer than 10 individuals, with five species observed only once during the study (Supplementary Information 3, Supplementary Information 4). In particular, September Edge sites were dominated by three highly abundant species with counts of over 130 individuals (Ypthima spp., Elymnias hypermnestra, Acraea terpsicore).
Lepidoptera density of individuals for the population as a whole varied significantly between habitats (estimate = 0.66, SE = 0.15, p = <0.001), and there was a significant interaction between habitat and season on density (estimate = 0.86, SE = 0.22, p = <0.001), but no effect of season alone (estimate = 0.25, SE = 0.16, p = 0.12). In particular, density was much higher in September Edge sites (mean = 25.25, SE = 2.91) than March Edge sites (mean = 7.86, SE = 0.79), but this difference between seasons was less marked in Core sites (February Core mean = 4.23, SE = 0.66; September Core mean = 4.84, SE = 0.41) (Figure 1).
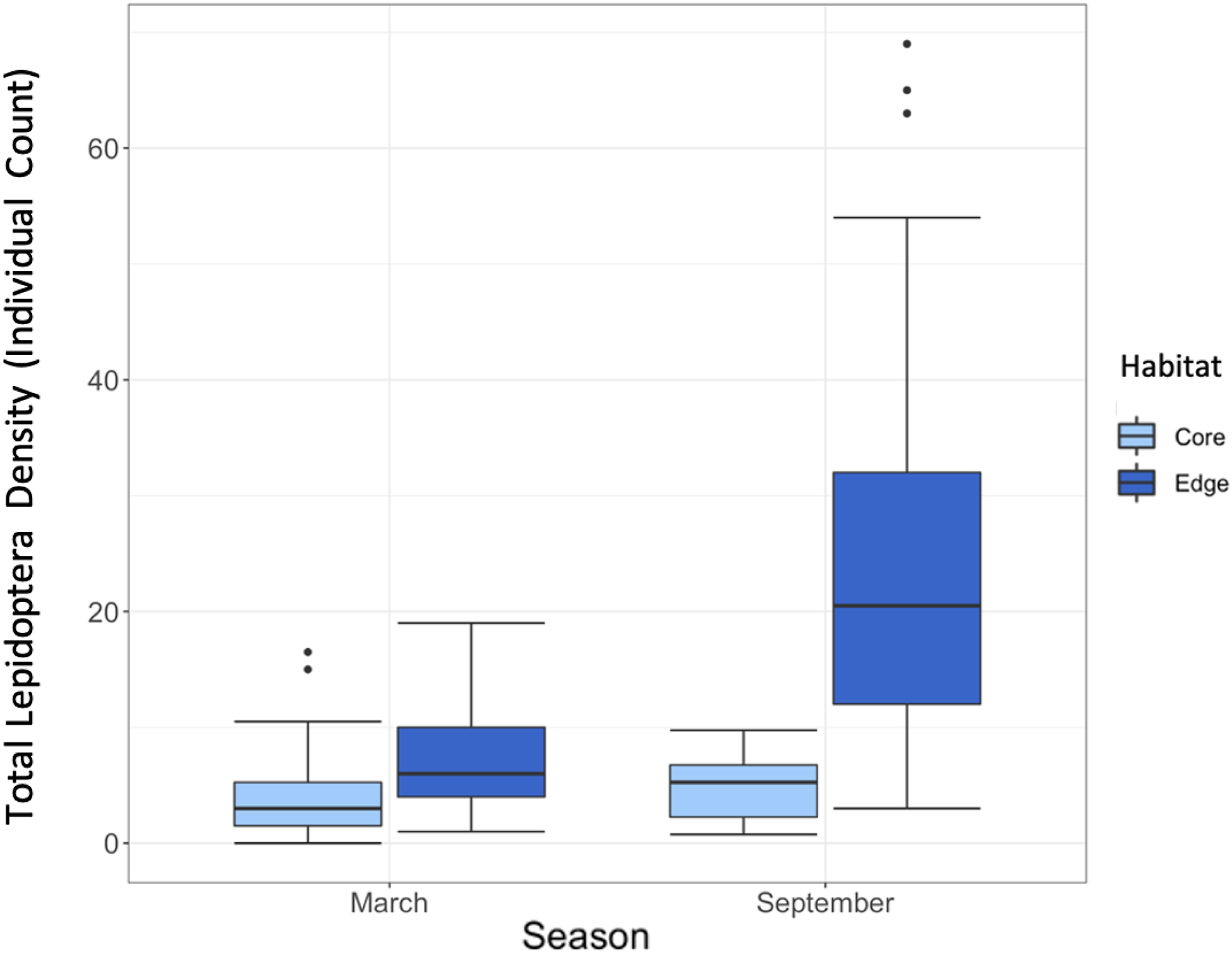
Figure 1. Box and whisker plots comparing Lepidoptera density in Core and Edge habitats in March and September seasons. Median, interquartile range, range, and outliers are given.
Effects were not uniform across the six most common species, with some species being found at higher densities in Core than Edge habitats, others vice versa, and others not showing a difference. Similarly, some species showed an increase in density in September compared to March in either Core or Edge habitats, while others did not.
Habitat type and the interaction between habitat and season had significant effects on Ypthima spp. and Elymnias hypermnestra density but not season alone (Ypthima spp. Habitat: Estimate = 2.17, SE = 0.46, p = <0.001; Season: Estimate = 0.39, SE = 0.57, p = 0.49; Interaction: Estimate = 1.28, SE = 0.59, p = 0.03; Elymnias hypermnestra Habitat: Estimate = 1.96, SE = 0.47, p = <0.001; Season: Estimate = −0.77, SE = 0.69, p = 0.26; Interaction: Estimate = 1.66, SE = 0.76, p = 0.03) (Figure 2A and B). Acraea terpsicore was not affected by either habitat or season nor their interaction (Habitat: Estimate = 1.29, SE = 128.69, p = 0.89; Season: Estimate = 0.01, SE = 210.13, p = 1.0; Interaction: Estimate = 3.29, SE = 210.13, p = 0.99) (Figure 2C). Leptosia nina was only significantly affected by habitat type (Habitat: Estimate = −0.81, SE = 0.37, p = 0.03; Season: Estimate = 0.19, SE = 0.31, p = 0.54; Interaction: Estimate = 0.77, SE = 0.48, p = 0.11) (Figure 2D). Amathusia spp. was significantly affected by only season (Habitat: Estimate = −16.28, SE = 284.01, p = 0.95; Season: Estimate = 2.01, SE = 0.38, p = <0.001; Interaction: Estimate = 15.75, SE = 284.01, p = 0.96) (Figure 2E). Tetragonus lycaenoides was affected by both season and habitat type independently, but not their interaction (Habitat: Estimate = −1.82, SE = 0.43, p = <0.001; Season: Estimate = −0.59, SE = 0.29, p = 0.04; Interaction: Estimate = 0.59, SE = 0.71, p = 0.93) (Figure 2F).
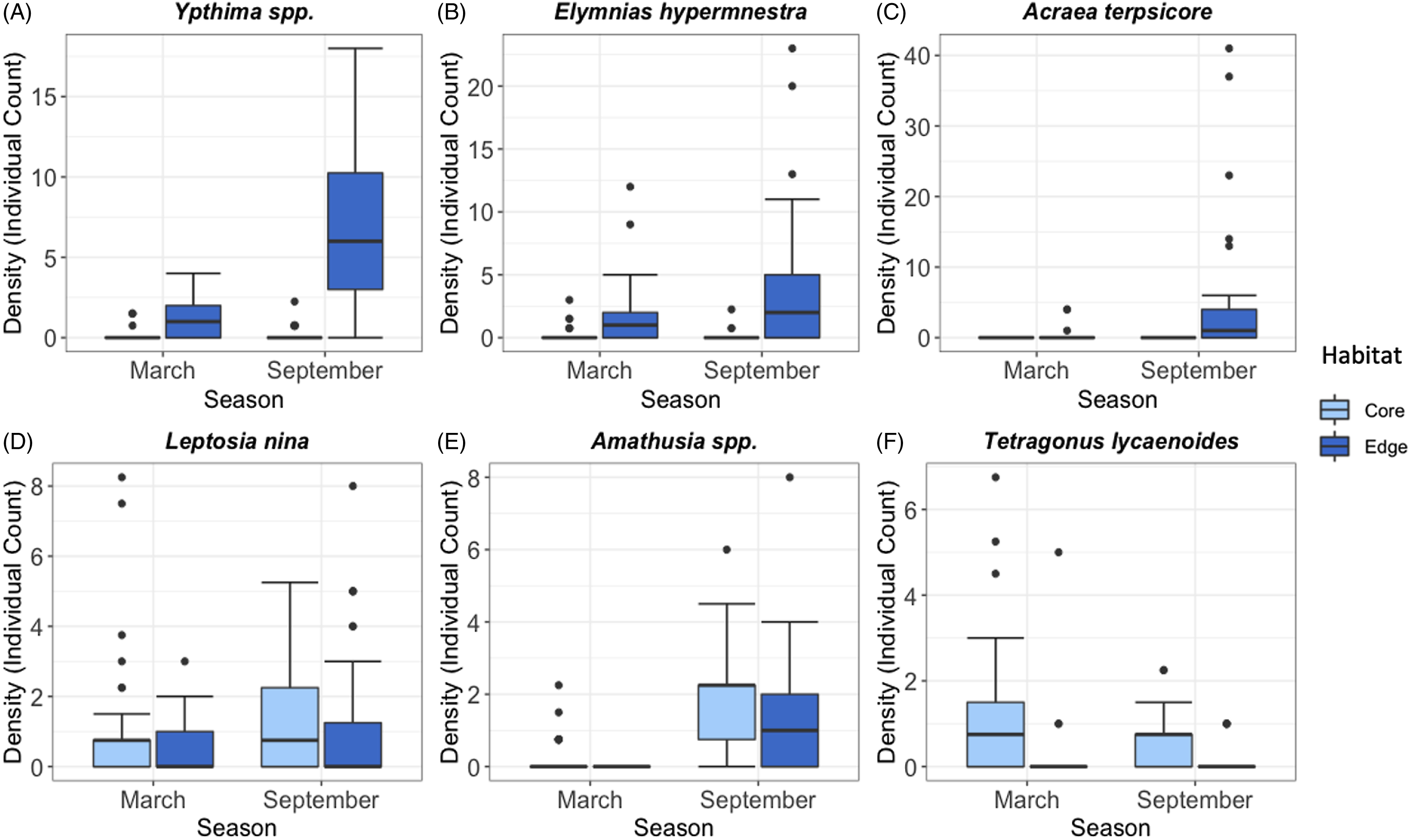
Figure 2. Comparisons of species density for the six most common species in Core and Edge habitats in March and September seasons (A–F): Ypthima spp., Elymnias hypermnestra, Acraea terpsicore, Leptosia nina, Amathusia spp., Tetragonus lycaenoides. Median, interquartile range, range, and outliers are given. Note: y-axis scales differ by species.
Lepidoptera behaviour
Both habitat and season had a significant effect on observed behaviour (Habitat: R = 0.29, p = <0.001; Season: R = 0.0622, p = 0.006, respectively) (Figure 3B), with foraging and mating being notably more common in Edge than Core habitats in both seasons. The most frequently observed behaviour was flying (59% of total observations; March Core = 59%, September Core = 65%, March Edge = 50%, September Edge = 58%) across all habitats and seasons (Figure 3A). For both Core and Edge habitats, the second most frequent behaviour was resting (March Core = 38%, September Core = 31%; March Edge = 19%, September Edge = 22%), followed by foraging (March Core = 4%, September Core = 3%, March Edge = 26%, September Edge = 13%).
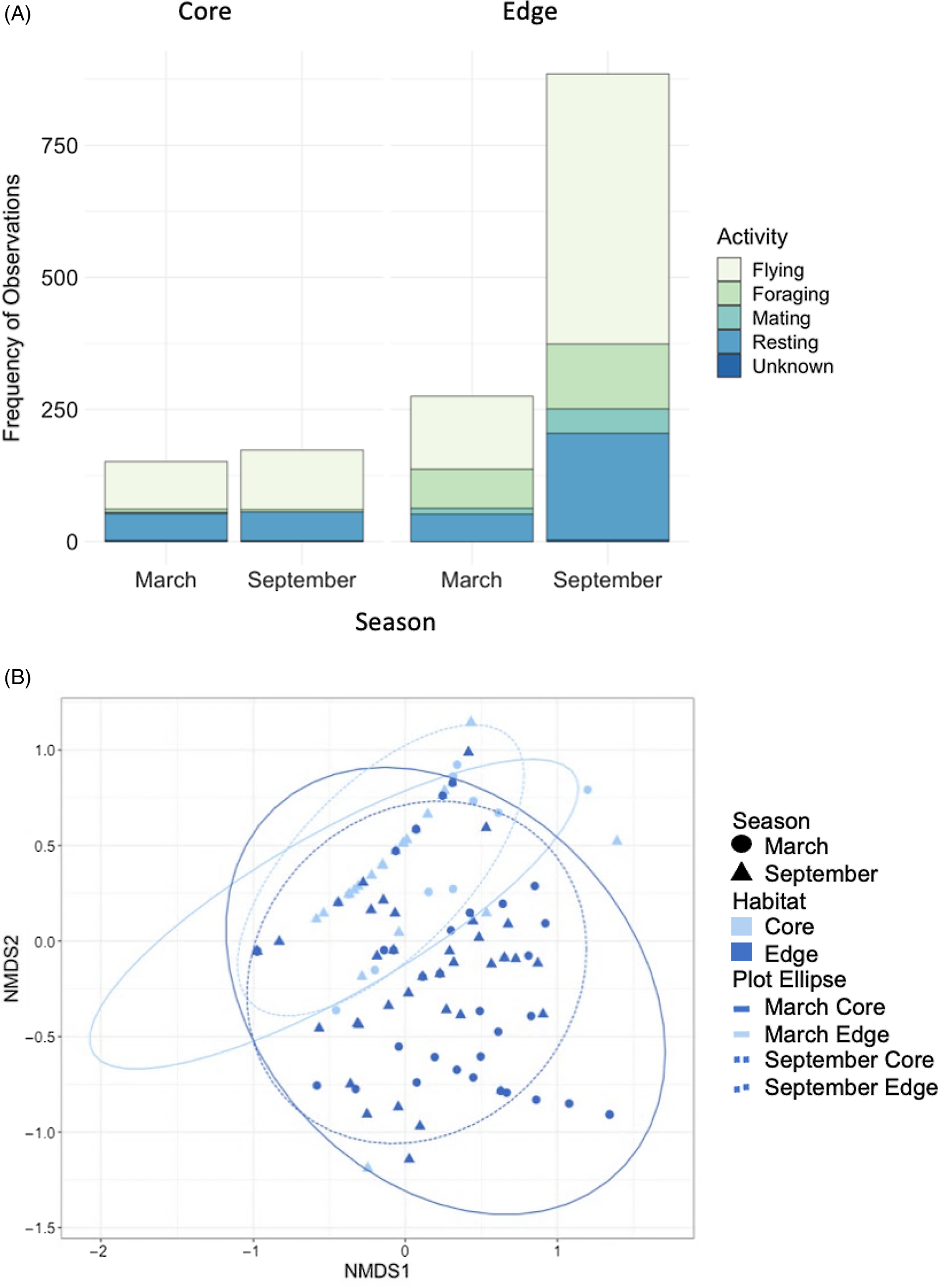
Figure 3. Bar charts showing the percentage of total observations by activity for the Lepidoptera community as a whole in Core and Edge habitats in both March and September seasons (A). Results of the non-metric multidimensional scaling, showing the effects of habitat and season on behaviour observed of butterflies and moths (B). Points are spherically grouped by site type (March Core, March Edge, September Core, September Edge), based on a 98% confidence interval.
The most frequently observed behaviours differed among the most abundant species (Figure 4A–F). Acraea terpsicore, Leptosia nina, and Amathusia spp. were nearly always recorded flying, while Tetragonous lycaenoides was most frequently recorded resting. Foraging was proportionally more common for Elymnias hypermnestra than other common species. Mating was not a commonly recorded behaviour for any species; however, when it was observed (for Ypthima spp, Elymnias hypermnesta, Acraea terpsicore, and Leptosia nina), it occurred most frequently in September Edge sites.
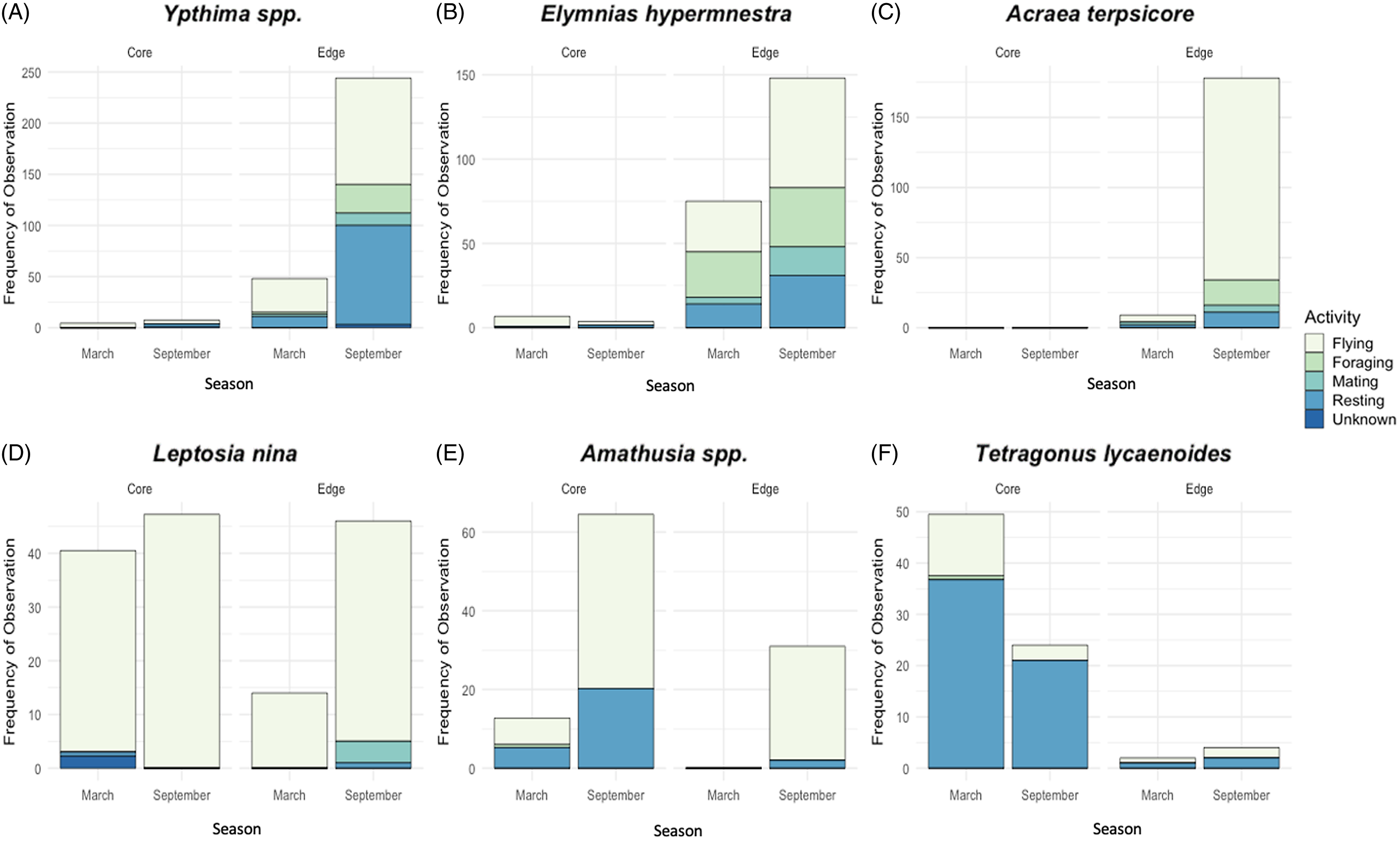
Figure 4. Bar charts showing the frequency of behaviour observed for the six most abundant species in Core and Edge habitats in March and September season (A–F): Ypthima spp., Elymnias hypermnestra, Acraea terpsicore, Leptosia nina, Amathusia spp., Tetragonous lycaenoides. Note: y-axis scales differ by species.
Both habitat and season had a significant effect on Ypthima spp. behaviour (Habitat: R = 0.23, p = 0.008; Season: R = 0.17, p = 0.002), with resting, foraging and mating behaviour being more common in September Edge sites. There was also a significant effect of season, but not habitat on Leptosia nina (Habitat: R = −0.03, p = 0.78; Season: R = 0.11, p = 0.008), Tetragonous lycaenoides (Habitat: R = 0.19, p = 0.07; Season: R = 0.08, p = 0.05), and Amathusia spp (Habitat: R = 0.03, p = 0.28; Season: R = 0.41, p > 0.001) behaviour, with differences varying between species (Figure 4). The behaviour of Acraea terpsicore was not significantly affected by either habitat or season (Habitat: NA as absent in Core habitats; Season: R = 0.15, p = 0.28).
Across species, the most commonly observed plants used for foraging were as follows: Asystasia gangetica subsp. micrantha flowers, Turnera ulmifolia flowers, and Melastoma affine fruit. In March, four out of a total of six observations were on Asystasia micrantha in Core areas, and eight out of a total of 80 observations were on Asystasia micrantha, 24 on Turnera ulmifolia, and 38 on Melastoma affine fruit in Edge areas. In September, as before, four out of a total of six observations were on Asystasia micrantha in Core areas, and 17 out of a total of 117 observations on Asystasia micrantha, 11 on Turnera ulmifolia (with a further three on Turnera subulata), and 36 on Melastoma affine fruit in Edge areas. In nearly all cases, individual butterflies feeding on Melastoma affine fruit were Elymnias hypermnestra (62 out of 74 cases) (Supplementary Information 4).
Discussion
In this study, we investigated the effects of microhabitat and season on butterfly and day-flying moth density and behaviour in industrial oil palm plantations. We found that oil palm plantations can support abundant Lepidoptera, with our surveys recording 1464 individuals from 41 species. We found a significantly higher density of Lepidoptera in Edge habitats, which were bordered on one side by plantation roads, than in Core habitats, located in the centre of plantation blocks surrounded on all sides by oil palm. While there was no significant difference between March and September surveys on overall butterfly density, there was an interaction between season and habitat, with density increasing more in September compared to March in Edge than Core areas. Of the six most abundant species, four showed a preference for either Edge or Core habitats, with Ypthima spp. and Elymnias hypermnestra being found at higher density in Edge, Leptosia nina and Tetragonas lycaenoides being found at a higher density in Core, and Acraea terpsicore and Amathusia spp. not differing between the two. The effects of season were similarly varied across species, with Amathusia sp being found at a higher density in September than in March, Ypthima spp. and Elymnias hypermnestra increasing more in September in Edge than Core habitats, Tetragonas lycaenoides being found at a higher density in March, and Acraea terpsicore not differing in density between seasons. The most common behaviour observed was flying in all plot types, and there was a significant effect of habitat and season on the overall behavioural time budget, with more active behaviours, such as foraging and mating, being recorded more commonly in Edge than Core habitats, particularly in September. As with density, individual species responses were varied, although season more commonly had an effect on behaviour than habitat, with the exception of Ypthima spp. where resting, foraging, and mating behaviour were again more common in Edge sites, particularly in September.
Lepidoptera community
Although we recorded a high abundance of Lepidoptera from over 40 species, this is a much lower number of species than would be expected from natural forest areas in the region (Hamer et al. Reference Hamer, Hill, Lace and Langan1997, Panjaitan et al. Reference Panjaitan, Drescher, Buchori, Peggie, Harahap, Scheu and Hidayat2020). In addition, we found no endemic or forest specialist species in our study, which emphasises the importance of conserving remaining forested areas for conservation of global Lepidoptera biodiversity. Over 60% of the butterflies we recorded were within the family Nymphalidae. This family contains many disturbance-tolerant species, which show a range of feeding strategies, broad habitat ranges, and high dispersal abilities (Armstrong, Reference Armstrong2010, Sousa et al. Reference Sousa, Sousa, Silva, Santos and Aranda2019, Uehara-Prado and Freitas, Reference Uehara-Prado and Freitas2006). For example, Ypthima spp. and Elymnias hypermnestra, by far the most common species in this study, are tolerant generalists capable of surviving in a wide range of disturbed habitats (Harmonis, Reference Harmonis2017). Ypthima spp. are particularly abundant in disturbed areas (Sing et al. Reference Sing, Jusoh, Hashim and Wilson2016), not unlike mature oil palm plantations, and Elymnias hypermnestra is commonly found in oil palm and coconut plantations in Southeast Asia (Khyade et al. Reference Khyade, Gaikwad and Vare2018). The success of such disturbance-tolerant species is likely to increase as agricultural land expands in the tropics (Laurance et al. Reference Laurance, Sayer and Cassman2014).
Effect of habitat on Lepidoptera density and behaviour
Edge habitats along roadsides had significantly, and sometimes substantially, higher Lepidoptera density than Core habitats in both seasons, and we recorded a greater proportion of active behaviours, such as foraging and mating, in Edge habitats. These findings indicate that the presence and behaviour of Lepidoptera are more heavily influenced by the habitat characteristics of a plantation than by seasonal conditions. It is likely that Edge conditions may have particularly benefitted the most abundant disturbance-tolerant species in our study, as seen in previous research (Hamer et al. Reference Hamer, Hill, Benedick, Mustaffa, Sherratt and Maryati2003; Willott et al. Reference Willott, Lim, Compton and Sutton2000). For example, the most frequent species we found in Edge habitats (Ypthtima spp.) is known to be most prevalent along forest edges, roads, and riverbanks (Laurance et al. Reference Laurance, Sayer and Cassman2014), and our results suggest that plantation roads may be behaving similarly to forest gaps (Hill et al. Reference Hill, Hamer, Tangah and Dawood2001). Edge and Core habitats differ in abiotic factors, such as greater sun and wind exposure in Edge, and in biotic factors, such as vegetation characteristics, including floral resources (Luke et al. Reference Luke, Purnomo, Advento, Aryawan, Naim, Pikstein, Ps, Rambe, Soeprapto, Caliman, Snaddon, Foster and Turner2019). For example, Edge habitats have a greater degree of canopy openness (Luke et al. Reference Luke, Purnomo, Advento, Aryawan, Naim, Pikstein, Ps, Rambe, Soeprapto, Caliman, Snaddon, Foster and Turner2019), which has been found to positively influence butterfly presence in previous studies (Vu, Reference Vu2009). In addition, the lack of direct sun exposure in Core areas could have made this habitat less favourable for butterflies, which are poikilothermic, and explains the greater proportion of time spent resting, when temperature was not sufficient to support energetically demanding behaviour. In addition, Edge habitats have a more diverse environment than Core areas, especially associated with the ditches, with features such as rocks, mud, and water that were rarely present in Core areas. This heterogeneity may also have contributed to a denser Lepidoptera population in Edge habitats, as it provided a wider range of conditions for species and increased opportunities for diverse behaviours. For example, mudpuddling is a foraging behaviour where butterflies congregate around mud and damp surfaces to drink moisture, salt, and other nutrients. This additional sodium uptake supports healthy butterfly physiology and can increase reproductive success in some species, as males transfer the nutrients to females during mating, and therefore has broader population impacts (Sculley and Boggs, Reference Sculley and Boggs1996). This behaviour was recorded only once throughout the study in a ditch in Edge habitat. Our study indicates that the creation of Edge habitats may offer an opportunity for plantation managers to foster higher numbers of Lepidoptera in oil palm and potentially healthier agricultural landscapes and so should be considered when undertaking landscape planning.
Previous research at the site has also found that edge habitats have a higher richness and biomass of native, non-native, and beneficial plant species than Core habitats (Luke et al. Reference Luke, Purnomo, Advento, Aryawan, Naim, Pikstein, Ps, Rambe, Soeprapto, Caliman, Snaddon, Foster and Turner2019). Butterflies and moths use vegetation throughout their lifecycle, with vegetative material being a food source for larvae, nectar and fruit being a food source for adults, and plants being used for perching, resting and hiding from predators (Hahn and Brühl, Reference Hahn and Brühl2016). Therefore, increased vegetation cover is likely to support greater Lepidoptera presence and higher incidences of mating and foraging behaviours. The difference in plant species composition between habitats may attract different butterfly species at both adult and larval phases, as they support foraging and breeding activities. In particular, Edge habitats have a higher abundance of the flowering plant Asystasia micrantha, in English commonly known as the Chinese violet, which is a larval host plant for Doleschallia bisaltidae and Junonia orithya wallacii. The Chinese violet is also an important adult nectar source for several species of Hesperiidae, Nymphalidae, and Pieridae and was the most common nectar source we observed in our behavioural observations. At our study sites, 30% of observed vegetation along Edge sites was Turnera ulmifolia, which does not naturally occur in plantations, but was planted along road edges as an additional nectar source to support parasitoid pest control species (Turner and Hinsch, Reference Turner, Hinsch and Rival2018). This greater abundance of nectar sources is also likely to contribute to the higher density of butterflies in Edge habitats and the higher incidence of nectar feeding we recorded. Larvae of Acraea terpsicore, one of the most common butterfly species we observed, also feed on Turnera ulmifolia, further explaining the higher counts of this species in Edge habitats (Abdullah and Rahim, Reference Abdullah and Rahim2018). Borreria latifolia (Subedi et al. Reference Subedi, Stewart, Neupane, Ghimire and Adhikari2020), a known food source for Ypthima, spp. with significantly greater density in Edge habitats, had an average biomass over 90 times greater in Edge habitats than in Core habitats (10.89 g per transect, 0.12 g per transect, respectively) (Luke et al. Reference Luke, Purnomo, Advento, Aryawan, Naim, Pikstein, Ps, Rambe, Soeprapto, Caliman, Snaddon, Foster and Turner2019). Similarly, vegetation in the Poaceae family upon which Elymnias hypermnestra is known to feed (Kunte, Reference Kunte2000) was significantly more abundant in Edge habitats, mirroring the significant effect of habitat on this species’ density.
However, Core habitats remained valuable for some species. We found higher densities of Tetragonus lycaenoides and Leptosia nina in Core habitats, likely due to their preference for dense and shady habitats (Aluthwattha et al. Reference Aluthwattha, Harrison, Ranawana, Xu, Lai and Chen2017). The relationship between host-plant biomass and butterfly density was not always consistent; while both adult and larval food sources of Leptosa nina were higher in Edge habitats (Luke et al. Reference Luke, Purnomo, Advento, Aryawan, Naim, Pikstein, Ps, Rambe, Soeprapto, Caliman, Snaddon, Foster and Turner2019), Leptosa nina density was significantly higher in Core habitats. This indicates that, while the more complex Edge habitats may host a higher density of Lepidoptera, a mix of microhabitats is needed to support the widest possible range of Lepidoptera species within plantations.
Effect of season on Lepidoptera density and behaviour
We recorded an interaction between season and habitat type for total butterfly density, with density increasing more in Edge than Core areas in September than March. Indeed, we recorded more individuals in September Edge sites than in all other site types combined. This may be because the host-plant dynamics in Edge habitats vary more with season than in Core habitats, perhaps because the greater number of plant species and greater light exposure in Edge areas mean that plants can respond more to changing environmental conditions in this habitat, influencing butterfly density as a result. As the full larval host-plant information and adult feeding information for most species in this study is not available, it is difficult to determine whether composition is driven more heavily by overall habitat complexity or the presence of specific food plants and the interaction of these factors with season. However, the overall higher frequency of interacting and foraging behaviour we observed in Edge transects in September supports the hypothesis that increased resources for foraging and mating may be driving this pattern. Density between seasons varied across the six most abundant species, with Amathusia sp, Ypthima spp. and Elymnias hypermnestra, all increasing in one or more habitats in September, but Tetragonas lycaenoides being found at a higher density in March, and Acraea terpsicore not differing in density between seasons. Effects of season on behaviour were similarly varied. These differences are likely also to reflect species-specific habitat requirements and resource availability that are influenced by season. More work is needed to quantify seasonal differences in flowering patterns and host-plant dynamics for the most common Lepidoptera species in different months to clarify this, such as the work already carried out by Koh and Sodhi (Reference Koh and Sodhi2004), and Scriven et al. (Reference Scriven, Benedick, Beale and Hill2017) at other locations.
Our surveys were conducted at eye level, meaning canopy species were not included. However, as the most abundant families prefer the understory and bush (Schulze et al. Reference Schulze, Linsenmair and Fiedler2001), we maintain that our findings are representative of the true population of butterflies and day-flying moths. Our analysis focused on the most common species, together representing over 70% of the population, giving us a clear indication of the species that are abundant enough to play a functional role in the ecosystem. All common species we found were generalists, with only Amathusia spp. feeding on oil palm in its larval phase but failing to reach an abundance high enough (over five larvae per frond) to be considered a pest by plantation managers (Mariau and Biggins, Reference Mariau and Biggins2021). Our findings therefore indicate that management decisions to support higher Lepidoptera numbers may be enacted without threatening yield. However, the most impactful Lepidopteran oil palm herbivores are nocturnal moths, that were not sampled in this study, and therefore, the generality of this finding needs further research.
Conclusions and implications for management
Our study shows that oil palm plantations can support a high density of butterflies and day-flying moths, although the number of species is far lower than in native forests, further confirming the importance of protecting intact natural ecosystems (Koh and Wilcove, Reference Koh and Wilcove2008, Kwatrina and Santosa, Reference Kwatrina and Santosa2019). However, the assemblage present in oil palm plantations underscores the potential of agricultural land to host robust populations of Lepidoptera, in particular disturbance-tolerant species. As butterflies are an indicator taxon of environmental health (Winarni et al. Reference Winarni, Dwiyahreni, Hartingtias, Sunaryo and Supriatna2020), this result may be indicative of the wider invertebrate community.
At a local scale, management practices may be manipulated to support more heterogenous plant communities and boost important resources, such as pollen and nectar, that may support a higher abundance of arthropods, including Lepidoptera and other flower-visiting species, including predatory and parasitic arthropods. Naturally occurring plant species such as Asystasia micrantha provided nectar sources in both Core and Edge habitats and, when allowed to flower under reduced clearing management regimes, may therefore support higher butterfly density and activity. Artificially planted Turnera ulmifolia also acted as an important nectar source in Edge habitats, suggesting that plantation managers may be able to carry out plantation management practices, such as targeted planting, which both positively affect the oil palm yield and foster healthy invertebrate biodiversity. Preference for Edge or Core habitats varied among the six most common species, highlighting the importance of having a range of both open and closed habitats in plantations. However, the higher density of all Lepidoptera and higher incidence of mating behaviours in Edge habitats indicates that including these areas in plantation design may increase Lepidoptera populations in plantations. To accurately determine management metrics such as the optimal ratio of open to closed habitats, distance to road or opening, and shape of intermittent structures, further research is needed on butterfly dispersal ability (Lucey and Hill, Reference Lucey and Hill2012) and response to a gradient of habitat heterogeneity (Kwatrina and Santosa, Reference Kwatrina and Santosa2019), while taking into account any impacts on yield.
Increasing butterfly presence in the plantations could have larger knock-on effects through their role in the ecosystem as herbivores, pollinators, and prey species. With a consideration for both conservation and production priorities, our findings are relevant to biodiversity-friendly management practices and may be useful to inform industry sustainability guidelines from organisations such as the Roundtable on Sustainable Palm Oil (2018).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467423000111
Acknowledgements
We thank RISTEK for permission to establish the BEFTA Understory Vegetation Project and to conduct research in Indonesia (permit numbers 426/SIP/FRP/SM/XI/2012, 72/EXT/ SIP/FRP/SM/IX/2013, 44/EXT/SIP/FRP/SM/IX/2014, 354/SIP/FRP/E5/Dit.KI/X/2016, 66/EXT/SIP/FRP/E5/Dit.KI/IX/2017,45/EXT/SIP/FRP/E5/Dit.KI/X/2018,431/E5/E5.4/SIP/2019, 53/E5/E5.4/SIP.EXT/2020, and 1/SIP.EXT/IV/FR/1/2022). We thank Pt Ivo Mas Tunggal and Golden Agri Resources, and Sinar Mas Agro Resources Technology Research Institute (SMARTRI) for allowing us to conduct research in their plantations, and we are grateful to the staff of SMARTRI for their help with fieldwork and support. This work was funded by The Isaac Newton Trust Cambridge, The Gates Cambridge Trust, Golden Agri Resources, and the Natural Environment Research Council [grant number NE/P00458X/1].
Author contributions
V.R.W led statistical analyses and writing of the manuscript, with S.H.L and E.C.T; J-P.C, J.S., W.A.F, S.P., and E.C.T designed the study; A.A.K.A., M.N., P., D.P., So, Su, R.S.T., R.W., T.D.S.R., and R.H.W. contributed to the sampling design and experimental protocols; A.D.A and E.C.T collected field data; V.R.W., A.D.A., and E.C.T identified and catalogued specimens. All authors approved the manuscript.
Financial support
Natural Environment Research Council, Grant/Award Number: NE/P00458X/1; The Gates Cambridge Trust; The Isaac Newton Trust Cambridge; Golden Agri Resources (GAR)
Competing interest
Co-authors with a Sinar Mas Agro Resources and Technology Research Institute (SMARTRI) affiliation were employed by SMARTRI, the research division of Golden Agri Resources (GAR), while research was conducted. SMARTRI and the University of Cambridge shared a Collaboration Agreement for this study that protects the intellectual property rights and data use of all researchers involved in this study. This research is therefore a full collaboration between the University of Cambridge and GAR.









