Abstract
Diabetes mellitus type 2 (DM2) is the most common chronic disease worldwide, characterized mainly by increased glucose concentration in the blood and affecting several organs’ functionality. The daily consumption of probiotic bacteria can help control diabetes and reduce the damage caused. Cell immobilization techniques are a powerful tool that provides physical cell protection to such probiotic bacteria against gastrointestinal conditions. We suggest that cell immobilization could be a significant vector for delivering a high quantity of viable probiotics to the gut, helping attenuate hyperglycemia in diabetic rats. Seventy male Wistar rats were used in this work. Nicotinamide was administrated via intraperitoneal injection 15 minutes before inducing type 2 diabetes (DM2), followed by a second intraperitoneal injection of streptozotocin to induce DM2. Rats were divided into seven groups. For 45 days, a specific treatment was applied to each group. The group of rats, supplied with immobilized Lactobacillus casei, showed a serum glucose concentration of 137 mg/dL, which was close to the one observed in the groups of healthy rats (117 mg/dL) and rats treated with metformin (155 mg/dL). The diabetic rats without treatment presented a higher serum glucose concentration (461 mg/dL). In the rats treated with immobilized L. casei, there was no biochemical parameter alteration, and the cell morphology of the analyzed tissues was similar to those of the healthy group. The consumption of immobilized L. casei could allow a high quantity of viable probiotics to be delivered to the gut, reducing serum glucose concentration by up to 70% compared to diabetic rats and reducing organ damage caused by diabetes.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a degenerative metabolic disorder, representing one of the leading causes of mortality and morbidity worldwide. By 2045, about 578 million people worldwide will suffer from this disease [1, 2]. The development of DM2 has been linked to an intestinal microbiota imbalance; apparently, some intestinal pathogenic microorganisms trigger inflammatory processes that reduce insulin sensitivity and stimulate the immune response that destroys the β-cells in the pancreas [3,4,5,6,7,8,9]. An alternative to restoring the intestinal microbiota is the consumption of probiotics from the genera Lactobacillus and Bifidobacterium. When ingested in adequate quantities, these bacteria benefit the host; probiotics compete with pathogenic microorganisms for adhesion sites and nutrients in the colon. However, they must remain viable during transit in the gastrointestinal system [10,11,12]. Cell immobilization techniques are a powerful tool that provides physical cell protection against digestive conditions [13,14,15]. Therefore, this study aims to demonstrate the effect of immobilized cells of Lactobacillus casei subsp. casei, which was used as a diet complement in diabetic rats. We suggest that cell immobilization could be a significant vector for delivering a high quantity of viable probiotics to the gut, which could help attenuate the DM2 complications.
Materials and Methods
Culture of Lactobacillus casei and Obtention of Free and Immobilized Cells
Lactobacillus. casei subsp. casei NRRL-1922 (L. casei) was donated by the Department of Agriculture of the USA. The strain was cultivated in MRS medium for 24 h at 37 °C and 120 rpm; the culture was then centrifuged at 6000 rpm for 10 min at 4 °C; the biomass concentration recovered was 1012 CFU/100 μL, which was suspended in phosphate-buffered saline (PBS). The cell immobilization procedure was carried out under aseptic conditions, as follows: The biomass from a liquid culture of L. casei previously incubated for 24 h at 37 °C was recovered by centrifugation at 6000 rpm for 10 min at 4 °C. The biomass was suspended in a 2% sodium alginate solution (Sigma-Aldrich). Subsequently, using a peristaltic pump adapted with an injector hose, the L. casei cell suspension in sodium alginate was added dropwise with gentle agitation into a CaCl2 solution (0.3 M). Each drop of the L. casei cell suspension in sodium alginate formed a microparticle of calcium alginate gel upon contact with the CaCl2 solution [16]. Spherical calcium alginate microparticles with a diameter of 2.1 ± 0.08 mm containing 1012 CFU/100 μL gel of L. casei were obtained from the cell immobilization process. Once the microparticles were formed, they were quantified, and it was established that 100 μL of sodium alginate containing the biomass formed 12 spherical microparticles containing a total of 1012 CFU of L. casei.
Evaluation of the Viability Loss of Lactobacillus casei Under In Vitro Simulated Gastrointestinal Conditions
Free and immobilized cells of L. casei were subjected to in vitro simulated physicochemical conditions of the stomach and small intestine; the composition of the digestive juices was set as follows:
In the stomach stage (100 mL): PBS (80 mL), mucin (4 g/L), pepsin (3 g/L), and cow’s milk (20 mL); the pH was adjusted to 2 by the addition of 5 M HCl; the duration of this stage was 90 min.
In the small intestine stage (100 mL): PBS (100 mL), pancreatin (1 g/L), hepatic bile (3 g/L), and mucin (4 g/L), the pH was adjusted to 6.8 by adding 1.5 N NaOH; the duration of this stage was 150 min. During the kinetics of viability loss, samples of 1 mL were taken every 30 min, either free or immobilized cells, to quantify, through dilutions, the number of viable cells in Petri dishes containing MRS culture media and agar, which were incubated for 48 h at 37 ºC. The gastrointestinal simulation was performed nine times.
Streptozotocin-Nicotinamide-Induced Type 2 Diabetes Mellitus in Rats
We used adult male Wistar rats of 8 weeks and 250 ± 50 g of weight; rodents were purchased from the FES Iztacala-UNAM. All rats were maintained under standard conditions of temperature (22–23 °C), light-dark cycles of 12 h/12 h, conventional food (LabDiet 500I) (Table 1), and purified water ad libitum. The experiments were done under the Official Mexican Standards (NOM-062-ZOO-1996 [17] and NOM-087-SEMARNAT-SSA1-2002 [18]). The National School of Medicine and Homeopathy Ethics Committee of the National Polytechnic Institute of Mexico verified that the study complied with international regulations and standards (approval number: CBE/024/2019).
To induce type 2 diabetes, the rats were deprived of food and water for 12 h. An intraperitoneal injection of nicotinamide (150 mg/kg) (Sigma-Aldrich) was administered to the rats 15 min before inducing type 2 diabetes. After this period, a second intraperitoneal injection of streptozotocin (50 mg/kg) (Sigma-Aldrich) was given [19]. After 72 h, the blood glucose concentration was measured with a glucometer (Optium Xceed); the rats were considered diabetic when the blood glucose concentration was higher than 125 mg/dL. Then, the rats were randomly divided into seven groups to evaluate the antihyperglycemic effect, each of 10 animals. The groups were as follows: group healthy control (H); group diabetic control (D); group diabetic treated daily with the first vehicle (300 μL PBS/day) (DPBS); group diabetic treated daily with a second vehicle (12 microparticles of calcium alginate plus 200 μL PBS/day) (DPBSA); group diabetic treated daily with metformin (100 mg/kg) (DMET); group diabetic treated daily with free cells of L. casei (1012 CFU in 300 μL PBS/day) using a gastric tube (DFLC); finally, group diabetic treated daily with immobilized cells of L. casei (12 calcium alginate microparticles containing a total of 1012 CFU in 200 μL PBS/day) using a gastric tube (DILC). After 45 days of treatment and food deprivation for 12 h, the rats were euthanized by CO2-induced asphyxia; then, blood, liver, kidney, large intestine, and pancreas samples were collected from the animals.
Biochemical Analyses
A blood sample was taken through a cardiac puncture with a syringe; the blood was centrifuged at 570 × g for 10 min at 4 °C and processed in the San Rafael Laboratory (Mexico City) in Autokem 11-KONTROLab equipment. The glucose concentration was determined. Also, a hepatic profile was performed. The direct, indirect, and total bilirubin were evaluated. The aspartate aminotransferase (AST), the alanine aminotransferase (ALT), and the alkaline phosphatase (ALP) were determined. On the other hand, lipidic profiles (cholesterol (Cl), triglycerides (Tg), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low lipoprotein (VLDL)) and renal profiles (urea (Ur) and creatinine (Cr)) were determined as well. Each parameter was evaluated in triplicate.
Histopathological Analysis
After dissecting the animals, small sections of the different organs, such as the liver, kidney, large intestine, and pancreas, were placed in 10% formaldehyde in PBS solution. Subsequently, samples of 4–6 µm were prepared, stained with hematoxylin-eosin (H&E), and examined by a microscope (×400). The histological samples obtained from all the experimental groups of rats were observed by the microscope set in 5 different sections in triplicate.
Statistical Analysis
Data statistical analysis was performed in GraphPad PRISM v8.0a software. A t-student analysis was carried out for the kinetics of the viability loss of L. casei. A one-way analysis of variance (ANOVA) was performed, and a post hoc Bonferroni test (p ≤ 0.05) was accomplished when there were significant statistical differences from the evaluation of the antihyperglycemic effect.
Results and Discussion
Kinetics of Viability Loss of Free and Immobilized Lactobacillus casei Under In Vitro Simulated Physicochemical Conditions of the Stomach and Small Intestine
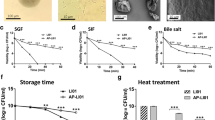
Free and immobilized cells of L. casei were subjected for 90 min to in vitro simulated stomach conditions and then subjected to 150 min to in vitro simulated physicochemical small bowel conditions. The results obtained are shown in Fig. 1.
At the beginning of the kinetics, the free and immobilized L. casei concentration was 12.55 ± 0.04 log10 CFU/mL and 12.25 ± 0.03 log10 CFU/mL of alginate gel, respectively, which represented 100% of viability. At the end of the stomach stage, it was observed that the viability percentage decreased in both cases. There were 22.57% (11.90 ± 0.11 log10 CFU/mL) of free viable cells and 80.83% (12.16 ± 0.01 log10 CFU/mL of alginate gel) of immobilized viable cells. At the end of the small intestine stage, only 2.1% of free cells (10.87 ± 0.01 log10 CFU/mL) and 51.9% of immobilized cells (11.97 ± 0.002 log10 CFU/mL alginate gel) remained viable. While at the end of the viability kinetics, the concentration of L. casei viable cells presented statistically significant differences between both treatments.
Evaluation of the Antihyperglycemic Effect of Free and Immobilized Lactobacillus casei in Diabetic Rats
Table 2 shows that after 45 days of treatment, the serum glucose concentration in the DILC group was similar to that of the H and DMET groups. Conversely, the serum glucose concentration was much higher in the D, DPBS, DPBSA, and DFLC groups with significant statistical differences. It is important to note that the group treated with immobilized L. casei (DILC group) showed a 67.8% reduction in serum glucose concentration compared to the group treated with free L. casei (DFLC group).
Hepatic, Lipidic, and Renal Profiles
Hepatic Profile
Table 3 shows the data obtained from the hepatic profile. Direct bilirubin presented normal values in groups H and DILC; on the contrary, it increased in groups D, DPBS, DPBSA, DMET, and DFLC. Regarding indirect bilirubin, the H group presented normal values; in contrast, it increased in the groups D, DPBS, DPBSA, DMET, DFLC, and DILC. It is important to note that the increase in indirect bilirubin was less steep in the DILC group. As for total bilirubin, it was found to be normal in the H and DILC groups; in contrast, it increased in the D, DPBS, DPBSA, DMET, and DFLC groups. On the other hand, AST enzyme activity was found to be normal in groups H, DPBS, DMET, and DILC. Particularly, there was an increase in groups D, DPBSA, and DFLC, showing significant statistical differences. ALT enzyme activity was slightly increased in groups H and DILC, while in groups D, DPBS, DPBSA, DMET, and DFLC, enzyme activity was higher, showing significant statistical differences. Finally, the enzymatic activity of ALP showed normal values in groups H and DILC; on the contrary, it increased in groups D, DPBS, DPBSA, and DFLC, showing significant statistical differences.
Lipidic Profile
Table 4 shows the data obtained from the lipid profile analysis. Total cholesterol concentration was found to be normal in groups H, DPBS, DPBSA, and DMET, while there was a slight increase in groups D, DFLC, and DILC. In particular, HDL showed similar concentrations in groups H and D; however, in groups DPBS, DPBSA, DMET, DFLC, and DILC, there was a slight decrease. Regarding LDL, the H, D, DPBS, and DPBSA groups showed very similar concentrations; on the contrary, they increased in the DMET, DFLC, and DILC groups. VLDL showed similar values in the H, DPBS, DMET, and DILC groups. On the other hand, the concentration of VLDL increased in the D, DPBSA, and DFLC groups, with statistically significant differences observed in the DFLC group only. Finally, Tg concentrations were found in similar and normal values in the H, DMET, and DILC groups; they increased in the D, DPBS, DPBSA, and DFLC groups, presenting significant statistical differences.
Renal Profile
Table 5 reports the data obtained from the renal profile analysis. Urea concentration showed normal values in the H, D, DMET, DFLC, and DILC groups; however, in the DPBS and DPBSA groups, it increased, presenting significant statistical differences. All groups showed normal values regarding creatinine; however, only in the DPBS group was there an increase, showing statistically significant differences.
Histological Analysis of Liver, Pancreas, Kidney, and Large Intestine
A histopathological examination was performed to demonstrate the effect of the consumption of free and immobilized L. casei in reducing the organ damage caused by diabetes in rats. Micrographs of the liver, pancreas, kidney, and large intestine were analyzed for the different experimental groups. For instance, Fig. 2 shows micrographs of liver histological sections, showing the central vein, sinusoid, and hepatocytes. In group H, the typical morphology of the liver was observed, while in groups D, DPBS, DPBSA, DMET, and DFLC, there was an increase in the size of the sinusoids; on the contrary, in group DILC, the tissue morphology was very similar to that observed in group H.
Figure 3 provides micrographs of kidney histological sections where the renal corpuscle, the medullary ray, the proximal convoluted tubule, and the capsule can be observed. In group H, the typical morphology of the kidney was observed. On the other hand, in groups D, DPBS, DPBSA, DMET, and DFLC, deformation of the corpuscles and an increase in the size of the capsule can be seen; on the contrary, in group DILC, the tissue morphology was very similar to that observed in group H.
Figure 4 represents micrographs of pancreas histological sections showing the islet of Langerhans, the serous acini, and the intralobular duct. Group H presented a typical pancreas morphology, while groups D, DPBS, DPBSA, and DMET showed small islets and were even broken, so it was difficult to observe them. The islets in the DFLC and DILC groups were similar to those observed in group H.
Finally, Fig. 5 shows micrographs of large intestine histological sections. The mucosa, which comprises the lamina propria, the crypt of Lieberkühn, the goblet cells, and the muscularis mucosae, can be observed. In group H, the typical morphology of the large intestine was observed, while in groups D, DPBS, DPBSA, and DMET, there was deformation in the upper part of the crypts and some disintegration. On the other hand, the DFLC and DILC groups presented tissue morphology similar to that observed in group H.
Discussion
A crucial fact for probiotic bacteria to provide health benefits to the host is their viability at the end of their passage through the digestive system [15, 22, 23]. The cell immobilization approach represents one way to mitigate the damage caused by gastrointestinal physicochemical conditions while preventing the losses of cell viability [13, 14, 22,23,24,25]. According to the results, after the in vitro simulation of the gastrointestinal conditions (CGI), the immobilized L. casei viable bacteria count was higher than the free form. The recommended daily intake of probiotics is at least 10 log10 CFU/mL to 12 log10 CFU/mL [26]; thus, cell immobilization meets the recommended probiotic dose. Similar results have been reported using free and immobilized L. delbrueckii, L. acidophilus, L. johnsonii, L. casei Shirota, and L. rhamnosus [23, 27, 28].
As for dysbiosis, it is defined as an imbalance of the intestinal microbiota, i.e., an increased number of pathogenic microorganisms in the intestines triggers biological processes, which potentiate the development and severity of diabetes [5, 7,8,9]. In this study, the rat’s intestinal microbiota was selectively modified by consuming free and immobilized L. casei. The results demonstrate that the treatments with L. casei reduce serum glucose concentration in diabetic rats. However, the decrease in serum glucose levels in diabetic rats treated with free L. casei (DFLC group) was only 7.5% compared to the diabetic group (D group), which was not significant; in similar studies carried out with various probiotic strains in free form, the reduction in serum glucose was found to be between 14.8 and 45.4% [29,30,31,32,33,34,35,36,37,38,39]. The serum glucose reduction in the group treated with immobilized L. casei (DILC group) was 70.3% compared to the diabetic group (D group). This reduction was significantly higher compared to the treatment with free L. casei (DFLC group) and exceeded the outcomes reported in previous studies involving free-form probiotics [3, 30,31,32,33,34,35,36,37,38,39]. L. casei immobilized showed similar serum glucose concentrations to the healthy group; we associate this behavior to cell immobilization with sodium alginate, as it provides physical protection to the probiotics [23, 24, 40] so that L. casei supported the CGI of the rats’ organism. A greater number of viable bacteria effectively carried out their beneficial actions, contributing to the mitigation of hyperglycemia.
To the best of our knowledge, no previous studies have explored the utilization of immobilized probiotics as a supplement for treating diabetes. Therefore, this research represents, for the first time, a pioneering effort to investigate and address this approach. The findings establish that cell immobilization serves as an important vector to protect probiotics from gastrointestinal challenges, enabling them to deliver their therapeutic benefits effectively.
To some extent, the right mechanism declaring probiotic effects in preventing and treating diabetes is unknown so far [41, 42]. However, it has been speculated that probiotic bacteria avoid synthesizing nitric oxide, which causes the formation of free radicals that lead to the deterioration of pancreatic β-cells [41, 43, 44]. On the other hand, Gram-negative bacteria membrane contains lipopolysaccharides (LPS) related to the production of endotoxins capable of crossing the intestinal barrier, causing inflammation and deterioration of the pancreas, liver, and kidney [45]. The consumption of probiotics balances the intestinal microbiota, inhibiting the proliferation of Gram-negative bacteria and reducing the permeability of the intestine [6, 45,46,47,48,49,50]. Probiotics also produce short-chain fatty acids (SCFA), such as propionate, acetate, and butyrate [3, 25, 30, 44, 45, 51,52,53], in which the latter compound interacts with intestinal L cells, leading to the overexpression of glucagon-like peptide (GLP-1), which is responsible for stimulating insulin secretion in the pancreas [6, 43, 45, 47, 51, 54,55,56,57]. Knowing that there is still some insulin production in DM2, it is likely that this mechanism occurred in our study.
When evaluating the liver profile, bilirubin concentration along with AST, ALT, and ALP enzyme activities, as reported in Table 2, the group treated with free L. casei (DFLC group) showed higher values than the group treated with immobilized L. casei (DILC group); other authors reported similar results when using probiotics in the free form [31, 33, 39]. This behavior is expected since diabetes is related to liver disease [58, 59]. Moreover, it can be affirmed that liver damage is indirectly reduced by enriching the diet with immobilized L. casei.
Concerning the lipid profile, the groups of rats treated with free and immobilized L. casei showed changes in lipid metabolism; this behavior has been already observed by several authors [3, 30,31,32, 37, 39, 43, 48, 60,61,62]. For instance, these results could be attributed to a metabolic disorder characteristic of diabetes known as dyslipidemia, caused by increased free fatty acids in the body, insulin resistance, and an increase in inflammatory adiposity [63].
We believe that the increase in urea concentration in the group treated with free L. casei was due to the high serum glucose concentration leading to the onset of renal damage; other authors have described similar behaviors using free probiotics [31, 32].
Although the group of rats treated with free L. casei did not show statistically significant differences in certain evaluated parameters, the results differed from the typical values. An opposite fact was observed in the group treated with immobilized L. casei, where only cholesterol was slightly increased. Although there are no reports of similar studies using immobilized probiotics, the data show that treatment with immobilized L. casei was more efficient than treatment with free L. casei.
As observed in the histopathological analysis, the microscopic morphology of the kidney, liver, pancreas, and large intestine of the DILC group showed no visual differences compared to the H group; similar behaviors have been reported in various studies using free probiotics [37]; however, in our work, the treatment with immobilized probiotics significantly reduced organ damage. In the micrographs of the pancreas, no differences were observed between the DFLC and DILC groups. However, according to the CGI simulations, the viability of free L. casei was strongly affected; for this reason, although the islet was observed, there was no significant reduction in the serum glucose concentration.
Conclusions
The results obtained in this work demonstrate that cell immobilization is an important vector to provide physical protection for probiotics, such as L. casei, from simulated gastrointestinal conditions in vitro, and quite possibly in vivo. Interestingly, the consumption of immobilized L. casei could allow delivering a high quantity of viable probiotics into the gut, reducing serum glucose concentration by up to 70% compared to diabetic rats and reducing organ damage caused by diabetes. The results also suggest that the consumption of immobilized L. casei may help control and treat DM2 by reducing glucose concentration, maintaining biochemical parameters at nominal values, and reducing the damage that DM2 generally causes to organs. However, further investigation into the mechanism of action needs to be addressed. To some extent, all these findings provide a basis for future clinical trials.
Data Availability
All data analyzed in this paper are already included herein. Any extra data of interest are available from the corresponding author on reasonable request.
References
IDF diabetes atlas 9th edition. Available online: https://diabetesatlas.org/en/resources/. Accessed 18 Jul 2022
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res and Clin Pract 87:4–14. https://doi.org/10.1016/j.diabres.2009.10.007
Khalid RA, Mathews SS, Büsselberg D (2023) The influence of gut microbial species on diabetes mellitus. Int J Mol Sci 24:8118. https://doi.org/10.3390/ijms24098118
Bora G, Gunna A, Kumar MM, Morya S, Godswill AC, Menaa F (2023) Gut microbiota and chronic diseases: role of probiotics. J Appl Nat Sci 15(2):692–703. https://doi.org/10.31018/jans.v15i2.4540
Houghton D, Hardy T, Stewart C, Errington L, Day CP, Trenell M et al (2018) Systematic review assessing the effectiveness of dietary intervention on gut microbiota in adults with type 2 diabetes. Diabetologia 61:1700–1711. https://doi.org/10.1007/s00125-018-4632-0
Sharma P, Bhardwaj P, Singh R (2016) Administration of Lactobacillus casei and Bifidobacterium bifidum ameliorated hyperglycemia, dyslipidemia, and oxidative stress in diabetic rats. Int J Prev Med 7:102. https://doi.org/10.4103/2008-7802.188870
Blandino G, Inturri R, Lazzara F, Di Rosa M, Malaguarnera L (2016) Impact of gut microbiota on diabetes mellitus. Diabetes Metab 1–13. https://doi.org/10.1016/j.diabet.2016.04.004.
Moghadam FN, Sedighi M, Khamseh ME, Shahmiri FA, Telebi M, Razavi S et al (2017) The association of type 2 diabetes with microbiota composition. Microb Pathog 10:630–636. https://doi.org/10.1016/j.micpath.2017.07.034
Sircana A, Framarin L, Leone N, Berrutti M, Catellino F, Parente R et al (2018) Altered gut microbiota in type 2 diabetes; just a coincidence? Curr DiabRep 18:1–11. https://doi.org/10.1007/s11892-018-1057-6
Zmora N, Zilberman G, Suez J, Mor U, Dori M, Bashiardes S et al (2018) Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 174:1388–1405. https://doi.org/10.1016/j.cell.2018.08.041
Sanders ME, Merenstein D, Marrifield CA, Hutkins R (2018) Probiotics for human use Nutr Bull 43:212–225. https://doi.org/10.1111/nbu.12334
Markowiak P, Slizewska K (2017) Effect of probiotics, prebiotics, and symbiotics on human health. Nutrients 9:1–30. https://doi.org/10.3390/nu9091021
Teixeira P, Martins L, Ragagnin C, Bona C, Hildebrand H, Oliveira B, Heldt M et al (2015) Microencapsulation of probiotics by spray drying evaluation of survival in simulated gastrointestinal conditions and availability under different storage temperatures. Cien Rural 45(7):1342–1347. https://doi.org/10.1590/0103-8478cr20140211
Rather SA, Akhter R, Masoodi FA, Gani A, Wani SM (2017) Effect of double alginate microencapsulation on in vitro digestibility and thermal tolerance of Lactobacillus plantarum NCDC201 and L. casei NCDC297. Food Technol 83:50–8. https://doi.org/10.1016/j.lwt.2017.04.036
Yao M, Wu J, Li B, Xiao H, McClements DJ, Li L (2017) Microencapsulation of Lactobacillus salivarious Li01 for enhanced storage viability and target delivery to gut microbiota. Food Hydrocoll 72:228–236. https://doi.org/10.1016/j.foodhyd.2017.05.033
Mendoza-Madrigal AG, Duran-Paramo E, del Toro GV, Chanona-Pérez JJ, Martínez-Ramírez OC, Arzate-Vázquez I (2017) Viability kinetics of free and immobilized Bifidobacterium bifidum in prensence of food samples under gastrointestinal in vitro conditions. Rev Mex Ing Quim 16(1):159–168
Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio Norma Oficial Mexicana NOM-062-ZOO-1999. Diario Oficial de la Federación, 22 de agosto de 2001. IOP Publishing GOB-MX. https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf. Accessed 23 Sep 2023
Protección ambiental-salud, ambiental-residuos peligroso, biológico-infecciosos-calcificación y especificaciones de manejo. Norma Oficial Mexicana NOM-087-ECOL-SSA1-2002. Diario Oficial de la Federación, 1 de noviembre de 2001. IOP Publishing COFEPRIS. https://dof.gob.mx/nota_detalle.php?codigo=704675&fecha=17/02/2003#gsc.tab=0. Accessed 23 Sep 2023.
Szkudelski T (2012) Streptozotocin-nicotinamide-induce diabetes in the rat. Characteristics of the experimental model EMB 273:481–490. https://doi.org/10.1258/ebm.2012.011372
Canadian Council on Animale Care Conseil Canadien de Protection Des Animaux (CCACCCPA). Available online: https://ccac.ca/Documents/Standards/Guidelines/Spanish/ANEX05.pdf. Accessed 18 Jul 2022
Oc.lm.ehu. Available online: http://www.oc.lm.ehu.es/Fundamentos/doctorado/cursos/CirExp/Tecnicas/F-044.pdf. Accessed 18 Jul 2022
Casarotti N, Todorov D, Penna B (2015) Effect of different matrices on probiotic resistance to in vitro simulated gastrointestinal conditions. Int J Dairy Technol 68(4):595–601. https://doi.org/10.1111/1471-0307.12215
Huang H, Tang Y, King V, Chou J, Tsen J (2015) Properties of Lactobacillus reuteri chitosan calcium-alginate encapsulation under simulated gastrointestinal conditions. Int Microbiol 18:61–69. https://doi.org/10.2436/20.1501.01.235
Mohammed M (2015) Effect of some traditional Saudi Arabian meals on the survival of probiotic bacteria in fermented milk under in vitro simulated gastrointestinal conditions. Biotechnology 14(6):260–266. https://doi.org/10.3923/biotech.2015.260.266
Reque PM, Brandelli A (2021) Encapsulation of probiotics and nutraceuticals: applications in functional food industry. Trends Food Sci Technol 114:1–10. https://doi.org/10.1016/j.tifs.2021.05.022
Fung W, Lye H, Lim T, Kuan C, Liong M (2011) Roles of probiotic on gut health. Probiotics, biology, genetics and health aspects, 1st edn. Sptringer, Germany, pp 88, 140–160
Martins IB, Deliza R, Dos Santos KM, Walter EH, Matins JM, Rosenthal A (2018) Viability of probiotics in goat cheese during storage and under simulated gastrointestinal conditions. Food Bioprocess Tech 11:853–863. https://doi.org/10.1007/s11947-018-2060-2
Aziz K, Haseeb A, Naseem H, Tariq M (2019) Lactobacillus fermentum strains of dairy-product origin adhere to mucin and survive digestive juice. J Med Microbiol 68:1771–1786. https://doi.org/10.1099/jmm.0.001090
Hendricks AA, Vella CA, New DD, Aunjum A, Antush M, Geidl R et al (2023) High-resolution taxonomic characterization reveals novel human microbial strains with potential as risk factors and probiotics for prediabetes and type 2 diabetes. Microorganisms 11:758. https://doi.org/10.3390/microorganisms11030758
Wulandari W, Yulianto WA, Pujimulyani D (2023) Probiotic potential of the Indonesian local variety of fermented parboiled rice (tape) improved the metabolic syndrome of diabetic rats. Food Res 7(2):96.106. https://doi.org/10.26656/fr.2017.7(2).845
Farida E, Nuraida L, Giriwono P, Jennie B (2020) Lactobacillus rhamnosus reduces blood glucose level through downregulation of gluconeogenesis gene expression in streptozotocin-induced diabetic rats. Int J Food Sci 6108575. https://doi.org/10.1155/2020/6108575
Toejing P, Khat-Udomkiri N, Intakhad J, Sirilun S, Chaiyasut C, Lailerd N (2020) Putative mechanisms responsible for the antihyperglycemic action of Lactobacillus paracasei HII01 in experimental type 2 diabetic rats. Nutrients 12:3015. https://doi.org/10.3390/nu12103015
Mihailović M, Živković M, Jovanović JA, Tolinački M, Sinadinović M, Rajić J et al (2017) Oral administration of probiotic Lactobacillus paraplantarum BGCG11attenuates diabetes-induced liver and kidney damage in rats. J Funct 38:427–437. https://doi.org/10.1016/j.jff.2017.09.0033
Wang G, Li X, Ahao J, Zhang H, Chen W (2017) Lactobacillus casei CCFM419 attenuates type 2 diabetes via gut microbiota dependent mechanism. Food Funct 8:3155–3164. https://doi.org/10.1039/c7fo00593h
Tian P, Li B, He C, Song W, Hou A, Tian S, Meng X et al (2016) Antidiabetic (type 2) effects of Lactobacillus G15 and Q14 in rats through regulation of intestinal permeability and microbiota. Food Funct 7:3789–3797. https://doi.org/10.1039/c6fo00831c
Aluwong T, Ayo J, Kpukple A, Olalekan OO (2016) Amelioration of hyperglycaemia, oxidative stress and dyslipidaemia in alloxan-induced diabetic Wistar rats treated with probiotic and vitamin C. Nutrients 8(5):151. https://doi.org/10.3390/nu8050151
Hsieh PS, Ho HH, Tsao SP, Hsieh SH, Lin WY, Chen JF, Kuo YW, Tsai SY, Huang HY (2021) Multi-strain probiotic supplement attenuates streptozotocin-induced type-2 diabetes by reducing inflammation and β-cell death in rats. PLoS ONE 16(6):e0251646. https://doi.org/10.1371/journals.pone.0251646
Archer AC, Muthukumar SP, Halami PM (2021) Lactobacillus fermentum MCC2759 and MCC2769 alleviate inflammation and intestinal function in hig-fat diet-fed and streptozotocin-induced diabetics rats. Probiotics Antimicrob Proteins 13:1068–1080. https://doi.org/10.1007/s12602-021-09744-0
Zhou X, Shang GS, Tan Q, He Q, Tan X, Park KY, Zhao X (2021) Effect of Lactobacillus fermentum TKSN041 on improving streptozotocin-induced type 2 diabetes in rats. Food Funct 12:7938–7953. https://doi.org/10.1039/D1FO01571K
Da Cruz RVC, Rocha FDAL, Da Carvalho FL, Moreira SF, Sartoratto A, Cabral L et al (2020) Modulation of the intestinal microbiota and the metabolites produced by the administration of ice cream and dietary supplement containing the same probiotics. Proc Nutr Soc 124(1):1–36. https://doi.org/10.1017/S0007114520000896
Bajinka O, Sylvain DK, Simbilyabo L, Cinteh I, Tan Y (2023) The predicted mechanism and evidence of probiotics on type 2 diabetes mellitus (T2DM). Arch Physiol Biochem. https://doi.org/10.1080/13813455.2022.2163260
Sharma VK, Singh TG, Dhiman S, Garg N (2022) Mechanisms of beneficial effects of probiotics in diabetes mellitus. In: Chopra, K., Bishnoi, M., Kondepudi, K.K. (eds) Probiotic research in therapeutics. Springer, Singapore. https://doi.org/10.1007/978-981-16-8444-9_6
Kumari VB C, Huligere SS, Alotaibi G, Al Mouslem AK, Bahauddin AA, Shivanandappa TB, Ramu R (2022) Probiotic intervention in the treatment of diabetes mellitus: a review. J Pre Appl Microbiol. https://doi.org/10.22207/JPAM.16.3.25
Bordalo L, Olbrich KM, Fortes CLL, Rocha SM, Lucursi L, Duarte HS (2017) Clinical application of probiotics in diabetes mellitus: therapeutics and new perspectives. Crit Rev Food Sci Nut 36(1):85–2. https://doi.org/10.1016/j.clnu.2015.11.011
Kheirkhah AH, Forouzani-Moghaddam MJ, Afkhami-Ardekani M (2023) Relationship between probiotics and type 2 diabetes mellitus: a review. IJDO 15(2):119–128. https://doi.org/10.18502/ijdo.v15i2.12971
Wang Y, Dilidaxi D, Wu Y, Sailike J, Sun X, Nabi X (2020) Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota an inducing GLP-1 secretion in db/db mice. Biomed Pharmacother 125:1–11. https://doi.org/10.1016/j.biopha.2020.109914
Kawata M, Segura LG, Nobre G, Bianchi F, Sivieri K (2019) Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus. Appl Microbiol Biotechnol 103:9229–9238. https://doi.org/10.1007/s00253-019-10156-y
Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A et al (2019) Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51:1–9
Miraghajani M, Shahraki S, Rafie N, Golpour S, Sabihi S, Ghiasvand R (2017) Potential mechanism linking probiotics to diabetes: a narrative review of the literature. Sao Paulo Med J 135(2):169–178. https://doi.org/10.1590/1516-3180.2016.0311271216
Zeng Z, Guo X, Zhang J, Yuan Q, Chen S (2021) Lactobacillus paracasei modulates the gut microbiota and improves inflammation in type 2 diabetic rats. Food Funct 12:6809. https://doi.org/10.1039/d1fo00515d
Wang Y, Wen L, Tang H, Qu J, Rao B (2023) Probiotics and prebiotics as dietary supplements of adjunctive treatment of type 2 diabetes. Pol J Microbiol 72(1):3–9. https://doi.org/10.33073/pjm-2023-013
Sun J, Buys NJ (2016) Glucose- and glycemic factor-lowering effects of probiotics on diabetes: a meta-analysis of randomized placebo-controlled trials. Br J Nutr 115:1167–1177. https://doi.org/10.1017/S0007114516000076
Nikbakht E, Khalesi S, Singh I, Therese L, West NP, Colson N (2016) Effect of probiotics and synbiotics on blood glucose: a systematic review and meta-analysis of controlled trials. Eur J Nutr 57:95–96. https://doi.org/10.1007/s00394-016-1300-3
Zhang Z, Liang X, Lv Y, Yi H, Chen Y, Bai L et al (2020) Evaluation of probiotics for improving and regulation metabolism relevant to type 2 diabetes in vitro. J Funct Foods 64:103664. https://doi.org/10.1016/j.jff.2019.103664
Hills RD, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Thenerge CR (2019) Gut microbiome: profound implications for diet and disease. Nutrients 11:1–40. https://doi.org/10.3390/nu11071623
Kim YA, Keogh JB, Clifton PM (2018) Probiotics, prebiotics, symbiotics and insulin sensitivity. Nutr Res Rev 31:35–51. https://doi.org/10.1017/S095442241700018X
Balakumar M, Prabhu D, Sathishkumar C, Prabu P, Rokana N, Kumar R et al (2018) Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut in high-fat diet-fed C57BL/6J mice. Eur J Nutr 57:279–295. https://doi.org/10.1007/s00394-016-1317-7
Dewidar B, Kahl S, Pafili K, Roden M (2020) Metabolic liver disease in diabetes- from mechanism to clinical trials. Metab Clin Exp 154299. https://doi.org/10.1016/j.metabol.2020.154299
Elkhale HA, Elsahar M, Elwan NM, El-Nakeep S, Naguib M, Hamed SH et al (2018) Managing diabetes and liver disease association. Arab J Gastroenterol 19(4):166–179. https://doi.org/10.1016/j.ajg.2018.08.003
Dang F, Jiang Y, Pan R, Zhou Y, Wu S, Wang R et al (2018) Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct. https://doi.org/10.1039/C8FO00081F
Memarrast F, Ghafouri-Fard S, Kolivand S, Jafary NS, Neyazi SE et al (2017) Comparative evaluation of probiotics effects on plasma glucose, lipid, and insulin levels in streptozotocin-induced diabetic rats. Diabetes Metab Res Rev 33(e2912):1–8. https://doi.org/10.1002/dmrr.2912
Zeng Z, Yuan Q, Yu R, Zhang J, Ma H, Chen S (2019) Ameliorative effects of probiotic Lactobacillus paracasei NL41 on insulin sensitivity, oxidative stress, and beta-cell function in a type 2 diabetes mellitus rat model. Mol Nut Food Res 63:1–9. https://doi.org/10.1002/mnfr.201900457
Chehade JM, Gladysz M, Mooradian AD (2013) Dyslipidemia in type 2 diabetes: prevalence, pathophysiology and management. Drugs 73:327–339. https://doi.org/10.1007/s40265-013-0023-5
Acknowledgements
The authors thank Ph.D. Jorge Cornejo Garrido for his support and advice in slaughtering rats, and thanks to the Agricultural Research Service Culture Collection of the United States Department of Agriculture for donating the strain Lactobacillus casei subsp. casei NRRL-1922. The authors thank the IPN for funding the research projects SIP-20196034 and SIP-20171616.
Author information
Authors and Affiliations
Contributions
José J. Arriaga-Morales: Design of methodology, reproducibility of results, application of statistics technics, data curation, writing the original draft, final approval of the manuscript to be submitted. Cynthia Ordaz-Pichardo: Research goals and aims, design of methodology, conducting the research and performing experiments. Roberto Castro‑Muñoz: writing, review, and editing of the published work. Enrique Durán-Páramo: Research goals and aims, design of methodology, conducting the research and performing experiments, provision of materials, animals, reagents and instrumentation, writing, review, and editing of the published work, coordination of the research work, and acquisition of financial support, final approval of the manuscript to be submitted.
Corresponding authors
Ethics declarations
Ethics Approval
The animal study protocol was approved by the National School of Medicine and Homeopathy Ethics Committee of the National Polytechnic Institute of Mexico, which verified that the study complied with international regulations and standards (approval number: CBE/024/2019).
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arriaga-Morales, J.J., Ordaz-Pichardo, C., Castro‑Muñoz, R. et al. Attenuation of Hyperglycemia in Diabetic Rats Assisted by Immobilized Probiotic in Sodium Alginate. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10166-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10166-3