Abstract
The formulation of polymer with embedded magnetic nanoparticles results in promising nanocomposites for smart and analytical applications. Nanocomposites containing polyacrylonitrile (PAN) and different mass contents of strontium hexaferrite (SFO) were prepared using the casting method. The nanocomposite samples were characterized by using different techniques such as field-emission scanning electron microscopy, Fourier transform infrared spectroscopy, X-ray diffraction, differential scanning calorimetry, and thermogravimetric analysis. Dielectric investigations of SFO/PAN nanocomposites showed that the permittivity and conductivity are considerably enhanced as the content of SFO increased. Optical properties revealed that the absorption and transmittance spectra were significantly affected by adding SFO nanoparticles to the PNA polymer matrix. To investigate the magnetic properties of the nanocomposite samples, the vibrating sample magnetometer was used. The magnetic hysteresis loops illustrated the ferromagnetic nature of SFO/PAN nanocomposites. Different magnetic parameters were given, and they depend on the content of PAN in the nanocomposites.
Similar content being viewed by others
Introduction
M-type hexaferrites are functional ceramic materials that have significant contributions to numerous technological and industrial fields. Depending on the alkaline metals (Sr, Ba, Ca, etc.) [1,2,3], these materials have different applications such as microwave and piezoelectric devices, gas-sensors, biomedicine and magnetic resonance imaging (MRI), and photocatalyst for water treatment. One of the most important M-type hexaferrite is SrFe12O19 (SFO). It has received extensive attention owing to its high coercivity, Curie temperature, saturation magnetization, and magnetocrystalline anisotropy [4, 5]. SFO has been utilized in applications for telecommunications, permanent magnets, recording media, and magneto-optical devices [6].
Polyacrylonitrile (PAN), a synthetic vinyl homopolymer, is extensively studied because of its high strength [7], thermal stability [8], abrasion resistance [9], and perfect precursor for carbon fabrication. PAN fibers have a higher melting point (Tm ~ 317 °C) and a high degree of molecular orientation. PAN is also totally non-toxic and appropriate for biological purposes and biomedical applications [10, 11].
Over the past 20 years, PAN has been the focus of various investigations. Researchers are interested in four primary types of PAN: films [12,13,14,15,16], membranes [17,18,19,20,21], composites [22,23,24,25,26,27], and carbon fibers or nanofibers [28,29,30,31]. Each type of PAN-based material requires a specific set of procedures and techniques. Besides all of these reasons to select PAN to form our studied composites, it can absorb different metal ions and assists the absorption materials for applications [32].
Nanomagnetic polymer composites (NPCs) describe a class of materials, where magnetic nanoparticles are embedded in polymer matrixes. Recently, nanomagnetic polymer composites (NPCs) based on polyacrylonitrile (PAN) are of great interest in materials science. These materials are known to offer a wide spectrum of applications due to their distinctive physicochemical characteristics such as improved mechanical properties [33], magnetic properties [34], and increased conductivity [35]. Recent studies on PAN magnetic nanocomposites have demonstrated their enormous potential applications in electronic [36, 37], biological, color imaging [38, 39], magnetic sensors, storage, and high-density magnetic recording systems.
According to the literature, PAN/magnetic fiber was synthesized by incorporating magnetic nanoparticles like iron, cobalt, ferrite [40], or magnetite [41] into the polymer matrix and then electrospinning. Wang et al. [42] synthesized CuFe2O4 fibers that could be used for catalytic oxidation. These fibers had a variable number of CuO nanoparticles on their surface by altering the ratio between the precursors of Co and Fe. In addition, Qing et. al [43] could prepare CoFe2O4/PAN nanofibers with various CoFe2O4 loadings. The results showed a superparamagnetic behavior of CoFe2O4/PAN nanofibers. Liu et al. [44] reported that the Fe3O4/PAN composite, prepared by the electrospinning and coating method, was successful in eliminating tetracycline. Different studies for hexaferrite/polymer composites were reported [45, 46]. La–Co-substituted barium hexaferrite/polyaniline nanocomposites were studied [45]. It was found that the magnetic properties of these composites showed a significant effect on radiation absorption. These results were also found for doped barium hexaferrite/barium titanate/polypyrrole nanocomposites [46].
Although a significant amount of efforts have been made to study PAN/magnetic composites, no more studies on the dielectric, optical, and ferromagnetic properties of these composites have been made yet. Incorporating SFO nanoparticles into the PAN matrix is expected to affect the dielectric, optical, and magnetic properties of the polymer matrix. In this work, we tried to throw light on the physical properties of SFO/PAN composites. To look for any applications of these composites, different characterization techniques are considered such as Fourier transform infrared (FTIR) spectroscopy, field-emission scanning electron microscopy (FE-SEM), and X-ray diffraction (XRD). The dielectric, optical, and magnetic properties were performed, and the results were discussed.
Experimental
Materials and preparation
Polyacrylonitrile powder of molecular weight (Mw ~ 150,000 g/mol) was purchased from (Polymer Laboratories, Essex, UK), as-prepared strontium hexaferrite SrFe12O19 (SFO), and N, N-dimethylformamide (DMF, 99.9%), Aldrich, Germany, was used as the solvent. The chemical reagents used in this work are of analytical grade. They were used directly as received without further purification. The as-prepared SFO nanoparticles were added in different x (wt% 0, 3, 5, 7, and 10) to polyacrylonitrile (PAN) according to:
where \({w}_{f}\) and \({w}_{p}\) stand for the corresponding weights of SFO and PAN.
The SFO/PAN nanocomposite films were prepared as follows: PAN powder and the desired amount of SFO nanoparticles were dissolved separately each of them in 25 mL of DMF solution. The PAN solution was placed under stirring for 24 h [47] at room temperature (RT) until a clear solution was obtained, while the SFO solution was subjected to ultra-sonication for 3 h at RT to stop the nanoparticles from agglomerating together. The solutions were mixed under ultrasonic stirring at RT for 3 h. Finally, the prepared mixture was placed in Petri dishes, and an 8-h solvent evaporation process was carried out inside an oven set to 70 °C.
Characterization techniques
To study the interactions between the PAN and the SFO nanoparticles a Fourier transform infrared (FTIR) spectrometer (JASCO, FT/IR-6200) was used in the wavenumber range 400–4000 cm−1. The surface morphology of pure and nanocomposite films was investigated by FE-SEM (Carl Zeiss Sigma 500VP, Holland), having an accelerating voltage of 20 kV. XRD patterns of SFO nanoparticles and nanocomposite samples were collected using a PAN analytical's X’Pert PRO having Cu Kα radiation. DSC measurements of the composites were evaluated using Perkin Elmer STA 6000 N2 atom DSC. The thermal decomposition behavior analysis (TGA) was done by TGA (Q50, TA instruments). A Hioki (Ueda, Nagano, Japan) model 3532 High Tester LCR was used for dielectric measurements. The optical spectra were captured using UV–vis spectrophotometer (JASCO 630) at RT in the wavelength (λ) range of 200–1200 nm. The magnetic characteristics of the nanocomposite films were examined at RT and a magnetic field up to ± 20 kOe using a vibrating sample magnetometer (VSM), LakeShore Model 7410.
Results and discussion
Characterizations
The bonding characteristics of the PAN/SFO nanocomposites were analyzed by FTIR spectroscopy. The FTIR spectrum of the pure PAN and PAN/SFO composites is shown in Fig. 1 There are different peaks including the strong stretching vibration of nitrile groups (–CN–) appearing at 2244 cm−1 and the peaks at 2930 and 1453 cm−1 which are corresponding to the stretching vibration and bending vibration of methylene (–CH2–), respectively [48]. The peak at 1670 cm−1 indicates the presence of carboxyl (C=O) groups which may be due to the oxidation of PAN in the air [48]. The peaks at 1251 and 1360 cm−1 are linked to CH and CH2 groups, respectively, which are the fundamental groups forming the backbone chain of the PAN [49]. By the incorporation of nanoparticles into the PAN matrix, the peaks at 2244 and 1095 cm−1 are associated with the nitrile groups which are observed to become stronger and sharper indicating a strong interaction between the PAN and the nanoparticles [50].
The surface morphology of the SFO nanoparticles and nanocomposite films were tested by FE-SEM as displayed in Fig. 2. SFO shows a hexagonal shape (Fig. 2a). A good dispersion of SFO into PAN and SFO was observed keeping the hexagonal shape of SFO as in the sample 3 wt% (see Fig. 2b). By increasing the SFO content, the morphology of the nanocomposite films has slightly changed may be due to the variation in the viscosity of the polymer and the tendency of the nanoparticles to agglomerate as seen in Fig. 2c, d. It was reported earlier [51] that the shape of the nanoparticles affected the morphology of the polyvinyl alcohol (PVA).
It is useful to estimate the average particle size (Dav) of the investigated samples by using the FE-SEM images. Figure 3d depicts the histograms of the particle distribution of pure SFO and some SFO/PAN nanocomposites based on ImageJ (Fiji) software. The Dav values of all studied samples are given in Table 1. One noticed a significant difference between the crystal size value of pure SFO nanoparticles and that of Dav because of the agglomeration of the particles in a powder. On the other hand, the Dav of SFO in the composites becomes lower than that of pure SFO. This result could be explained in terms of decreasing the agglomeration of SFO nanoparticles and the separation between these nanoparticles by the polymer chains in composites.
The XRD patterns of pure SFO and PAN loaded with SFO nanoparticles are displayed in Fig. 4. It is observed that pure PAN and SFO/PAN nanocomposites have the same diffraction peak at 2θ = 17°. According to the literature, this peak indicates the crystalline phase of PAN that is assigned to the (110) plane [52]. The main characteristic peaks of SFO (see the lower panel of Fig. 4) could be easily observed in the XRD pattern of the as-prepared SFO/PAN nanocomposites (see the upper panel of Fig. 4). The intensity of the SFO peaks increased significantly with increasing its content inside the PAN matrix. The average size of crystallites of SFO nanoparticles is estimated based on the Debye–Scherrer formula as [53]:
where D stands for the grain size in nanometers (nm), λ is the X-ray radiation's wavelength (Cu Kα = 0.14460 nm), k is the Scherrer constant (~ 0.9), θ is the X-ray diffraction angle, and β represents the full-width at half-maximum (FWHM) of the peak. The crystal size of SFO was estimated to be 48.96 nm. This value is different compared to that observed by FE-SEM because of the agglomeration of the nanoparticles. To estimate the effect of nanoparticles on the PAN's crystal structure, the degree of crystallinity (Xc) of the composites was estimated using Hinrichsen’s approach [54]:
where Ia is the intensity dispersed by the amorphous fraction, which is the area of the diffractogram outside the peaks, and Ic is the integral intensity of the crystalline fraction, which is the area under the diffraction peaks and background curve. The calculated values of the degree of crystallinity are summarized in Table 1. As seen in Table 1, the Xc value of PAN was altered by adding SFO nanoparticles. Such a result is accepted in composites when crystalline nanofillers are added to semicrystalline polymeric materials. Such a result could be attributed to some reasons either due to the added amount of the crystalline nanofiller to the polymer or the alignment of the PAN polymeric chains [55].
The effect of SFO NPs on the thermal stability of PAN was investigated using TGA and DSC techniques. Figure 5 displays the DSC thermograms of pure PAN and SFO/PAN nanocomposites. The pure PAN shows a sharp thermic peak at (Tp ~ 576 K); this peak is due to an exothermic reaction occurring due to the cyclization of nitrile groups [56]. By increasing the SFO content, this peak is shifted toward lower temperatures. The relative parameters obtained from the DSC curves are listed in Table 1. As seen from Table 1, there is a decrease in enthalpy value with increasing SFO content which indicates the intensified disorder with the addition of the SFO NPs.
TGA was used to check the thermal stability of pure PAN and SFO/PAN nanocomposites. The TGA curves are displayed in Fig. 6. The degradation process of all samples can be expressed as five stages. In stage I, a slight loss of mass took place owing to the water molecules and decomposition of low molecular weight components [57]. In the second II and third III stages, the weight loss in the temperature between 543 and 643 K, the nanocomposites have a smaller weight loss rate compared to that of pure PAN which leaves a residual weight of 66% up to 643 K. Besides, the nanocomposite samples showed a residual weight of 78.3% indicating that they have easily undergone cyclization reaction compared to pure PAN. Such results are consistent with the results from the DSC analysis. The fourth stage IV, between 643 and 738 K, corresponds to 12% weight loss of nanocomposites and 8% weight loss of pure PAN owing to PAN’s thermal degradation [58]. In the final stage V, 4–5% of weight loss was seen for all studied samples due to pyrolytic degradation of PAN leaving residual masses near 873 K, 58%, 60.3%, 62.9%, and 64.1% for pure PAN, and SFO/PAN nanocomposites (5,7, and 10 wt%), respectively. This is evidence that the samples containing higher SFO content have greater thermal stability compared to that of pure PAN. Similar results were reported for polyhedral oligomeric silsesquioxane (POSS) nanoparticles doped polypropylene [59].
Dielectric properties
The dielectric properties of the as-prepared PAN and SFO/PAN composites were studied. One noticed that the dielectric permittivity (ε' = d C/εoA, where C is the capacitance, d is the sample thickness, εo is the free space permittivity, and A is the sample cross-sectional area) decreases with increasing the frequency for all samples as shown in Fig. 7a–d. In addition, the ε' showed high values for all temperatures at lower frequencies; then, it started to decrease again as the frequency increased because the dipoles were no longer able to follow the field and hence their contribution to the polarization decreased. The ε′ of PAN is considerably enhanced by doping with SFO nanoparticles. The increase in ε′ for PAN can be ascribed to the formation of extra molecular dipoles in the nanocomposites.
Figure 8a–d displays the temperature dependence of ε′ for pure PAN and PAN loaded with SFO nanoparticles at some selected frequencies. The behavior of ε′ can be explained as follows: at lower temperatures, the number of dipoles rotating in parallel with the applied electric field was limited as the thermal energy absorbed by the polymer is small [60]. By increasing temperature the polymer’s viscosity reduced and the dipoles gain enough energy to be easily oriented with the applied electric field, thus ε′ increased and reached a maximum near 373 K related to a peak is observed due to the micro-Brownian motion corresponding to α-relaxation process [61].
The value of the dielectric loss is significant for capacitor applications. The usage of dielectric materials in high-performance capacitor applications could be constrained by the high levels of dielectric loss. Figure 9a–c shows the variation of the dielectric loss, ε″, where ε″ = ε′ tan δ), for pure PAN and some nanocomposites at some selected temperatures. One noted that ε″ increased rapidly at lower frequencies revealing the existence of electrode polarizations [62]. On the other hand, as the frequency increases, the amount of polarization, by charge accumulation, reduces and then the value of the dielectric loss decreased.
Figure 10a–d displays the temperature dependence of the ε″ for pure PAN and some nanocomposites at some selected frequencies. The ε″(T) shows a relaxation peak due to α-relaxation in PAN. This peak appears in the temperature range from 360 to 400 K [63] because of the micro-Brownian motion of segments of polymeric chains. Such peak becomes broader and shifts to a higher temperature side for higher frequencies. This broadness indicates dispersion in relaxation time as a result of the increase in the density of dipoles.
To analyze the dielectric behavior of the nanocomposite samples and prevent the impact electrode polarizations, the electric modulus can be used [64]:
where M′ and M″ represent the real and imaginary parts of the electric modulus, respectively. The variation of M′ with the temperature at some selected frequencies is shown in Fig. 11a–d. It is noticed that by increasing temperatures, the values of M′ decreased because the dielectric permittivity is thermally activated [65]. This result is consistent with the increase of ε′ of pure PAN with adding the SFO nanoparticles.
It is useful to throw light on the relaxation process in PAN after adding SFO nanoparticles. Figure 12a–e displays the frequency dependence of M′′ for some nanocomposites as an example. A relaxation peak known as the conductivity-relaxation peak of the mobile ions (αc-relaxation) could be seen [66]. A possible explanation for the low-frequency region of the αc-relaxation is the DC conductivity resulting from charge carriers that are continuously hopping over long distances. In the high-frequency region, the ions are constrained to their potential wells corresponding to a short-range motion. Consequently, the M″ relaxation peak results from the transition from long-range to short-range mobility [67]. Also, the M peak shifts toward higher frequencies as temperature increases due to an increase in the density of dipoles contributing to the relaxing process.
Based on Fig. 13, the activation energy of the relaxation was determined using the maximum frequency, fmax, described by the Arrhenius equation [68]:
where Ea is the activation energy, k is the Boltzmann constant, and fo is a distinctive constant parameter for a specific relaxation process. Figure 13 depicts the change of fmax for pure PAN and some SFO/PAN nanocomposites versus 1000/T and the calculated values of Ea are shown in Table 2.
The ac conductivity values of pure PAN and SFO/PAN nanocomposites are calculated as:
where ω is the angular frequency. The calculated values of the ac conductivity are shown in Table 2. As seen, the conductivity increased by increasing the SFO content. This might be attributed to the formation of conductive networks throughout the composite as a result of the effect of SFO nanoparticles. These networks enable the charge carriers to hop from one site to another within the nanocomposites.
Optical properties
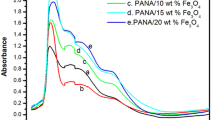
The UV–vis spectra of radiation absorption and transmittance (T) of pure PAN and SFO/PAN nanocomposites are presented in Fig. 14a–b. The absorbance spectra of films show a sharp edge at the ultraviolet region at 300 nm with low absorption at the long wavelength region. This edge is probably caused by n—π* electronic transition [69]. The films appear low absorption at the long wavelength region. Also, the absorbance increases with increasing the content of SFO nanoparticles SFO. On the other hand, the transmittance of PAN decreased by adding SFO nanoparticles because they act as scattering sites for the incident photon. This means that the transparency of the polymeric materials can be changed by nanofillers. It is also noticed that T increased with increasing λ. The reason for this increase is the energy of the incident photons, which can excite the electrons from the valence band to the conduction band.
The refractive index (n) is calculated from the values of the reflectance, R, according to the relation: [70]
while the variation of n with λ can be expressed as [71]:
where n∞ is the refractive index at infinite wavelength, and λo is the average interband oscillator wavelength. Figure 15 represents the variation of (n2−1)−1 against λ−2. The fitting parameters, n∞ and λo, are given and listed in Table 3. From these parameters, the average oscillator strength (So) where So = \(({n}_{\infty }^{2}-1)/ {\lambda }_{o}^{2})\). It is noticed that the refractive index of PAN was changed by adding SFO nanoparticles to the PAN polymer matrix which is useful for applications. Similarly, the values of other optical parameters are So and λo.
Magnetic properties
The room-temperature hysteresis loops of SFO nanoparticles and PAN loaded with different ratios of SFO are shown in Fig. 16a–d. The magnetization curves revealed that the as-prepared SFO/PAN nanocomposites exhibit ferromagnetic behavior. The magnetization loops are not saturated within the studied range of the applied magnetic field. Different magnetic parameters for the investigated samples are given in Table 4. It was observed from Table 4 that the Mr values are enhanced with increasing SFO content owing to the magnetic nature of these nanofillers. The coercivity of the samples slightly changes with the change in the SFO content.
Stoner–Wohlfarth (S–W) model was used [72, 73]:
where β is a parameter related to the magnetocrystalline anisotropy of the samples. Figure 17a, b displays the plots of M against 1/H2 for pure SFO and all nanocomposites. From the linear fitting of Eq. 10, Ms was estimated by extrapolating M versus 1/H2 to zero. The values of Ms and β are given and listed in Table 4. SFO nanoparticles revealed a high value of saturation magnetization (Ms) (see Fig. 17a), whereas, in the case of SFO/PAN nanocomposites, the Ms depends on the content of the nanoparticles. This can be explained owing to the existence of a diamagnetic phase of PAN in the nanocomposites, which may affect the uniformity of magnetization by excluding the surface magnetic moment. Also, the Mr/Ms ratio is related to the grain size and the distribution of particles [74]. It was found to be (Mr/Ms > 0.5) for the samples under study. The values of the crystal size and magnetic properties of SFO are comparable with those of similar published materials [75].
A plot of M as a function of 1/H2 for pure SFO (a) and (b) the as-prepared SFO/PAN nanocomposites. The solid lines represent the fitting, according to Eq. 10
Conclusions
Strontium hexaferrite (SFO) nanoparticles were prepared and incorporated into a polyacrylonitrile (PAN) matrix. The impact of SFO on the dielectric, optical, and magnetic properties of PAN was investigated. The XRD spectrum for PAN shows a semicrystalline structure while the nanocomposites exhibited the peaks of the SFO nanofillers. The FE-SEM showed a good dispersion of SFO on the surface of PAN, especially at low content. DSC data also showed that SFO/PAN nanocomposite samples had a broader thermic temperature range and a lower rate of evolving heat. The TGA results exhibited that with increasing the SFO content, the SFO/PAN nanocomposites are more thermally stable than pure PAN.
The incorporation of the SFO nanoparticles enhances the dielectric permittivity and ac conductivity promising for high-performance dielectric capacitors. Within the studied range of temperature and frequency, a relaxation process was observed for pure PAN and PAN loaded with SFO nanoparticles. Based on the optical properties, the transparency of PAN was changed by adding SFO nanoparticles because the absorption and transmittance were changed. Also, the refractive index of pure PAN increased with loading with SFO nanoparticles. Different optical parameters are given.
Finally, different magnetic parameters were given for pure SFO and SFO/PAN nanocomposites. It is useful to keep the magnetic properties of a polymeric matrix by adding some magnetic nanoparticles such as SFO. All nanocomposites exhibited ferromagnetic behavior at RT. All together. i.e., the outcome results of this work are, the physical properties of PAN can be controlled by adding a suitable amount of SFO nanoparticles. Therefore, the studied nanocomposites could be useful for applications such as capacitors, anti-coating reflection, and recording data, depending on the specific change in the physical properties of the pristine samples.
References
Godara SK, Singh M, Kaur V, Narang SB, Sood AK (2021) Effect of calcium solubility on structural, microstructural and magnetic properties of M-type barium hexaferrite. Ceram Int 47(14):20399–20406. https://doi.org/10.1016/j.ceramint.2021.04.048
Godara SK, Kaur MP, Kaur V, Malhi PS, Singh M, Verma S, Jasrotia R, Ahmed J, Tamboli MS, Sood AK (2022) Investigation of microstructural and magnetic properties of Ca2+ doped strontium hexaferrite nanoparticles. J King Saud Univ-Sci 34(4):101963. https://doi.org/10.1016/j.jksus.2022.101963
Godara SK, Kaur V, Narang SB, Singh G, Singh M, Bhadu GR (2021) Tailoring the magnetic properties of M-type strontium ferrite with synergistic effect of co-substitution and calcinations temperature. J Asian Ceram Soc 9(2):686–698. https://doi.org/10.1080/21870764.2021.1911059
Jahn L, Müller HG (1969) The coercivity of hard ferrite single crystals. Phys Status Solidi (b) 35(2):723–730. https://doi.org/10.1002/pssb.19690350222
Dhage VN, Mane ML, Keche AP, Birajdar CT, Jadhav KM (2011) Structural and magnetic behaviour of aluminium doped barium hexaferrite nanoparticles synthesized by solution combustion technique. Phys B 406(4):789–793. https://doi.org/10.1016/j.physb.2010.11.094
Zi ZF, Sun YP, Zhu XB, Yang ZR, Song WH (2008) Structural and magnetic properties of SrFe12O19 hexaferrite synthesized by a modified chemical co-precipitation method. J Magn Magn Mater 320(21):2746–2751. https://doi.org/10.1016/j.jmmm.2008.06.009
Xu Q, Xu L, Cao W, Wu S (2005) A study on the orientation structure and mechanical properties of polyacrylonitrile precursors. Polym Adv Technol 16(8):642–645. https://doi.org/10.1002/pat.625
Musale DA, Kumar A (2000) Solvent and pH resistance of surface cross linked chitosan/poly (acrylonitrile) composite nano filtration membranes. J Appl Polym Sci 77(8):1782–1793. https://doi.org/10.1002/1097-4628(20000822)77:8%3e1822::AID20%3c3.0.CO;2-5
Aoki Y, Watanabe M (1992) Morphological, thermal, and rheological properties of nylon/acrylonitrile-butadiene-styrene alloys. Polym Eng Sci 32(13):878–885. https://doi.org/10.1002/pen.760321307L
Wan LS, Xu ZK, Huang XJ, Wang ZG, Ye P (2005) Hemocompatibility of poly (acrylonitrile-co-N-vinyl-2-pyrrolidone) s: swelling behavior and water states. Macromol Biosci 5(3):229–236. https://doi.org/10.1002/mabi.200400157
Wang ZG, Wan LS, Xu ZK (2007) Surface engineerings of polyacrylonitrile-based asymmetric membranes towards biomedical applications: an overview. J Membr Sci 304(1–2):8–23. https://doi.org/10.1016/j.memsci.2007.05.012
Deki Sh, Nabika H, Akamatsu K, Mizuhata M, Kajinami A (2001) Pereparation and characterization of metal nanoparticles dispersed in Polyacrylonitrile thin film. Scr Mater 44(9–8):1879–1882. https://doi.org/10.1016/S1359-6462(01)00734-5
Yu DM, Nam SW, Yoon S, Kim TH, Lee JY, Nam SY, Hong YT (2015) Edge protection using polyacrylonitrile thin-films for hydrocarbon-based membrane electrode assemblies. J Ind Eng Chem 28:190–196
Zheng H, Xue H, Zhang Y, Shen Z (2002) A glucose biosensor based on microporous polyacrylonitrile synthesized by single rare-earth catalyst. Biosens Bioelectron 17(6–7):541–545. https://doi.org/10.1016/S0956-5663(02)00010-6H
Xue H, Shen Z, Li C (2005) Improved selectivity and stability of glucose biosensor based on in situ electropolymerized polyaniline–polyacrylonitrile composite film. Biosens Bioelectron 20(11):2330–2334. https://doi.org/10.1016/j.bios.2004.07.018
Ouyang Q, Cheng L, Wang H, Li K (2008) Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym Degrad Stab 93(8):1415–1421. https://doi.org/10.1016/j.polymdegradstab.2008.05.021
Jung B (2004) Preparation of hydrophilic polyacrylonitrile blend membranes for ultrafiltration. J Membr Sci 229(1–2):129–136. https://doi.org/10.1016/j.memsci.2003.10.020
Dalwani M, Benes NE, Bargeman G, Stamatialis D, Wessling M (2011) Effect of pH on the performance of polyamide/polyacrylonitrile based thin film composite membranes. J Membr Sci 372(1–2):228–238. https://doi.org/10.1016/j.memsci.2011.02.012
Khamforoush M, Pirouzram O, Hatami T (2015) The evaluation of thin film composite membrane composed of an electrospun polyacrylonitrile nanofibrous mid-layer for separating oil–water mixture. Desalination 359:14–21. https://doi.org/10.1016/j.desal.2014.12.016
Panda SR, Mukherjee M, Sirshendu D (2015) Preparation, characterization and humic acid removal capacity of chitosan coated iron-oxide polyacrylonitrile mixed matrix membrane. J Water Process Eng 6:93–104. https://doi.org/10.1016/j.jwpe.2015.03.007
Wang Q, Song WL, Fan LZ, Song Y (2015) Facile fabrication of polyacrylonitrile/alumina composite membranes based on triethylene glycol diacetate-2-propenoic acid butyl ester gel polymer electrolytes for high-voltage lithium-ion batteries. J Membr Sci 486:21–28. https://doi.org/10.1016/j.memsci.2015.03.022
El-Ghamaz NA, Diab MA, Zoromba MS, El-Sonbati AZ, El-Shahat O (2013) Conducting polymers. VI. Effect of doping with iodine on the dielectrical and electrical conduction properties of polyacrylonitrile. Solid State Sci 24:140–146. https://doi.org/10.1016/j.solidstatesciences.2013.07.016S
İnan S, Altaş Y (2011) Preparation of zirconium–manganese oxide/polyacrylonitrile (Zr–Mn oxide/PAN) composite spheres and the investigation of Sr (II) sorption by experimental design. Chem Eng J 168(3):1263–1271. https://doi.org/10.1016/j.cej.2011.02.038
Doan TNL, Ghaznavi M, Zhao Y, Zhang Y, Konarov A, Sadhu M, Chen P (2013) Binding mechanism of sulfur and dehydrogenated polyacrylonitrile in sulfur/polymer composite cathode. J Power Sources 241:61–69. https://doi.org/10.1016/j.jpowsour.2013.04.113
Hwang JJ, Ma TW (2012) Preparation, morphology, and antibacterial properties of polyacrylonitrile/montmorillonite/silver nanocomposites. Mater Chem Phys 136(2–3):613–623. https://doi.org/10.1016/j.matchemphys.2012.07.034
He X, Pu W, Wang L, Ren J, Jiang C, Wan C (2007) Synthesis of spherical nano tin encapsulated pyrolytic polyacrylonitrile composite anode material for Li–ion batteries. Solid State Ion 178(11–12):833–836. https://doi.org/10.1016/j.ssi.2007.02.013
Korobeinyk AV, Whitby RL, Mikhalovsky SV (2012) High temperature oxidative resistance of polyacrylonitrile-methylmethacrylate copolymer powder converting to a carbonized monolith. Eur Polym J 48(1):97–104. https://doi.org/10.1016/j.eurpolymj.2011.10.006
Chen IH, Wang CC, Chen CY (2010) Fabrication and characterization of magnetic cobalt ferrite/polyacrylonitrile and cobalt ferrite/carbon nanofibers by electrospinning. Carbon 48(3):604–611. https://doi.org/10.1016/j.carbon.2009.09.062
Nataraj SK, Yang KS, Aminabhavi TM (2012) Polyacrylonitrile-based nanofibers—a state-of-the-art review. Prog Polym Sci 37(3):487–513. https://doi.org/10.1016/j.progpolymsci.2011.07.001
Lee JH, Manuel J, Choi H, Park WH, Ahn JH (2015) Partially oxidized polyacrylonitrile nanofibrous membrane as a thermally stable separator for lithium ion batteries. Polymer 68:335–343. https://doi.org/10.1016/j.polymer.2015.04.055
Kozicki M, Sąsiadek E, Karbownik I, Maniukiewicz W (2015) Doped polyacrylonitrile fibres as UV radiation sensors. Sens Actuators B Chem 213:234–243. https://doi.org/10.1016/j.snb.2015.02.087
Liu D, Chen H, Yin P, Ji N, Zong G, Qu R (2011) Synthesis of polyacrylonitrile by single-electron transfer-living radical polymerization using Fe (0) as catalyst and its adsorption properties after modification. J Polym Sci Part A Polym Chem 49(13):2916–2923. https://doi.org/10.1002/pola.24727
Guo Z, Kim TY, Lei K, Pereira T, Sugar JG, Hahn HT (2008) Strengthening and thermal stabilization of polyurethane nanocomposites with silicon carbide nanoparticles by a surface-initiated-polymerization approach. Compos Sci Technol 68(1):164–170. https://doi.org/10.1016/j.compscitech.2007.05.031
McHenry ME, Johnson F, Okumura H, Ohkubo T, Ramanan VRV, Laughlin DE (2003) The kinetics of nanocrystallization and microstructural observations in FINEMET, NANOPERM and HITPERM nanocomposite magnetic materials. Scr Mater 48(7):881–887. https://doi.org/10.1016/S1359-6462(02)00597-3
Stankovich S, Dikin DA, Dommett GH, Kohlhaas KM, Zimney EJ, Stach EA, Ruoff RS (2006) Graphene-based composite materials. Nature 442(7100):282–286. https://doi.org/10.1038/nature04969
Fan N, Ma X, Ju Z, Li J (2008) Formation, characterization and magnetic properties of carbon-encapsulated iron carbide nanoparticles. Mater Res Bull 43(6):1549–1554. https://doi.org/10.1016/j.materresbull.2007.06.017
Guo Z, Moldovan M, Young DP, Henry LL, Podlaha EJ (2007) Magnetoresistance and annealing behaviors of particulate Co–Au nanocomposites. Electrochem Solid-State Lett 10(12):E31. https://doi.org/10.1149/1.2794685
Hung MT, Choi O, Ju YS, Hahn HT (2006) Heat conduction in graphite-nanoplatelet-reinforced polymer nanocomposites. Appl Phys Lett 89(2):023117. https://doi.org/10.1063/1.2221874
Sun XC, Reyes-Gasga J, Dong XL (2001) Microstructure of carbon encapsulated superparamagnetic Co nanoparticles. MRS Online Proc Libr (OPL). https://doi.org/10.1557/PROC-704-W6.26.1
Wang B, Sun Y, Wang H (2010) Preparation and properties of electrospun PAN/Fe3O4 magnetic nanofibers. J Appl Polym Sci 115(3):1781–1786. https://doi.org/10.1002/app.31288
Blachowicz T, Ehrmann A (2021) Influence of clustering round magnetic nano-dots on magnetization reversal. J Phys Conf Ser 1730(1):012034. https://doi.org/10.1088/1742-6596/1730/1/012034
Wang P, Dong T, Li M, Yang P (2019) Controlling growth of CuO nanoparticles on CuFe2O4 nanotubes and their adsorption kinetics. J Nanosci Nanotechnol 19(8):4474–4480. https://doi.org/10.1166/jnn.2019.16481
Qing-Qing Ni, , Hong Xia, Xudong Jin , Fan Liu. (2014) Application of electrospun nanofibers in electromagnetic interference shielding. In: Electrospun nanofibers for energy and environmental applications nanostructure science and technology, pp 497–525. https://doi.org/10.1007/978-3-642-54160-5_20
Liu Q, Zhong LB, Zhao QB, Frear C, Zheng YM (2015) Synthesis of Fe3O4/polyacrylonitrile composite electrospun nanofiber mat for effective adsorption of tetracycline. ACS Appl Mater Interfaces 7(27):14573–14583. https://doi.org/10.1021/acsami.5b04598
Kaur T, Kumar S, Sharma J, Srivastava AK (2015) Radiation losses in the microwave Ku band in magneto-electric nanocomposites. Beilstein J Nanotechnol 6(1):1700–1707. https://doi.org/10.3762/bjnano.6.173
Kaur T, Kumar S, Narang SB, Srivastava AK (2016) Radiation losses in microwave Ku region by conducting pyrrole/barium titanate and barium hexaferrite based nanocomposites. J Magn Magn Mater 420:336–342. https://doi.org/10.1016/j.jmmm.2016.07.058
Munajat NA, Nurfaizey AH, Bahar AAM, You KY, Fadzullah SHSM, Omar G (2018) High-frequency dielectric analysis of carbon nanofibers from pan precursor at different pyrolysis temperatures. Microw Opt Technol Lett 60(9):2198–2204. https://doi.org/10.1002/mop.31326
Zhang D, Chung R, Karki AB, Li F, Young DP, Guo Z (2010) Magnetic and magnetoresistance behaviors of solvent extracted particulate iron/polyacrylonitrile nanocomposites. J Phys Chem C 114(1):212–219. https://doi.org/10.1021/jp908844r
Panapoy M, Dankeaw A, Ksapabutr B (2008) Electrical conductivity of PAN-based carbon nanofibers prepared by electrospining method. Thammasat Int J Sci Technol 13:11–17. https://doi.org/10.1177/1528083715598654
Ji L, Medford AJ, Zhang X (2009) Electrospun polyacrylonitrile/zinc chloride composite nanofibers and their response to hydrogen sulfide. Polymer 50(2):605–612. https://doi.org/10.1016/j.polymer.2008.11.016
Mousa M, Dong Y (2020) The role of nanoparticle shapes and structures in material characterisation of polyvinyl alcohol (PVA) bionanocomposite films. Polymers 12(2):264. https://doi.org/10.3390/polym12020264
Palade S, Pantazi A, Vulpe S, Berbecaru C, Ţucureanu V, Oprea O, Dragoman D (2017) Tunable dielectric properties in polyacrylonitrile/multiwall carbon nanotube composites. Polym Compos 38(8):1741–1748. https://doi.org/10.1002/pc.23744
Iqbal MJ, Ashiq MN (2008) Physical and electrical properties of Zr–Cu substituted strontium hexaferrite nanoparticles synthesized by co-precipitation method. Chem Eng J 136(2–3):383–389. https://doi.org/10.1016/j.cej.2007.05.046
Hinrichsen G (1972) Structural changes of drawn polyacrylonitrile during annealing. J Polym Sci Part C Polym Symp 38(1):303–314. https://doi.org/10.1002/polc.5070380123
Samimi-Sohrforozani E, Azimi S, Abolhasani A, Malekian S, Arbab S, Zendehdel M, Abolhasani MM, Nia NY (2021) Development of porous polyacrylonitrile composite fibers: new precursor fibers with high thermal stability. Electron Mater 2:454–465. https://doi.org/10.3390/electronicmat2040031
Furushima Y, Nakada M, Takahashi H, Ishikiriyama K (2014) Study of melting and crystallization behavior of polyacrylonitrile using ultrafast differential scanning calorimetry. Polymer 55(13):3075–3081. https://doi.org/10.1016/j.polymer.2014.05.015
Semsarzadeh MA, Molaeei A (1997) Thermal reactions and analysis of polyacrylonitrile films. Iran Polym J 6(2):113
Van Krevelen DW, Te Nijenhuis K (2009) Properties of polymers: their correlation with chemical structure: their numerical estimation and prediction from additive group contributions, 4th Revised. Elsevier Science, Amsterdam
Kumar S, Butola BS, Joshi M (2013) POSS/polypropylene hybrid nanocomposite monofilaments by reactive extrusion. Fibers Polym 14(3):428–435. https://doi.org/10.1007/s12221-013-0428-5
Mahrous S (1996) Dielectric analysis of the α-relaxation of PVC stabilized with cadmium laurate. Polym Int 40(4):261–267. https://doi.org/10.1002/(SICI)1097-0126(199608)40:4%3e261::AID-PI549%3c3.0.CO;2-C
Elashmawi IS, Abdelrazek EM, Ragab HM, Hakeem NA (2010) Structural, optical and dielectric behavior of PVDF films filled with different concentrations of iodine. Phys B 405(1):94–98. https://doi.org/10.1016/j.physb.2009.08.037
Gupta AK, Chand N, Singh R, Mansingh A (1979) Dielectric study of polyacrylonitrile, poly-2-hydroxyethyl methacrylate and their copolymers. Eur Polym J 15(2):129–136. https://doi.org/10.1016/0014-3057(79)90197-6
Gupta AK, Chand N (1980) Glass transition in polyacrylonitrile: analysis of dielectric relaxation data. J Polym Sci Polym Phys Ed 18(5):1125–1136. https://doi.org/10.1002/pol.1980.180180516
Mahrous S, Hanfy TA (2009) Dielectric analysis of chlorinated polyvinyl chloride stabilized with di-n-octyltin maleate. J Appl Polym Sci 113(1):316–320. https://doi.org/10.1002/app.29490
Mahrous S, Hanfy TA, Sobhy MS (2007) Dielectric relaxation of chlorinated polyvinyl chloride (CPVC) stabilized with cyanoguanidine. Curr Appl Phys 7(6):629–635. https://doi.org/10.1016/j.cap.2007.01.002
Khiar ASA, Anuar MRS, Parid M (2016) Effect of 1-ethyl-3-methylimidazolium nitrate on the electrical properties of starch/chitosan blend polymer electrolyte. In: Materials Science Forum, vol 846. Trans Tech Publications Ltd. pp 510–516. https://doi.org/10.4028/www.scientific.net/MSF.846.510
Sengwa RJ, Sankhla S (2008) Ionic conduction and dielectric dispersion study on chain dynamics of poly (vinyl pyrrolidone)–glycerol blends. Ind J Appl Phys 46:513–520
Hanafy TA, Elbanna K, El-Sayed S, Hassen A (2011) Dielectric relaxation analysis of biopolymer poly (3-hydroxybutyrate). J Appl Polym Sci 121(6):3306–3313. https://doi.org/10.1002/app.33950T
Tański T, Snopiński P, Borek W (2017) Strength and structure of AlMg3 alloy after ECAP and post-ECAP processing. Mater Manuf Process 32(12):1368–1374. https://doi.org/10.1080/10426914.2016.1257131
Abed AS, Ziadan KM, Abdullah AQ (2014) Some optical properties of polyurethane. Iraqi J of Polym 17(1):18–28
Rashidian M, Dorranian D (2014) Low-intensity UV effects on optical constants of PMMA film. J Theor Appl Phys 8(2):1–7. https://doi.org/10.1007/s40094-014-0121-0
Amir M, Geleri M, Güner S, Baykal A, Sözeri H (2015) Magneto optical properties of FeBxFe2− xO4 nanoparticles. J Inorg Organomet Polym Mater 25(5):1111–1119. https://doi.org/10.1007/s10904-015-0217-2
Auwal IA, Güngüneş H, Güner S, Shirsath SE, Sertkol M, Baykal A (2016) Structural, magneto-optical properties and cation distribution of SrBixLaxYxFe12− 3xO19 (0.0≤ x≤ 0.33) hexaferrites. Mater Res Bull 80:263–272. https://doi.org/10.1016/j.materresbull.2016.03.028
Sadaiyandi K, Kennedy A, Sagadevan S, Chowdhury ZZ, Johan M, Bin R, Thamiz SR (2018) Influence of Mg doping on ZnO nanoparticles for enhanced photocatalytic evaluation and antibacterial analysis. Nanoscale Res Lett 13(1):1–13. https://doi.org/10.1186/s11671-018-2643-x
El-Sayed S, Hashhash A, Refai HS, Rutkauskas AV, Baleidy WS, Lis ON, Hassen A (2021) The detailed studies of the structural and magnetic properties of hexaferrites Ba1− xSrxFe12O19 for 0.0≤ x≤ 0.75. J Mater Sci Mater Electron 32(8):10977–10990. https://doi.org/10.1007/s10854-021-05757-1
Acknowledgements
The authors would like to thank Prof. A. Hashhash, Dr. H. S. Refai, and my student Mr. W. S. Baleidy, Egyptian Atomic Energy Authority, Cairo, Egypt, for the preparation of the pure SFO sample.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to this work. WMS prepared the nanocomposites and completed the experimental measurements. Both AH and SM shared writing and followed the performance of the experiments. AH suggested the idea and contributed to completing the paper writing, analyzing the data, and validation. He offered valuable advice on the performance of the experiments.
Corresponding author
Ethics declarations
Conflict of interest
My collaborators and I would like to declare that we have no conflict of interest for our submitted manuscript. Moreover, the manuscript is approved by us for publication. All data are available upon any request from the BOU Journal by contacting the corresponding author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shoubak, W.M., Hassan, A., Mahrous, S. et al. Controlling the physical properties of polyacrylonitrile by strontium hexaferrite nanoparticles. Polym. Bull. 81, 697–718 (2024). https://doi.org/10.1007/s00289-023-04736-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04736-2