Recent Advances in Using Natural Antibacterial Additives in Bioactive Wound Dressings
Abstract
:1. Introduction
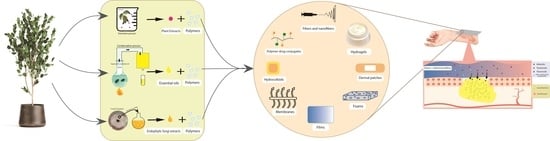
2. Bioactive Wound Dressings (Polymer + Additives)
2.1. Hydrogels
2.2. Hydrocolloids
2.3. Foams
2.4. Films
2.5. Dermal Patches
2.6. Fibers and Nanofibers-Based Electrospun Polymers
2.7. Membranes
2.8. Polymer-Drug Conjugates
2.9. Other Polymer Wound Dressings
3. Plant-Based Bio-Additives as Novel Antibacterial and Antimicrobial Additives to the Polymer Wound Dressings
3.1. Plant Extracts
3.2. Essential Oils
3.3. Endophytes: A Novel Source of Bioactive Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Carr, N.J. The pathology of healing and repair. Surgery 2022, 40, 13–19. [Google Scholar] [CrossRef]
- Jones, V.; Grey, J.; Harding, K. Wound dressings. BMJ 2006, 332, 777. [Google Scholar] [CrossRef]
- Sen, C.; Roy, S.; Gordillo, G. Wound Healing (Neligan Plastic Surgery: Volume One); Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- McCosker, L.; Tulleners, R.; Cheng, Q.; Rohmer, S.; Pacella, T.; Graves, N.; Pacella, R. Chronic wounds in Australia: A systematic review of key epidemiological and clinical parameters. Int. Wound J. 2019, 16, 84–95. [Google Scholar] [CrossRef]
- Dart, A.; Bhave, M.; Kingshott, P. Antimicrobial Peptide-Based Electrospun Fibers for Wound Healing Applications. Macromol. Biosci. 2019, 19, e1800488. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.; Tomaselli, N. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Chapter 6—Efficacy of Polymer-Based Wound Dressings in Chronic Wounds. In Modeling and Control of Drug Delivery Systems; Azar, A.T., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 79–110. [Google Scholar]
- Sikka, M.P.; Midha, V.K. 16—The role of biopolymers and biodegradable polymeric dressings in managing chronic wounds. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 463–488. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Zeng, D.; Shen, S.; Fan, D. Molecular design, synthesis strategies and recent advances of hydrogels for wound dressing applications. Chin. J. Chem. Eng. 2021, 30, 308–320. [Google Scholar] [CrossRef]
- Agarwal, A.; McAnulty, J.F.; Schurr, M.J.; Murphy, C.J.; Abbott, N.L. 8—Polymeric materials for chronic wound and burn dressings. In Advanced Wound Repair Therapies; Farrar, D., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 186–208. [Google Scholar]
- Leveriza-Oh, M.; Phillips, T. Chapter 32—Dressings and postoperative carea. In Lower Extremity Soft Tissue & Cutaneous Plastic Surgery, 2nd ed.; Dockery, G.D., Crawford, M.E., Eds.; W.B. Saunders: Oxford, UK, 2012; pp. 471–488. [Google Scholar]
- Nielsen, J.; Fogh, K. Clinical utility of foam dressings in wound management: A review. Chronic Wound Care Manag. Res. 2015, 2, 31–38. [Google Scholar]
- Kirwan, H.; Pignataro, R. Chapter 2—The Skin and Wound Healing. In Pathology and Intervention in Musculoskeletal Rehabilitation, 2nd ed.; Magee, D.J., Zachazewski, J.E., Quillen, W.S., Manske, R.C., Eds.; W.B. Saunders: Oxford, UK, 2016; pp. 25–62. [Google Scholar]
- Weller, C. 4—Interactive dressings and their role in moist wound management. In Advanced Textiles for Wound Care; Rajendran, S., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 97–113. [Google Scholar]
- Chaganti, P.; Gordon, I.; Chao, J.H.; Zehtabchi, S. A systematic review of foam dressings for partial thickness burns. Am. J. Emerg. Med. 2019, 37, 1184–1190. [Google Scholar] [CrossRef]
- Namviriyachote, N.; Lipipun, V.; Akkhawattanangkul, Y.; Charoonrut, P.; Ritthidej, G.C. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J. Pharm. Sci. 2019, 14, 63–77. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef]
- Peles, Z.; Zilberman, M. Novel soy protein wound dressings with controlled antibiotic release: Mechanical and physical properties. Acta Biomater. 2012, 8, 209–217. [Google Scholar] [CrossRef]
- Srivastava, C.M.; Purwar, R.; Kannaujia, R.; Sharma, D. Flexible silk fibroin films for wound dressing. Fibers Polym. 2015, 16, 1020–1030. [Google Scholar] [CrossRef]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef]
- Levin, A. The Clinical Epidemiology of Cardiovascular Diseases in Chronic Kidney Disease: Clinical Epidemiology of Cardiovascular Disease in Chronic Kidney Disease Prior to Dialysis. Semin. Dial. 2003, 16, 101–105. [Google Scholar] [CrossRef]
- Cilurzo, F.; Musazzi, U.M.; Franzé, S.; Fedele, G.; Minghetti, P. Design of in vitro skin permeation studies according to the EMA guideline on quality of transdermal patches. Eur. J. Pharm. Sci. 2018, 125, 86–92. [Google Scholar] [CrossRef]
- Auda, S.H.; Mahrous, G.M.; Ibrahim, M.A.; Shazly, G.A.; Salem-Bekhit, M.M. Novel chlorhexidine dermal patches, preparation characterization and antimicrobial evaluation. Polym. Bull. 2017, 74, 3995–4007. [Google Scholar] [CrossRef]
- Suhaeri, M.; Noh, M.; Moon, J.; Kim, I.; Oh, S.; Ha, S.; Lee, J.; Park, K. Novel skin patch combining human fibroblast-derived matrix and ciprofloxacin for infected wound healing. Theranostics 2018, 8, 5025–5038. [Google Scholar] [CrossRef]
- Nilani, P.; Pranavi, A.; Duraisamy, B.; Damodaran, P.; Subhashini, V.; Elango, K. Formulation and evaluation of wound healing dermal patch. Afr. J. Pharm. Pharmacol. 2011, 5, 1252–1257. [Google Scholar]
- Guglielmi, P.; Pontecorvi, V.; Rotondi, G. Natural compounds and extracts as novel antimicrobial agents. Expert Opin. Ther. Pat. 2020, 30, 949–962. [Google Scholar] [CrossRef]
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun Nanofibrous Polyurethane Membrane as Wound Dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 675–679. [Google Scholar] [CrossRef]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.; Park, Y.J.; Hong, S.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef]
- Ju, H.W.; Lee, O.J.; Lee, J.M.; Moon, B.M.; Park, H.J.; Park, Y.R.; Lee, M.C.; Kim, S.H.; Chao, J.R.; Ki, C.S.; et al. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int. J. Biol. Macromol. 2016, 85, 29–39. [Google Scholar] [CrossRef]
- Lin, J.; Li, C.; Zhao, Y.; Hu, J.; Zhang, L. Co-electrospun Nanofibrous Membranes of Collagen and Zein for Wound Healing. ACS Appl. Mater. Interfaces 2012, 4, 1050–1057. [Google Scholar] [CrossRef]
- Venugopal, J.R.; Zhang, Y.; Ramakrishna, S. In Vitro Culture of Human Dermal Fibroblasts on Electrospun Polycaprolactone Collagen Nanofibrous Membrane. Artif. Organs 2006, 30, 440–446. [Google Scholar] [CrossRef]
- Coelho, D.S.; Veleirinho, B.; Alberti, T.; Maestri, A.; Yunes, R.; Dias, P.F.; Maraschin, M. Electrospinning technology: Designing nanofibers toward wound healing application. In Nanomaterials-Toxicity, Human Health and Environment; Intechopen: London, UK, 2018; pp. 1–19. [Google Scholar]
- Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. Electrospinning of Nanofibers and Their Applications for Energy Devices. J. Nanomater. 2015, 2015, 140716. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-Based Electrospun Fibers for Wound Healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Abrigo, M.; Kingshott, P.; McArthur, S. Electrospun Polystyrene Fiber Diameter Influencing Bacterial Attachment, Proliferation, and Growth. ACS Appl. Mater. Interfaces 2015, 7, 7644–7652. [Google Scholar] [CrossRef]
- Genevro, G.M.; Gomes Neto, R.J.; de Paulo, L.A.; Lopes, P.S.; de Moraes, M.A.; Beppu, M.M. Glucomannan asymmetric membranes for wound dressing. J. Mater. Res. 2019, 34, 481–489. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- López-Mata, M.A.; Gastelum-Cabrera, M.; Valbuena-Gregorio, E.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E.; Morales-Figueroa, G.G.; Quihui-Cota, L.; Juárez-Onofre, J.E. Physicochemical properties of novel pectin/Aloe gel membranes. Iran. Polym. J. 2018, 27, 545–553. [Google Scholar] [CrossRef]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69B, 216–222. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Pires, A.L.R.; de Azevedo Motta, L.; Dias, A.M.A.; de Sousa, H.C.; Moraes, Â.M.; Braga, M.E.M. Towards wound dressings with improved properties: Effects of poly(dimethylsiloxane) on chitosan-alginate films loaded with thymol and beta-carotene. Mater. Sci. Eng. C 2018, 93, 595–605. [Google Scholar] [CrossRef]
- Bueno, C.Z.; Moraes, A.J. Development of porous lamellar chitosan-alginate membranes: Effect of different surfactants on biomaterial properties. Appl. Polym. Sci. 2011, 122, 624. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Kimna, C.; Tamburaci, S.; Tihminlioglu, F. Novel zein-based multilayer wound dressing membranes with controlled release of gentamicin. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2057–2070. [Google Scholar] [CrossRef]
- Popa, G.-M.L.; Truşcă, R.D.; Ilie, C.-I.; Țiplea, R.E.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Dițu, L.-M. Antibacterial Activity of Bacterial Cellulose Loaded with Bacitracin and Amoxicillin: In Vitro Studies. Molecules 2020, 25, 4069. [Google Scholar]
- Cheng, C.-F.; Wu, K.; Chen, Y.; Hung, S. Bacterial adhesion to antibiotic-loaded guided tissue regeneration membranes—A scanning electron microscopy study. J. Formos. Med. Assoc. 2015, 114, 35–45. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Li, P.; Zhu, C.; Lin, Z.; Mackey, V.; Coy, D.H.; He, Q. The wound dressings and their applications in wound healing and management. Health Sci. J. 2019, 13, 1–8. [Google Scholar]
- Pasut, G.; Veronese, F. Polymer–drug conjugation, recent achievements and general strategies. Prog. Polym. Sci. 2007, 32, 933–961. [Google Scholar] [CrossRef]
- Rohini, N.A.; Joseph, A.; Mukerji, A. Polymeric prodrugs: Recent achievements and general strategies. J. Antivir. Antiretrovir. 2013, 2, S15. [Google Scholar]
- Abuchowski, A.; van Es, T.; Palczuk, N.C.; Davis, F.F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977, 252, 3578–3581. [Google Scholar] [CrossRef]
- Abuchowski, A.; McCoy, J.R.; Palczuk, N.C.; van Es, T.; Davis, F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977, 252, 3582–3586. [Google Scholar] [CrossRef]
- Schoemaker, N.E.; van Kesteren, C.; Rosing, H.; Jansen, S.; Swart, M.; Lieverst, J.; Fraier, D.; Breda, M.; Pellizzoni, C.; Spinelli, R.; et al. A phase I and pharmacokinetic study of MAG-CPT, a water-soluble polymer conjugate of camptothecin. Br. J. Cancer 2002, 87, 608–614. [Google Scholar] [CrossRef]
- Kim, C.J.; Lee, Y.S.; Lee, K.H.; Jeong, B.; Kim, T.W.; Kang, T.H.; Kim, H.S.; Park, J. Effect of topical Paclitaxel using PEG/PLGA polymer on the animal model of cervical cancer. Korean J. Gynecol. Oncol. 2008, 19, 68–74. [Google Scholar] [CrossRef]
- Maeda, H. SMANCS and polymer-conjugated macromolecular drugs: Advantages in cancer chemotherapy. Adv. Drug Deliv. Rev. 2001, 46, 169–185. [Google Scholar] [CrossRef]
- Balasubramaniam, M.P.; Murugan, P.; Chenthamara, D.; Ramakrishnan, S.G.; Salim, A.; Lin, F.; Robert, B.; Subramaniam, S. Synthesis of chitosan-ferulic acid conjugated poly(vinyl alcohol) polymer film for an improved wound healing. Mater. Today Commun. 2020, 25, 101510. [Google Scholar] [CrossRef]
- Hardwicke, J.T.; Hart, J.; Bell, A.; Duncan, R.; Thomas, D.W.; Moseley, R. The effect of dextrin–rhEGF on the healing of full-thickness, excisional wounds in the (db/db) diabetic mouse. J. Control. Release 2011, 152, 411–417. [Google Scholar] [CrossRef]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- Alam, M.M.; Islam, M.N.; Hossain Hawlader, M.D.; Ahmed, S.; Wahab, A.; Islam, M.; Uddin, K.M.R.; Hossain, A. Prevalence of multidrug resistance bacterial isolates from infected wound patients in Dhaka, Bangladesh: A cross-sectional study. Int. J. Surg. Open 2021, 28, 56–62. [Google Scholar] [CrossRef]
- Unalan, I.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Frank, G.; Boccaccini, A.R. Physical and Antibacterial Properties of Peppermint Essential Oil Loaded Poly (ε-caprolactone) (PCL) Electrospun Fiber Mats for Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 346. [Google Scholar] [CrossRef]
- Javanbakht, S.; Nabi, M.; Shadi, M.; Amini, M.M.; Shaabani, A. Carboxymethyl cellulose/tetracycline@UiO-66 nanocomposite hydrogel films as a potential antibacterial wound dressing. Int. J. Biol. Macromol. 2021, 188, 811–819. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Jasovský, D.; Littmann, J.; Zorzet, A.; Cars, O. Antimicrobial resistance—A threat to the world’s sustainable development. Upsala J. Med. Sci. 2016, 121, 159–164. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and Characterization of PVA/Starch Hydrogel Membranes Incorporating Essential Oils Aimed to be Used in Wound Dressing Applications. J. Polym. Environ. 2021, 29, 156–174. [Google Scholar] [CrossRef]
- Syed, I.; Garg, S.; Sarkar, P. 5—Entrapment of essential oils in hydrogels for biomedical applications. In Polymeric Gels; Pal, K., Banerjee, I., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 125–141. [Google Scholar]
- Mahmood, H.; Khan, I.U.; Asif, M.; Khan, R.U.; Asghar, S.; Khalid, I.; Khalid, S.H.; Irfan, M.; Rehman, F.; Shahzad, Y.; et al. In vitro and in vivo evaluation of gellan gum hydrogel films: Assessing the co impact of therapeutic oils and ofloxacin on wound healing. Int. J. Biol. Macromol. 2021, 166, 483–495. [Google Scholar] [CrossRef]
- Chinnaiyan, S.K.; Pandiyan, R.; Natesan, S.; Chindam, S.; Gouti, A.K.; Sugumaran, A. Fabrication of basil oil Nanoemulsion loaded gellan gum hydrogel—Evaluation of its antibacterial and anti-biofilm potential. J. Drug Deliv. Sci. Technol. 2022, 68, 103129. [Google Scholar] [CrossRef]
- Ghosh, B.; Bhattacharya, D.; Mukhopadhyay, M. A hydrogel sheet mask with tea tree essential oil entrapment and targeted dose delivery capability. Mater. Today Proc. 2022, 57, 77–83. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; Nicoli, S.; Padula, C.; Santi, P.; Rossi, F.; de Holanda e Silva, K.G.; Mansur, C.R.E. Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: Permeation and stability studies. Eur. J. Pharm. Biopharm. 2017, 116, 38–50. [Google Scholar] [CrossRef]
- Goudoulas, T.B.; Vanderhaeghen, S.; Germann, N. Micro-dispersed essential oils loaded gelatin hydrogels with antibacterial activity. LWT 2022, 154, 112797. [Google Scholar] [CrossRef]
- Shabkhiz, M.A.; Khalil Pirouzifard, M.; Pirsa, S.; Mahdavinia, G.R. Alginate hydrogel beads containing Thymus daenensis essential oils/Glycyrrhizic acid loaded in β-cyclodextrin. Investigation of structural, antioxidant/antimicrobial properties and release assessment. J. Mol. Liq. 2021, 344, 117738. [Google Scholar] [CrossRef]
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of Apigenin loaded gellan gum–chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119. [Google Scholar] [CrossRef]
- Aelenei, P.; Luca, S.V.; Horhogea, C.E.; Rimbu, C.M.; Dimitriu, G.; Macovei, I.; Silion, M.; Aprotosoaie, A.C.; Miron, A. Morus alba leaf extract: Metabolite profiling and interactions with antibiotics against Staphylococcus spp. including MRSA. Phytochem. Lett. 2019, 31, 217–224. [Google Scholar] [CrossRef]
- Akhmetova, A.; Saliev, T.; Allan, I.U.; Illsley, M.J.; Nurgozhin, T.; Mikhalovsky, S. A Comprehensive Review of Topical Odor-Controlling Treatment Options for Chronic Wounds. J. Wound Ostomy Cont. Nurs. 2016, 43, 598–609. [Google Scholar] [CrossRef]
- Jin, S.G.; Kim, K.S.; Yousaf, A.M.; Kim, D.W.; Jang, S.W.; Son, M.; Kim, Y.H.; Yong, C.S.; Kim, J.O.; Choi, H. Mechanical properties and in vivo healing evaluation of a novel Centella asiatica-loaded hydrocolloid wound dressing. Int. J. Pharm. 2015, 490, 240–247. [Google Scholar] [CrossRef]
- Thangavel, A.; Ayyanar, M.; Justin Koilpillai, Y.; Sekar, T. Phytochemical Screening and Antibacterial activity of leaf and callus extracts of Centella asiatica. Bangladesh J. Pharmacol. 2011, 6, 55–60. [Google Scholar]
- Kuo, C.-W.; Chiu, Y.-F.; Wu, M.-H.; Li, M.-H.; Wu, C.-N.; Chen, W.-S.; Huang, C.-H. Gelatin/Chitosan Bilayer Patches Loaded with Cortex Phellodendron amurense/Centella asiatica Extracts for Anti-Acne Application. Polymers 2021, 13, 579. [Google Scholar] [CrossRef]
- Phaechamud, T.; Yodkhum, K.; Charoenteeraboon, J.; Tabata, Y. Chitosan-aluminum monostearate composite sponge dressing containing asiaticoside for wound healing and angiogenesis promotion in chronic wound. Mater. Sci. Eng. C 2015, 50, 210–225. [Google Scholar] [CrossRef]
- Oh, J.; Kim, H.; Beuchat, L.R.; Ryu, J.-H. Inhibition of Staphylococcus aureus on a laboratory medium and black peppercorns by individual and combinations of essential oil vapors. Food Control 2022, 132, 108487. [Google Scholar] [CrossRef]
- He, Q.; Zhang, L.; Yang, Z.; Ding, T.; Ye, X.; Liu, D.; Guo, M. Antibacterial mechanisms of thyme essential oil nanoemulsions against Escherichia coli O157:H7 and Staphylococcus aureus: Alterations in membrane compositions and characteristics. Innov. Food Sci. Emerg. Technol. 2022, 75, 102902. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.P.; Barros Ferreira, R.S.; Lizárraga, E.; Tapia-Blácido, D.R.; Silva, N.C.C.; Angelats-Silva, L.; Siche, R. Bioactive Andean sweet potato starch-based foam incorporated with oregano or thyme essential oil. Food Packag. Shelf Life 2020, 23, 100457. [Google Scholar] [CrossRef]
- Koga, A.Y.; Pereira, A.V.; Lipinski, L.C.; Oliveira, M.R.P. Evaluation of wound healing effect of alginate films containing Aloe vera (Aloe barbadensis Miller) gel. J. Biomater. Appl. 2018, 32, 1212–1221. [Google Scholar] [CrossRef]
- Atiba, A.; Nishimura, M.; Kakinuma, S.; Hiraoka, T.; Goryo, M.; Shimada, Y.; Ueno, H.; Uzuka, Y. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-β and fibroblast growth factor production. Am. J. Surg. 2011, 201, 809–818. [Google Scholar] [CrossRef]
- Guilherme, E.D.; de Souza, C.W.; Bernardo, M.P.; Zenke, M.; Mattoso, L.H.; Moreira, F.K. Antimicrobially active gelatin/[Mg-Al-CO3]-LDH composite films based on clove essential oil for skin wound healing. Mater. Today Commun. 2021, 27, 102169. [Google Scholar]
- Razavi, M.S.; Golmohammadi, A.; Nematollahzadeh, A.; Rovera, C.; Farris, S. Cinnamon Essential Oil Encapsulated into a Fish Gelatin-Bacterial Cellulose Nanocrystals Complex and Active Films Thereof. Food Biophys. 2021, 17, 38–46. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef]
- Kavoosi, G.; Dadfar, S.M.; Purfard, A.M. Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci. 2013, 78, E244–E250. [Google Scholar] [CrossRef]
- Otoni, C.G.; Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Roberts, M.S. Solute-Vehicle-Skin Interactions in Percutaneous Absorption: The Principles and the People. Ski. Pharmacol. Physiol. 2013, 26, 356–370. [Google Scholar] [CrossRef]
- Sroczyk, E.A.; Berniak, K.; Jaszczur, M.; Stachewicz, U. Topical electrospun patches loaded with oil for effective gamma linoleic acid transport and skin hydration towards atopic dermatitis skincare. Chem. Eng. J. 2022, 429, 132256. [Google Scholar] [CrossRef]
- Tuter, M.; Secundo, F.; Riva, S.; Aksoy, H.A.; Ustun, G. Partial purification of Nigella sativa L. Seed lipase and its application in transesterification reactions. J. Am. Oil Chem. Soc. 2003, 80, 43–48. [Google Scholar] [CrossRef]
- Foster, R.H.; Hardy, G.; Alany, R. Borage oil in the treatment of atopic dermatitis. Nutrition 2010, 26, 708–718. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, J.; Lee, S. The Therapeutic Effect of Evening Primrose Oil in Atopic Dermatitis Patients with Dry Scaly Skin Lesions Is Associated with the Normalization of Serum Gamma-Interferon Levels. Ski. Pharmacol. Physiol. 2002, 15, 20–25. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Dhiman, S.; Borah, S.; Rabha, B.; Chaurasia, A.K.; Veer, V. Essential oil based polymeric patch development and evaluating its repellent activity against mosquitoes. Acta Trop. 2015, 147, 45–53. [Google Scholar] [CrossRef]
- Shan, Y.-H.; Peng, L.-H.; Liu, X.; Chen, X.; Xiong, J.; Gao, J.-Q. Silk fibroin/gelatin electrospun nanofibrous dressing functionalized with astragaloside IV induces healing and anti-scar effects on burn wound. Int. J. Pharm. 2015, 479, 291–301. [Google Scholar] [CrossRef]
- Safaee-Ardakani, M.R.; Hatamian-Zarmi, A.; Sadat, S.M.; Mokhtari-Hosseini, Z.B.; Ebrahimi-Hosseinzadeh, B.; Rashidiani, J.; Kooshki, H. Electrospun Schizophyllan/polyvinyl alcohol blend nanofibrous scaffold as potential wound healing. Int. J. Biol. Macromol. 2019, 127, 27–38. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Faraji, S.; Nowroozi, N.; Nouralishahi, A.; Shayeh, J.S. Electrospun poly-caprolactone/graphene oxide/quercetin nanofibrous scaffold for wound dressing: Evaluation of biological and structural properties. Life Sci. 2020, 257, 118062. [Google Scholar] [CrossRef]
- Ramalingam, R.; Dhand, C.; Leung, C.M.; Ong, S.T.; Annamalai, S.K.; Kamruddin, M.; Verma, N.K.; Ramakrishna, S.; Lakshminarayanan, R.; Arunachalam, K.D. Antimicrobial properties and biocompatibility of electrospun poly-ε-caprolactone fibrous mats containing Gymnema sylvestre leaf extract. Mater. Sci. Eng. C 2019, 98, 503–514. [Google Scholar] [CrossRef]
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Darban, S.A.; Bazzaz, B.S.F.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2020, 162, 645–656. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.; Abdel-Rahman, R.; Kubena, I.; Kobera, L.; Spotz, Z.; Zboncak, M.; Prikryl, R.; Brus, J.; Jancar, J. Chitosan-glucan complex hollow fibers reinforced collagen wound dressing embedded with aloe vera. Part I: Preparation and characterization. Carbohydr. Polym. 2020, 230, 115708. [Google Scholar] [CrossRef]
- Dong, W.-H.; Liu, J.-X.; Mou, X.-J.; Liu, G.-S.; Huang, X.-W.; Yan, X.; Ning, X.; Russell, S.J.; Long, Y.-Z. Performance of polyvinyl pyrrolidone-isatis root antibacterial wound dressings produced in situ by handheld electrospinner. Colloids Surf. B Biointerfaces 2020, 188, 110766. [Google Scholar] [CrossRef]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Khan, G.; Pandey, V.K.; Bakade, B.V.; Mishra, B. Biomimetic PCL-gelatin based nanofibers loaded with ciprofloxacin hydrochloride and quercetin: A potential antibacterial and anti-oxidant dressing material for accelerated healing of a full thickness wound. Int. J. Pharm. 2019, 567, 118480. [Google Scholar] [CrossRef]
- Urena-Saborio, H.; Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Gunasekaran, S. Electrospun plant mucilage nanofibers as biocompatible scaffolds for cell proliferation. Int. J. Biol. Macromol. 2018, 115, 1218–1224. [Google Scholar] [CrossRef]
- Jafari, A.; Amirsadeghi, A.; Hassanajili, S.; Azarpira, N. Bioactive antibacterial bilayer PCL/gelatin nanofibrous scaffold promotes full-thickness wound healing. Int. J. Pharm. 2020, 583, 119413. [Google Scholar] [CrossRef]
- Ardekani, N.T.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef]
- Abou Zekry, S.S.; Abdellatif, A.; Azzazy, H.M. Fabrication of pomegranate/honey nanofibers for use as antibacterial wound dressings. Wound Med. 2020, 28, 100181. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Liu, X.; Mao, J.; Gu, S.; Xu, W. Electrospinning of carboxyethyl chitosan/poly(vinyl alcohol)/silk fibroin nanoparticles for wound dressings. Int. J. Biol. Macromol. 2013, 53, 88–92. [Google Scholar] [CrossRef]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Mahto, S.K. Soy protein isolate supplemented silk fibroin nanofibers for skin tissue regeneration: Fabrication and characterization. Int. J. Biol. Macromol. 2020, 160, 112–127. [Google Scholar] [CrossRef]
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers—A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar] [CrossRef]
- Yousefi, I.; Pakravan, M.; Rahimi, H.; Bahador, A.; Farshadzadeh, Z.; Haririan, I. An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater. Sci. Eng. C 2017, 75, 433–444. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int. J. Pharm. 2013, 452, 333–343. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Özlem, E. Production of lavender oil loaded antibacterial polymeric membranes. Cumhur. Sci. J. 2020, 41, 160–168. [Google Scholar]
- Chomachayi, M.D.; Solouk, A.; Akbari, S.; Sadeghi, D.; Mirahmadi, F.; Mirzadeh, H. Electrospun nanofibers comprising of silk fibroin/gelatin for drug delivery applications: Thyme essential oil and doxycycline monohydrate release study. J. Biomed. Mater. Res. Part A 2018, 106, 1092–1103. [Google Scholar] [CrossRef]
- Son, B.C.; Park, C.; Kim, C. Fabrication of antimicrobial nanofiber air filter using activated carbon and cinnamon essential oil. J. Nanosci. Nanotechnol. 2020, 20, 4376–4380. [Google Scholar] [CrossRef]
- Liakos, I.L.; Holban, A.M.; Carzino, R.; Lauciello, S.; Grumezescu, A.M. Electrospun fiber pads of cellulose acetate and essential oils with antimicrobial activity. Nanomaterials 2017, 7, 84. [Google Scholar] [CrossRef]
- Rieger, K.A.; Schiffman, J. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561–568. [Google Scholar] [CrossRef]
- Bharathi, B.S.; Stalin, T. Cerium oxide and peppermint oil loaded polyethylene oxide/graphene oxide electrospun nanofibrous mats as antibacterial wound dressings. Mater. Today Commun. 2019, 21, 100664. [Google Scholar]
- Bazargani, M.M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016, 61, 156–164. [Google Scholar] [CrossRef]
- Eğri, Ö.; Erdemir, N. Production of Hypericum perforatum oil-loaded membranes for wound dressing material and in vitro tests. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1404–1415. [Google Scholar] [CrossRef]
- Xiang, F.; Bai, J.; Tan, X.; Chen, T.; Yang, W.; He, F. Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl. et Van. var. argyi cv. Qiai. Ind. Crops Prod. 2018, 125, 582–587. [Google Scholar] [CrossRef]
- Li, T.-T.; Li, J.; Zhang, Y.; Huo, J.-L.; Liu, S.; Shiu, B.-C.; Lin, J.-H.; Lou, C.-W. A study on artemisia argyi oil/sodium alginate/PVA nanofibrous membranes: Micro-structure, breathability, moisture permeability, and antibacterial efficacy. J. Mater. Res. Technol. 2020, 9, 13450–13458. [Google Scholar] [CrossRef]
- Lamarra, J.; Bucci, P.; Giannuzzi, L.; Montanari, J.; Rivero, S.; Pinotti, A. Biomaterial-based dressings as vehicle for chitosan-encapsulated cabreuva essential oil: Cytotoxicity and regenerative activity. React. Funct. Polym. 2020, 156, 104728. [Google Scholar] [CrossRef]
- Khan, A.u.R.; Huang, K.; Jinzhong, Z.; Zhu, T.; Morsi, Y.; Aldalbahi, A.; El-Newehy, M.; Yan, X.; Mo, X. PLCL/Silk fibroin based antibacterial nano wound dressing encapsulating oregano essential oil: Fabrication, characterization and biological evaluation. Colloids Surf. B: Biointerfaces 2020, 196, 111352. [Google Scholar] [CrossRef]
- Özkalp, B.; Sevgi, F.; Özcan, M.; Özcan, M. The antibacterial activity of essential oil of oregano (Origanum vulgare L.). J. Food Agric. Environ. 2010, 8, 6–8. [Google Scholar]
- Lu, M.; Dai, T.; Murray, C.K.; Wu, M.X. Bactericidal Property of Oregano Oil Against Multidrug-Resistant Clinical Isolates. Front. Microbiol. 2018, 9, 2329. [Google Scholar] [CrossRef]
- Greco, F.; Vicent, M. Combination therapy: Opportunities and challenges for polymer–drug conjugates as anticancer nanomedicines. Adv. Drug Deliv. Rev. 2009, 61, 1203–1213. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Design and therapeutic efficacy of polymer based drug delivery systems for antimalarials. Polym. Sci. Res. Adv. Pract. Appl. Educ. Asp. 2016, 188–198. [Google Scholar]
- Sanchis, J.; Canal, F.; Lucas, R.; Vicent, M.J. Polymer–drug conjugates for novel molecular targets. Nanomedicine 2010, 5, 915–935. [Google Scholar] [CrossRef]
- Trifković, K.T.; Milašinović, N.Z.; Djordjević, V.B.; Krušić, M.T.K.; Knežević-Jugović, Z.D.; Nedović, V.A.; Bugarski, B.M. Chitosan microbeads for encapsulation of thyme (Thymus serpyllum L.) polyphenols. Carbohydr. Polym. 2014, 111, 901–907. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; Gómez y Gómez, Y. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef]
- Francesko, A.; Fernandes, M.; Rocasalbas, G.; Gautier, S.; Tzanov, T. Polymers in Wound Repair. In Advanced Polymers in Medicine; Springer: Cham, Switzerland, 2015; pp. 401–431. [Google Scholar]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Muthukumar, T.; Senthil, R.; Sastry, T. Synthesis and characterization of biosheet impregnated with Macrotyloma uniflorum extract for burn/wound dressings. Colloids Surf B Biointerfaces 2013, 102, 694–699. [Google Scholar] [CrossRef]
- Silva, S.S.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Effect of crosslinking in chitosan/aloe vera-based membranes for biomedical applications. Carbohydr Polym 2013, 98, 581–588. [Google Scholar] [CrossRef]
- Agarwal, R.; Alam, S.; Gupta, B. Preparation of Curcumin Loaded Poly(Vinyl Alcohol)-Poly(Ethylene Oxide)-Carboxymethyl Cellulose Membranes for Wound Care Application. J. Biomater. Tissue Eng. 2013, 3, 273–283. [Google Scholar] [CrossRef]
- Yang, J.Y.; Singh, D.; Singh, D.; Lee, E.; Choi, S.; Han, S.S.; Park, S.J. Terminalia bellirica Extracts Loaded on Stimuli Responsive HEMA-DEA Hydrogel for Enhanced Growth and Proliferation of Mesenchymal Stem Cells. J. Biomater. Tissue Eng. 2014, 4, 37–45. [Google Scholar] [CrossRef]
- Ilhan, E.; Cesur, S.; Guler, E.; Topal, F.; Albayrak, D.; Guncu, M.M.; Cam, M.E.; Taskin, T.; Sasmazel, H.T.; Aksu, B.; et al. Development of Satureja cuneifolia-loaded sodium alginate/polyethylene glycol scaffolds produced by 3D-printing technology as a diabetic wound dressing material. Int. J. Biol. Macromol. 2020, 161, 1040–1054. [Google Scholar] [CrossRef]
- Ali Khan, B.; Ullah, S.; Khan, M.K.; Alshahrani, S.M.; Braga, V.A. Formulation and evaluation of Ocimum basilicum-based emulgel for wound healing using animal model. Saudi Pharm. J. 2020, 28, 1842–1850. [Google Scholar] [CrossRef]
- Razdan, K.; Kanta, S.; Chaudhary, E.; Kumari, S.; Rahi, D.K.; Yadav, A.K.; Sinha, V.R. Levofloxacin loaded clove oil nanoscale emulgel promotes wound healing in Pseudomonas aeruginosa biofilm infected burn wound in mice. Colloids Surf. B Biointerfaces 2023, 222, 113113. [Google Scholar] [CrossRef]
- Eid, A.M.; Jaradat, N.; Issa, L.; Abu-Hasan, A.; Salah, N.; Dalal, M.; Mousa, A.; Zarour, A. Evaluation of anticancer, antimicrobial, and antioxidant activities of rosemary (Rosmarinus Officinalis) essential oil and its Nanoemulgel. Eur. J. Integr. Med. 2022, 55, 102175. [Google Scholar] [CrossRef]
- Ting, T.C.; Amat Rahim, N.F.; Che Zaudin, N.A.; Abdullah, N.H.; Mohamad, M.; Shoparwe, N.F.; Mhd Ramle, S.F.; Aimi, Z.; Abdul Hamid, Z.A.; Yusof, A.H. Development and Characterization of Nanoemulgel Containing Piper betle Essential Oil as Active Ingredient. IOP Conf. Ser. Earth Environ. Sci. 2020, 596, 012032. [Google Scholar] [CrossRef]
- Barrett, J.F. Can biotech deliver new antibiotics? Curr. Opin. Microbiol. 2005, 8, 498–503. [Google Scholar] [CrossRef]
- Pallavali, R.R.; Avula, S.; Degati, V.L.; Penubala, M.; Damu, A.G.; Durbaka, V.R.P. Data of antibacterial activity of plant leaves crude extract on bacterial isolates of wound infections. Data Brief 2019, 24, 103896. [Google Scholar] [CrossRef]
- Algreiby, A.A.; Hammer, K.A.; Durmic, Z.; Vercoe, P.; Flematti, G.R. Antibacterial compounds from the Australian native plant Eremophila glabra. Fitoterapia 2018, 126, 45–52. [Google Scholar] [CrossRef]
- Smith, J.E.; Tucker, D.; Watson, K.; Jones, G. Identification of antibacterial constituents from the indigenous Australian medicinal plant Eremophila duttonii F. Muell. (Myoporaceae). J. Ethnopharmacol. 2007, 112, 386–393. [Google Scholar] [CrossRef]
- Revathi, P.; Parimelazhagan, T.; Manian, S. Quantification of phenolic compounds, in vitro antioxidant analysis and screening of chemical compounds using GC-MS in Acalypha alnifolia Klein ex willd.: A leafy vegetable. Int. J. Pharma Biosci. 2013, 4, 973–986. [Google Scholar]
- Noumedem, J.A.; Tamokou, J.D.; Teke, G.N.; Momo, R.C.; Kuete, V.; Kuiate, J.R. Phytochemical analysis, antimicrobial and radical-scavenging properties of Acalypha manniana leaves. SpringerPlus 2013, 2, 503. [Google Scholar] [CrossRef]
- Evanjelene, V.K.; Natarajan, D. Evaluation of antioxidant, phytochemical and antibacterial properties of Acalypha alnifolia Klein ex Willd. J. Chem. Pharm. Res. 2013, 5, 205–212. [Google Scholar]
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 2006, 6, 35. [Google Scholar] [CrossRef]
- Rao, R.V.; Rao, G.; Rao, B. Anti-inflammatory activity of the leaves and bark of delonix elata. Anc. Sci. Life 1997, 17, 141–143. [Google Scholar]
- Amabye, T.; Bezabh, A.; Mekonen, F. Phytochemical Constituents and Antioxidant Activity of Delonix elata L. Flower Extract. J. Anal. Pharm. Res. 2016, 2, 00006. [Google Scholar]
- Gopal, M.; Shamanna, M. Anti-arthritic and immune modifying potential of delonix elata bark extracts. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1642–1648. [Google Scholar]
- Kumarappan, M.; Salwe, K.; Shetty, H. Evaluation of Protective Effect of Delonix Elata on Chronic Inflammation and Comparison of its Ulcerogenic Potential with Ibuprofen. J. Pharma Biosci. 2011, 2, 237–243. [Google Scholar]
- Vijayasanthi, M.; Kannan, V. Antimicrobial activities of Delonix elata (Bojer ex Hook.) Raf. and Spathodea campanulata P. Beauv. Afr. J. Microbiol. Res. 2014, 8, 697–701. [Google Scholar]
- Singh, S.; Kumar, S. A review: Introduction to genus Delonix. World J. Pharm. Pharm. Sci. 2014, 3, 2042–2055. [Google Scholar]
- Modi, A.; Mishra, V.; Bhatt, A.; Jain, A.; Mansoori, M.H.; Gurnany, E.; Kumar, V. Delonix regia: Historic perspectives and modern phytochemical and pharmacological researches. Chin. J. Nat. Med. 2016, 14, 31–39. [Google Scholar]
- Rahman, M.; Hasan, N.; Das, A.K.; Hossain, T.; Jahan, R.; Khatun, A.; Rahmatullah, M. Effect of Delonix regia leaf extract on glucose tolerance in glucose-induced hyperglycemic mice. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 34–36. [Google Scholar]
- Sama, K.; Xavier, V.; Raja, A. Preliminary phytochemical screening of root bark of Delonix regia. Int. J. Pharm. Life Sci. 2011, 2, 42–43. [Google Scholar]
- Kumar, A.R.; Shaik, R.; Yeshwanth, D. Phytochemical evaluation of Delonix regia, Samanea saman, Bauhinia variegatga. Int. J. Res. Pharm. Chem. 2013, 3, 768–772. [Google Scholar]
- Shiramane, R.S.; Biradar, K.V.; Chivde, B.V.; Shambhulingayya, H.; Goud, V. In-vivo antidiarrhoeal activity of ethanolic extract of Delonix regia flowers in experimental induced diarrhoea in wistar albino rats. Int. J. Res. Pharm. Chem. 2011, 1, 2231–2781. [Google Scholar]
- Shewale, V.D.; Deshmukh, T.A.; Patil, L.S.; Patil, V.R. Anti-inflammatory activity of delonix regia (boj. Ex. Hook). Adv. Pharmacol. Sci. 2011, 2012, 789713. [Google Scholar]
- Shabir, G.; Anwar, F.; Sultana, B.; Khalid, Z.M.; Afzal, M.; Khan, Q.M.; Ashrafuzzaman, M. Antioxidant and antimicrobial attributes and phenolics of different solvent extracts from leaves, flowers and bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf.]. Molecules 2011, 16, 7302–7319. [Google Scholar] [CrossRef]
- Adjé, F.; Lozano, Y.F.; Lozano, P.; Adima, A.; Chemat, F.; Gaydou, E.M. Optimization of anthocyanin, flavonol and phenolic acid extractions from Delonix regia tree flowers using ultrasound-assisted water extraction. Ind. Crops Prod. 2010, 32, 439–444. [Google Scholar] [CrossRef]
- Vivek, M.N.; Sachidananda Swamy, H.C.; Manasa, M.; Pallavi, S.; Yashoda Kambar Asha, M.M.; Chaithra, M.; Prashith Kekuda, T.R.; Mallikarjun, N.; Onkarappa, R. Antimicrobial and antioxidant activity of leaf and flower extract of Caesalpinia pulcherrima, Delonix regia and Peltaphorum ferrugineum. J. Appl. Pharm. Sci. 2013, 3, 64. [Google Scholar]
- Gupta, R.; Chandra, S. Chemical investigation of Delonix regia Raf. flowers. Indian J. Pharm. 1971, 33, 75. [Google Scholar]
- Khan, M.A.; Saxena, A.; Fatima, F.T.; Sharma, G.; Goud, V.; Husain, A. Study of wound healing activity of Delonix regia flowers in experimental animal models. Am. J. PharmTech Res. 2012, 2, 380–390. [Google Scholar]
- Parekh, J.; Jadeja, D.; Chanda, S. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol. 2006, 29, 203–210. [Google Scholar]
- Swamy, H.S.; Asha, M.M.; Chaithra, M.; Vivek, M.N.; Yoshoda Kambar, P.K.T.R. Antibacterial activity of flower extract of Caesalpinia pulcherrima, Delonix regia and Peltaphorum ferrugineum against urinary tract pathogens. Int. Res. J. Biol. Sci. 2014, 3, 80–83. [Google Scholar]
- Mathad, P.; Mety, S.S. Phytochemical and Antimicrobial Activity of Digera Muricata (L.) Mart. E-J. Chem. 2010, 7, 509254. [Google Scholar] [CrossRef]
- Ghaffar, A.; Tung, B.T.; Rahman, R.; Nadeem, F.; Idrees, M. Botanical Specifications, Chemical Composition and Pharmacological Applications of Tartara (Digera muricata L.)–A Review. Int. J. Chem. Biochem. Sci. 2019, 16, 17–22. [Google Scholar]
- Khan, M.R.; Ahmed, D. Protective effects of Digera muricata (L.) Mart. on testis against oxidative stress of carbon tetrachloride in rat. Food Chem. Toxicol. 2009, 47, 1393–1399. [Google Scholar] [CrossRef]
- Raj, V.P.; Chandrasekhar, R.H.; Vijayan, P.; Dhanaraj, S.A.; Rao, M.C.; Rao, V.J.; Nitesh, K. In vitro and in vivo hepatoprotective effects of the total alkaloid fraction of Hygrophila auriculata leaves. Indian J. Pharmacol. 2010, 42, 99–104. [Google Scholar]
- Ahmed, S.; Riaz, M.; Malik, A.; Shahid, M. Effect of seed extracts of Withania somnifera, Croton tiglium and Hygrophila auriculata on behavior and physiology of Odontotermes obesus (Isoptera, Termitidae). Biologia 2007, 62, 770–773. [Google Scholar] [CrossRef]
- Govindachari, T.; Nagarajan, K.; Pai, B. Isolation of lupeol from the root of Asteracantha longifolia Nees. Indian J. Sci. Res. B 1957, 16, 72p. [Google Scholar]
- Nabèrè, O.; Adama, H.; Samson, G.; Kiessoum, K.; Patrice, Z.; Roland, M.N.-T.; Moussa, C.; Martin, K.; Jea, M.F. Antibacterial and phytochemical studies of three Acanthaceae species used in Burkina Faso traditional medicine. J. Appl. Pharm. Sci. 2013, 3, 49. [Google Scholar]
- Godbole, N.; Gunde, B.; Srivastava, P. An investigation of oil from seed of Hygrophila spinosa. Oil Soap 1941, 18, 206–207. [Google Scholar] [CrossRef]
- Parashar, V.; Singh, H. Investigation of Astercantha longifolia Nees. Indian J. Pharmacol. 1965, 27, 109–113. [Google Scholar]
- Shanmugasundaram, P.; Venkataraman, S. Hepatoprotective and antioxidant effects of Hygrophila auriculata (K. Schum) Heine Acanthaceae root extract. J. Ethnopharmacol. 2006, 104, 124–128. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Govindarajan, R.; Rao, G.M.; Rao, C.V.; Shirwaikar, A.; Mehrotra, S.; Pushpangadan, P. Action of Hygrophila auriculata against streptozotocin-induced oxidative stress. J. Ethnopharmacol. 2006, 104, 356–361. [Google Scholar] [CrossRef]
- Esther, V.C.J.; Saraswathi, R.; Dhanasekar, S. In vitro antibacterial and antifungal activities along with X-ray irradiation studies of medicinal plant Hygrophila Auriculata. Int. J. Pharm. Pharm. Sci. 2012, 4, 352–358. [Google Scholar]
- Vlietinck, A.; Van Hoof, L.; Totte, J.; Lasure, A.; Berghe, D.V.; Rwangabo, P.C.; Mvukiyumwami, J. Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J. Ethnopharmacol. 1995, 46, 31–47. [Google Scholar] [CrossRef]
- Laxmichand, B.H.; Modi, D. A Comprehensive Review on Maerua Oblongifolia. Int. J. Res. Advent Technol. 2019, 7, 721–727. [Google Scholar]
- Abdel-Mogib, M. A lupane triterpenoid from Maerua oblongifolia. Phytochemistry 1999, 51, 445–448. [Google Scholar] [CrossRef]
- Arulanand Raj, N.; Gopal, V.; Dhivya, S.; Jayabalan, G. Evaluation of wound healing effect of Maerua Oblongifolia in albino rats. World J. Pharm. Res. 2018, 8, 1380–1385. [Google Scholar]
- Sasi Priya, S. Maerua oblongifolia–What do we really know? Overview, Progress and Perspectives. J. PeerScientist 2020, 2, e1000012. [Google Scholar]
- Manjunatha, B. Antibacterial activity of Pterocarpus santalinus. Indian J. Pharm. Sci. 2006, 68, 115. [Google Scholar] [CrossRef]
- Karthick, M.; Parthiban, K. Chemical characterization of Pterocarpus santalinus wood using GC-MS. J. Pharmacogn. Phytochem. 2019, 8, 380–382. [Google Scholar]
- Stella, J.; Krishnamoorthy, P.; Mohamed, A.J.; Anand, M. Free Radical Scavenging and Antibacterial Evaluation of Pterocarpus Santalinus Leaf In-Vitro Study. Int. J. Pharm. Sci. Res. 2011, 2, 1204. [Google Scholar]
- Chagas, V.T.; França, L.M.; Malik, S.; Paes, A.M.A. Syzygium cumini (L.) skeels: A prominent source of bioactive molecules against cardiometabolic diseases. Front. Pharmacol. 2015, 6, 259. [Google Scholar] [CrossRef]
- Bandiola, T.; Ignacio, G.B.; Yunson, E.G.; Bandiola, P.D. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents, toxicity studies, and traditional and pharmacological uses. Int. J. Appl. Pharm. Biol. Res. 2017, 2, 15–23. [Google Scholar]
- Ayyanar, M.; Subash-Babu, P. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac. J. Trop. Biomed. 2012, 2, 240–246. [Google Scholar]
- Gowri, S.S.; Vasantha, K. Phytochemical screening and antibacterial activity of Syzygium cumini (L.)(Myrtaceae) leaves extracts. Int. J. Pharm. Tech. Res. 2010, 2, 1569–1573. [Google Scholar]
- McKenzie, A.; Price, J. The alkaloids of Gyrocarpus americanus Jacq. Aust. J. Chem. 1953, 6, 180–185. [Google Scholar] [CrossRef]
- Bradacs, G.; Maes, L.; Heilmann, J. In vitro cytotoxic, antiprotozoal and antimicrobial activities of medicinal plants from Vanuatu. Phytother. Res. 2010, 24, 800–809. [Google Scholar] [CrossRef]
- Steenkamp, V.; Fernandes, A.C.; van Rensburg, C.E.J. Antibacterial activity of Venda medicinal plants. Fitoterapia 2007, 78, 561–564. [Google Scholar] [CrossRef]
- Prakash, C.V.S.; Prakash, I. Bioactive chemical constituents from pomegranate (Punica granatum) juice, seed and peel—A review. Int. J. Res. Chem. Environ. 2011, 1, 1–18. [Google Scholar]
- Jurenka, J. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Schubert, S.Y.; Lansky, E.P.; Neeman, I. Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids. J. Ethnopharmacol. 1999, 66, 11–17. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Jayaprakasha, G.K.; Singh, R.P. Studies on Antioxidant Activity of Pomegranate (Punica granatum) Peel Extract Using in Vivo Models. J. Agric. Food Chem. 2002, 50, 4791–4795. [Google Scholar] [CrossRef]
- Prashanth, D.; Asha, M.; Amit, A. Antibacterial activity of Punica granatum. Fitoterapia 2001, 72, 171–173. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The antimicrobial capacity of Cistus salviifolius and Punica granatum plant extracts against clinical pathogens is related to their polyphenolic composition. Sci. Rep. 2021, 11, 588. [Google Scholar] [CrossRef]
- Falodun, A.; Okunrobo, L.; Uzoamaka, N. Short Communication—Phytochemical screening and anti-inflammatory evaluation of methanolic and aqueous extracts of Euphorbia heterophylla Linn (Euphorbiaceae). Afr. J. Biotechnol. 2006, 5, 529–531. [Google Scholar]
- Onwuka, G.I. Food Analysis and Instrumentation: Theory and Practice; Napthali Prints: Lagos, Nigeria, 2005. [Google Scholar]
- Akinmutimi, A. Nutritive value of raw and processed jack fruit seeds (Artocarpus heterophyllus). Chem. Analysis. Agric. J. 2006, 1, 266–271. [Google Scholar]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- James, O.; Friday, E.T. Phytochemical composition, bioactivity and wound healing potential of Euphorbia heterophylla (Euphorbiaceae) leaf extract. Int. J. Pharm. Biomed. Res. 2010, 1, 54–63. [Google Scholar]
- Ughachukwu, P.; Ezenyeaku, C.C.T.; Ochiogu, B.C.; Ezeagwuna, D.A.; Anahalu, I.C. Evaluation of antibacterial activities of Euphorbia heterophylla. IOSR J. Dent. Med. Sci. 2014, 13, 69–75. [Google Scholar]
- Falodun, A.; Agbakwuru, E.; Ukoh, G. Antibacterial Activity of Euphor-Bia Heterophylla Linn (Family-Euphorbiaceae). Biol. Sci. PJSIR 2003, 46, 471–472. [Google Scholar]
- Hosseinzadeh, M.H.; Ghalavand, A.; Boojar, M.M.-A.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A. Application of manure and biofertilizer to improve soil properties and increase grain yield, essential oil and ω3 of purslane (Portulaca oleracea L.) under drought stress. Soil Tillage Res. 2021, 205, 104633. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Gozari, M.; Alborz, M.; El-Seedi, H.R.; Jassbi, A.R. Chemistry, biosynthesis and biological activity of terpenoids and meroterpenoids in bacteria and fungi isolated from different marine habitats. Eur. J. Med. Chem. 2021, 210, 112957. [Google Scholar] [CrossRef]
- Tripathi, N.; Asthana, A.; Dixit, S. Toxicity of some terpenoids against fungi infesting fruits and seeds of Capsicum annuum L. during storage. J. Phytopathol. 1984, 110, 328–335. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Zhou, J.; Huang, C.-G.; Hu, Q.-F.; Huang, X.-Z.; Wang, W.; Zhang, L.-Z.; Li, G.-P.; Xia, F.-T. Two novel antiviral terpenoids from the cultured Perovskia atriplicifolia. Tetrahedron 2015, 71, 3844–3849. [Google Scholar] [CrossRef]
- Isah, M.B.; Tajuddeen, N.; Umar, M.I.; Alhafiz, Z.A.; Mohammed, A.; Ibrahim, M.A. Terpenoids as emerging therapeutic agents: Cellular targets and mechanisms of action against protozoan parasites. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 227–250. [Google Scholar]
- Aziz, Z.A.; Ahmad, A.; Setapar, S.H.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical and Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Ge, L.; Lin, B.; Mo, J.; Chen, Q.; Su, L.; Li, Y.; Yang, K. Composition and antioxidant and antibacterial activities of essential oils from three yellow Camellia species. Trees 2019, 33, 205–212. [Google Scholar] [CrossRef]
- Ghaderi, L.; Aliahmadi, A.; Ebrahimi, S.N.; Rafati, H. Effective Inhibition and eradication of Pseudomonas aeruginosa biofilms by Satureja khuzistanica essential oil nanoemulsion. J. Drug Deliv. Sci. Technol. 2021, 61, 102260. [Google Scholar] [CrossRef]
- Zheng, G.-Q.; Kenney, P.M.; Lam, L.K.T. Sesquiterpenes from Clove (Eugenia caryophyllata) as Potential Anticarcinogenic Agents. J. Nat. Prod. 1992, 55, 999–1003. [Google Scholar] [CrossRef]
- Beuchat, L. Control of Foodborne Pathogens and Spoilage Microorganisms by Naturally Occurring Antimicrobials. In Microbial Food Contamination; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Anwer, M.K.; Jamil, S.; Ibnouf, E.O.; Shakeel, F. Enhanced antibacterial effects of clove essential oil by nanoemulsion. J. Oleo Sci. 2014, 63, 347–354. [Google Scholar] [CrossRef]
- Miyazawa, M.; Hisama, M. Suppression of chemical mutagen-induced SOS response by alkylphenols from clove (Syzygium aromaticum) in the Salmonella typhimurium TA1535/pSK1002 umu test. J. Agric. Food Chem. 2001, 49, 4019–4025. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- Cressy, H.K.; Jerrett, A.R.; Osborne, C.M.; Bremer, P.J. A novel method for the reduction of numbers of Listeria monocytogenes cells by freezing in combination with an essential oil in bacteriological media. J. Food Prot. 2003, 66, 390–395. [Google Scholar] [CrossRef]
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Łysakowska, M.; Pastuszka, M.; Bienias, W.; Kowalczyk, E. The potential of use basil and rosemary essential oils as effective antibacterial agents. Molecules 2013, 18, 9334–9351. [Google Scholar] [CrossRef]
- Amani, F.; Sami, M.; Rezaei, A. Characterization and Antibacterial Activity of Encapsulated Rosemary Essential Oil within Amylose Nanostructures as a Natural Antimicrobial in Food Applications. Starch Stärke 2021, 73, 2100021. [Google Scholar] [CrossRef]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Özbek, H.; Uğraş, S.; Dülger, H.; Bayram, I.; Tuncer, I.; Öztürk, G.; Öztürk, A. Hepatoprotective effect of Foeniculum vulgare essential oil. Fitoterapia 2003, 74, 317–319. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; De Lampasona, M.P.; Catalan, C. Chemical constituents, antifungal and antioxidative potential of Foeniculum vulgare volatile oil and its acetone extract. Food Control 2006, 17, 745–752. [Google Scholar] [CrossRef]
- Tognolini, M.; Ballabeni, V.; Bertoni, S.; Bruni, R.; Impicciatore, M.; Barocelli, E. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol. Res. 2007, 56, 254–260. [Google Scholar] [CrossRef]
- Choi, E.-M.; Hwang, J.-K. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia 2004, 75, 557–565. [Google Scholar] [CrossRef]
- Abou El-Soud, N.; El-Laithy, N.; El-Saeed, G.; Wahby, M.; Khalil, M.; Morsy, F.; Shaffie, N. Antidiabetic activities of Foeniculum vulgare Mill. essential oil in streptozotocin-induced diabetic rats. Maced. J. Med. Sci. 2011, 4, 139–146. [Google Scholar]
- Pradhan, M.; Sribhuwaneswari, S.; Karthikeyan, D.; Minz, S.; Sure, P.; Chandu, A.N.; Mishra, U.; Kamalakannan, K.; Saravanankumar, A.; Sivakumar, T. In-vitro cytoprotection activity of Foeniculum vulgare and Helicteres isora in cultured human blood lymphocytes and antitumour activity against B16F10 melanoma cell line. Res. J. Pharm. Technol. 2008, 1, 450–452. [Google Scholar]
- Lee, H.-S. Acaricidal activity of constituents identified in Foeniculum vulgare fruit oil against Dermatophagoides spp.(Acari: Pyroglyphidae). J. Agric. Food Chem. 2004, 52, 2887–2889. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Donos, N.; Netuschil, L.; Reich, E.; Sculean, A. Clinical and antibacterial effect of tea tree oil—A pilot study. Clin. Oral Investig. 2000, 4, 70–73. [Google Scholar] [CrossRef]
- Lee, C.-J.; Chen, L.-W.; Chen, L.-G.; Chang, T.-L.; Huang, C.-W.; Huang, M.-C.; Wang, C.-C. Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. J. Food Drug Anal. 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Cassone, A.; Salvatore, G. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and-resistant human pathogenic Candida species. BMC Infect. Dis. 2006, 6, 158. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63. [Google Scholar] [CrossRef]
- Yang, K.; Liu, A.; Hu, A.; Li, J.; Zen, Z.; Liu, Y.; Tang, S.; Li, C. Preparation and characterization of cinnamon essential oil nanocapsules and comparison of volatile components and antibacterial ability of cinnamon essential oil before and after encapsulation. Food Control 2021, 123, 107783. [Google Scholar] [CrossRef]
- Seyed Ahmadi, S.G.; Farahpour, M.R.; Hamishehkar, H. Topical application of Cinnamon verum essential oil accelerates infected wound healing process by increasing tissue antioxidant capacity and keratin biosynthesis. Kaohsiung J. Med. Sci. 2019, 35, 686–694. [Google Scholar] [CrossRef]
- Radünz, M.; Mota Camargo, T.; Santos Hackbart, H.C.; Inchauspe Correa Alves, P.; Radünz, A.L.; Avila Gandra, E.; da Rosa Zavareze, E. Chemical composition and in vitro antioxidant and antihyperglycemic activities of clove, thyme, oregano, and sweet orange essential oils. LWT 2021, 138, 110632. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Shen, R.; Zhang, R.; Liu, L.; Yang, X. Konjac glucomannan-based edible films loaded with thyme essential oil: Physical properties and antioxidant-antibacterial activities. Food Packag. Shelf Life 2021, 29, 100700. [Google Scholar] [CrossRef]
- Govaris, A.; Solomakos, N.; Pexara, A.; Chatzopoulou, P.S. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. Int. J. Food Microbiol. 2010, 137, 175–180. [Google Scholar] [CrossRef]
- Cattelan, M.G.; de Castilhos, M.B.M.; Sales, P.J.P.; Hoffmann, F.L. Antibacterial activity of oregano essential oil against foodborne pathogens. Nutr. Food Sci. 2013, 43, 169–174. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crop. Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Beatovic, D.; Krstic-Milosevic, D.; Trifunovic, S.; Siljegovic, J.; Glamoclija, J.; Ristic, M.; Jelacic, S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod. 2015, 9, 62. [Google Scholar]
- Gaio, I.; Saggiorato, A.G.; Treichel, H.; Cichoski, A.J.; Astolfi, V.; Cardoso, R.I.; Toniazzo, G.; Valduga, E.; Paroul, N.; Cansian, R.L. Antibacterial activity of basil essential oil (Ocimum basilicum L.) in Italian-type sausage. J. Verbrauch. Lebensm. 2015, 10, 323–329. [Google Scholar] [CrossRef]
- Sharafati Chaleshtori, R.; Rokni, N.; Rafieian-Kopaei, M.; Deris, F.; Salehi, E. Antioxidant and antibacterial activity of basil (Ocimum basilicum L.) essential oil in beef burger. J. Agric. Sci. Technol. 2015, 17, 817–826. [Google Scholar]
- Van Dat, D.; Van Cuong, N.; Le, P.H.A.; Anh, T.T.L.; Viet, P.T.; Huong, N.T.L. Orange Peel Essential Oil Nanoemulsions Supported by Nanosilver for Antibacterial Application. Indones. J. Chem. 2020, 20, 430–439. [Google Scholar]
- Do Evangelho, J.A.; da Silva Dannenberg, G.; Biduski, B.; el Halal, S.L.M.; Kringel, D.H.; Gularte, M.A.; Fiorentini, A.M.; da Rosa Zavareze, E. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Desam, N.R.; Al-Rajab, A.J.; Sharma, M.; Mylabathula, M.M.; Gowkanapalli, R.R.; Albratty, M. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha×Piperita L. (peppermint) essential oils. J. King Saud Univ. Sci. 2019, 31, 528–533. [Google Scholar] [CrossRef]
- Afsharypuor, S.; Rahiminezhad, M.; Ghaemmaghami, L.; Soleimani, M.S.; Khanmohammadic, M.; Afsharipour, N. Essential Oil Constituents of Leaves of the Male and Female Shrubs of Juniperus chinensis L. from Isfahan. Iran. J. Pharm. Sci. 2007, 3, 177–180. [Google Scholar]
- Majumder, S.; Ghosh, A.; Bhattacharya, M. Natural anti-inflammatory terpenoids in Camellia japonica leaf and probable biosynthesis pathways of the metabolome. Bull. Natl. Res. Cent. 2020, 44, 141. [Google Scholar] [CrossRef]
- Feás, X.; Estevinho, L.M.; Salinero, C.; Vela, P.; Sainz, M.J.; Vázquez-Tato, M.P.; Seijas, J.A. Triacylglyceride, Antioxidant and Antimicrobial Features of Virgin Camellia oleifera, C. reticulata and C. sasanqua Oils. Molecules 2013, 18, 4573–4587. [Google Scholar] [CrossRef]
- Chung, I.-M.; Kim, M.Y.; Park, W.-H.; Moon, H.-I. Antiatherogenic activity of Dendropanax morbifera essential oil in rats. Die Pharm. Int. J. Pharm. Sci. 2009, 64, 547–549. [Google Scholar]
- Werka, J.S.; Boehme, A.K.; Setzer, W.N. Biological Activities of Essential Oils from Monteverde, Costa Rica. Nat. Prod. Commun. 2007, 2, 1934578X0700201204. [Google Scholar] [CrossRef]
- Chaleshtori, R.S.; Kopaei, M.R.; Salehi, E. Bioactivity of Apium petroselinum and Portulaca oleracea Essential Oils as Natural Preservatives. Jundishapur J. Microbiol. 2015, 8, e20128. [Google Scholar] [CrossRef]
- Pang, J.; Dong, W.; Li, Y.; Xia, X.; Liu, Z.; Hao, H.; Jiang, L.; Liu, Y. Purification of Houttuynia cordata Thunb. Essential Oil Using Macroporous Resin Followed by Microemulsion Encapsulation to Improve Its Safety and Antiviral Activity. Molecules 2017, 22, 293. [Google Scholar] [CrossRef]
- Lu, H.; Wu, X.; Liang, Y.; Zhang, J. Variation in Chemical Composition and Antibacterial Activities of Essential Oils from Two Species of Houttuynia Thunb. Chem. Pharm. Bull. 2006, 54, 936–940. [Google Scholar] [CrossRef]
- Ali, E.M. Phytochemical composition, antifungal, antiaflatoxigenic, antioxidant, and anticancer activities of Glycyrrhiza glabra L. and Matricaria chamomilla L. essential oils. J. Med. Plants Res. 2013, 7, 2197–2207. [Google Scholar]
- Bahrami, A.; Fattahi, R. Biodegradable carboxymethyl cellulose–polyvinyl alcohol composite incorporated with Glycyrrhiza Glabra L. essential oil: Physicochemical and antibacterial features. Food Sci. Nutr. 2021, 9, 4974–4985. [Google Scholar] [CrossRef]
- Yong, C.; Zhang, Y.-Q. Advances in the Studies of Chemical Constituents of Genus Ziziphus. Nat. Prod. Res. Dev. 2011, 23, 979–982. [Google Scholar]
- Al-Reza, S.M.; Rahman, A.; Lee, J.; Kang, S.C. Potential roles of essential oil and organic extracts of Zizyphus jujuba in inhibiting food-borne pathogens. Food Chem. 2010, 119, 981–986. [Google Scholar] [CrossRef]
- Kasuya, H.; Hata, E.; Satou, T.; Yoshikawa, M.; Hayashi, S.; Masuo, Y.; Koike, K. Effect on Emotional Behavior and Stress by Inhalation of the Essential oil from Chamaecyparis obtusa. Nat. Prod. Commun. 2013, 8, 1934578X1300800428. [Google Scholar] [CrossRef]
- Yang, J.-K.; Choi, M.-S.; Seo, W.-T.; Rinker, D.L.; Han, S.W.; Cheong, G.-W. Chemical composition and antimicrobial activity of Chamaecyparis obtusa leaf essential oil. Fitoterapia 2007, 78, 149–152. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef]
- Caruso, D.J.; Palombo, E.A.; Moulton, S.E.; Zaferanloo, B. Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds. Microorganisms 2022, 10, 1990. [Google Scholar] [CrossRef]
- Zaferanloo, B.; Mahon, P.J.; Palombo, E.A. Endophytes from medicinal plants as novel sources of bioactive compounds. In Medicinal Plants: Diversity and Drugs; CRC Press: Boca Raton, FL, USA, 2012; Volume 355, p. 411. [Google Scholar]
- Pasrija, P.; Girdhar, M.; Kumar, M.; Arora, S.; Katyal, A. Endophytes: An unexplored treasure to combat Multidrug resistance. Phytomedicine Plus 2022, 2, 100249. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Dufossé, L.; Chhipa, H.; Saxena, S.; Mahajan, G.B.; Gupta, M.K. Fungal Endophytes: A Potential Source of Antibacterial Compounds. J. Fungi 2022, 8, 164. [Google Scholar] [CrossRef]
- Samadzadeh, S.; Farzaneh, M.; Shahsavari, Z.; Ebrahimi, S.N.; Asadollahi, M.; Mirjalili, M.H. Characterization and antimicrobial activity of fungal endophytes from Crocus caspius (Iridaceae). Biocatal. Agric. Biotechnol. 2022, 43, 102429. [Google Scholar] [CrossRef]
- Dos Santos Lacerda, Í.C.; Polonio, J.C.; Golias, H.C. ENDophytic Fungi as Sources of Antiviral Compounds—A Review. Chem. Biodivers. 2022, 19, e202100971. [Google Scholar]
- Macías-Rubalcava, M.L.; Hernández-Bautista, B.E.; Jiménez-Estrada, M.; González, M.C.; Glenn, A.E.; Hanlin, R.T.; Hernández-Ortega, S.; Saucedo-García, A.; Muria-González, J.M.; Anaya, A.L. Naphthoquinone spiroketal with allelochemical activity from the newly discovered endophytic fungus Edenia gomezpompae. Phytochemistry 2008, 69, 1185–1196. [Google Scholar] [CrossRef]
- Wu, S.H.; Huang, R.; Miao, C.P.; Chen, Y.W. Two new steroids from an endophytic fungus Phomopsis sp. Chem. Biodivers. 2013, 10, 1276–1283. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; del Carmen Rocha-Granados, M.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Mishra, P.; Verekar, S.; Kulkarni-Almeida, A.; Roy, S.; Jain, S.; Balakrishnan, A.; Vishwakarma, R.; Deshmukh, S. Anti-inflammatory and anti-diabetic naphtha quinones from an endophytic fungus Dendryphion nanum (Nees) S. Hughes. Indian J. Chem. Sect. B 2013, 52B, 565–567. [Google Scholar]
- Toghueo, R.M.K. Anti-leishmanial and anti-inflammatory agents from endophytes: A review. Nat. Prod. Bioprospecting 2019, 9, 311–328. [Google Scholar] [CrossRef]
- Banyal, A.; Thakur, V.; Thakur, R.; Kumar, P. Endophytic microbial diversity: A new hope for the production of novel anti-tumor and anti-HIV agents as future therapeutics. Curr. Microbiol. 2021, 78, 1699–1717. [Google Scholar] [CrossRef]
- Zaferanloo, B.; Pepper, S.A.; Coulthard, S.A.; Redfern, C.P.F.; Palombo, E.A. Metabolites of endophytic fungi from Australian native plants as potential anticancer agents. FEMS Microbiol. Lett. 2018, 365, fny078. [Google Scholar] [CrossRef]
- Jung, C.; Arnold, A.E. The Effects of Endohyphal Bacteria on Anti-Cancer and Anti-Malaria Metabolites of Endophytic Fungi. Bachelor’s Thesis, The University of Arizona, Tucson, AZ, USA, 2012. [Google Scholar]
- Kyeremeh, K.; Owusu, K.B.; Ofosuhene, M.; Ohashi, M.; Agyapong, J.; Camas, A.S.; Camas, M. Anti-Proliferative and Anti-Plasmodia Activity of Quinolactacin A2, Citrinadin A and Butrecitrinadin co-isolated from a Ghanaian Mangrove Endophytic Fungus Cladosporium oxysporum strain BRS2A-AR2F. J. Chem. Appl. 2017, 3, 12. [Google Scholar]
- Qin, J.-C.; Zhang, Y.-M.; Gao, J.-M.; Bai, M.-S.; Yang, S.-X.; Laatsch, H.; Zhang, A.-L. Bioactive metabolites produced by Chaetomium globosum, an endophytic fungus isolated from Ginkgo biloba. Bioorganic Med. Chem. Lett. 2009, 19, 1572–1574. [Google Scholar] [CrossRef]
- Kim, S.; Shin, D.S.; Lee, T.; Oh, K.B. Periconicins, two new fusicoccane diterpenes produced by an endophytic fungus Periconia sp. with antibacterial activity. J. Nat. Prod. 2004, 67, 448–450. [Google Scholar] [CrossRef]
- Dai, J.; Krohn, K.; Flörke, U.; Draeger, S.; Schulz, B.; Kiss-Szikszai, A.; Antus, S.; Kurtán, T.; van Ree, T. Metabolites from the endophytic fungus Nodulisporium sp. from Juniperus cedre. Eur. J. Org. Chem. 2006, 2006, 3498–3506. [Google Scholar] [CrossRef]
- 299. Han, Z.; Mei, W.; Cui, H.; Zeng, Y.; Lin, H.; Hong, K.; Dai, H. Antibacterial constituents from the endophytic fungus Penicillium sp. of mangrove plant Cerbera manghas. Chem. J. Chin. Univ. 2008, 29, 752. [Google Scholar]
- Lu, H.; Zou, W.X.; Meng, J.C.; Hu, J.; Tan, R.X. New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci. 2000, 151, 67–73. [Google Scholar] [CrossRef]
- Dissanayake, R.K.; Ratnaweera, P.B.; Williams, D.E.; Wijayarathne, C.D.; Wijesundera, R.L.C.; Andersen, R.J.; de Silva, E.D. Antimicrobial activities of endophytic fungi of the Sri Lankan aquatic plant Nymphaea nouchali and chaetoglobosin A and C, produced by the endophytic fungus Chaetomium globosum. Mycology 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Wang, F.W.; Jiao, R.H.; Cheng, A.B.; Tan, S.H.; Song, Y.C. Antimicrobial potentials of endophytic fungi residing in Quercus variabilis and brefeldin A obtained from Cladosporium sp. World J. Microbiol. Biotechnol. 2007, 23, 79–83. [Google Scholar] [CrossRef]
- Mousa, W.K.; Raizada, M.N. The diversity of anti-microbial secondary metabolites produced by fungal endophytes: An interdisciplinary perspective. Front. Microbiol. 2013, 4, 65. [Google Scholar] [CrossRef]
- Kim, I.H.; Takashima, S.; Hitotsuyanagi, Y.; Hasuda, T.; Takeya, K. New Quassinoids, Javanicolides C and D and Javanicosides B−F, from Seeds of Brucea javanica. J. Nat. Prod. 2004, 67, 863–868. [Google Scholar] [CrossRef]
- Singh, M.P.; Janso, J.E.; Luckman, S.W.; Brady, S.F.; Clardy, J.; Greenstein, M.; Maiese, W.M. Biological activity of guanacastepene, a novel diterpenoid antibiotic produced by an unidentified fungus CR115. J. Antibiot. 2000, 53, 256–261. [Google Scholar] [CrossRef]
- Huang, Z.; Cai, X.; Shao, C.; She, Z.; Xia, X.; Chen, Y.; Yang, J.; Zhou, S.; Lin, Y. Chemistry and weak antimicrobial activities of phomopsins produced by mangrove endophytic fungus Phomopsis sp. ZSU-H76. Phytochemistry 2008, 69, 1604–1608. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Roth, S.; Deyrup, S.T.; Gloer, J.B. A protective endophyte of maize: Acremonium zeae antibiotics inhibitory to Aspergillus flavus and Fusarium verticillioides1 1Dedicated to John Webster on the occasion of his 80th birthday. Mycol. Res. 2005, 109, 610–618. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Poling, S.M. Antimicrobial activity of pyrrocidines from Acremonium zeae against endophytes and pathogens of maize. Phytopathology 2009, 99, 109–115. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Sommart, U.; Phongpaichit, S.; Sakayaroj, J.; Kirtikara, K. Metabolites from the endophytic fungus Phomopsis sp. PSU-D15. Phytochemistry 2008, 69, 783–787. [Google Scholar] [CrossRef]
- Phongpaichit, S.; Rungjindamai, N.; Rukachaisirikul, V.; Sakayaroj, J. Antimicrobial activity in cultures of endophytic fungi isolated from Garcinia species. FEMS Immunol. Med. Microbiol. 2006, 48, 367–372. [Google Scholar] [CrossRef]
- Noble, H.M.; Langley, D.; Sidebottom, P.J.; Lane, S.J.; Fisher, P.J. An echinocandin from an endophytic Cryptosporiopsis sp. and Pezicula sp. in Pinus sylvestris and Fagus sylvatica. Mycol. Res. 1991, 95, 1439–1440. [Google Scholar] [CrossRef]
- Schulz, B.; Sucker, J.; Aust, H.J.; Krohn, K.; Ludewig, K.; Jones, P.G.; Döring, D. Biologically active secondary metabolites of endophytic Pezicula species. Mycol. Res. 1995, 99, 1007–1015. [Google Scholar] [CrossRef]
- Tejesvi, M.; Segura, D.; Schnorr, K.; Sandvang, D.; Mattila, S.; Olsen, P.; Neve, S.; Kruse, T.; Kristensen, H.; Pirttilä, A.M. An antimicrobial peptide from endophytic Fusarium tricinctum of Rhododendron tomentosum Harmaja. Fungal Divers. 2013, 60, 153–159. [Google Scholar] [CrossRef]
- Cui, H.B.; Mei, W.L.; Miao, C.D.; Lin, H.P.; Hong, K.; Dai, H.F. Antibacterial constituents from the endophytic fungus Penicillium sp. 0935030 of mangrove plant Acrostichum aureurm. Chin. J. Antibiot. 2008, 33, 407–410. [Google Scholar]
- Kjer, J.; Wray, V.; Edrada-Ebel, R.; Ebel, R.; Pretsch, A.; Lin, W.; Proksch, P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. isolated from the mangrove plant Sonneratia alba. J. Nat. Prod. 2009, 72, 2053–2057. [Google Scholar] [CrossRef]
- Aly, A.H.; Edrada-Ebel, R.; Wray, V.; Müller, W.E.; Kozytska, S.; Hentschel, U.; Proksch, P.; Ebel, R. Bioactive metabolites from the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermum picroides. Phytochemistry 2008, 69, 1716–1725. [Google Scholar] [CrossRef]
- Brady, S.F.; Bondi, S.M.; Clardy, J. The guanacastepenes: A highly diverse family of secondary metabolites produced by an endophytic fungus. J. Am. Chem. Soc. 2001, 123, 9900–9901. [Google Scholar] [CrossRef]
- Brady, S.F.; Singh, M.P.; Janso, J.E.; Clardy, J. Guanacastepene, a fungal-derived diterpene antibiotic with a new carbon skeleton. J. Am. Chem. Soc. 2000, 122, 2116–2117. [Google Scholar] [CrossRef]
- Souza, J.J.d.; Vieira, I.J.C.; Rodrigues-Filho, E.; Braz-Filho, R. Terpenoids from Endophytic Fungi. Molecules 2011, 16, 10604–10618. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities With Multifunctional Prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Yang, X.; Strobel, G.; Stierle, A.; Hess, W.M.; Lee, J.; Clardy, J. A fungal endophyte-tree relationship: Phoma sp. in Taxus wallachiana. Plant Sci. 1994, 102, 1–9. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.-K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites* *Paper presented at the British Mycological Society symposium on Fungal Bioactive Compounds, held at the University of Wales Swansea on 22–27 April 2001. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Wiyakrutta, S.; Sriubolmas, N.; Panphut, W.; Thongon, N.; Danwisetkanjana, K.; Ruangrungsi, N.; Meevootisom, V. Endophytic fungi with anti-microbial, anti-cancer and anti-malarial activities isolated from Thai medicinal plants. World J. Microbiol. Biotechnol. 2004, 20, 265–272. [Google Scholar] [CrossRef]
- Strobel, G.A. Microbial gifts from rain forests1. Can. J. Plant Pathol. 2002, 24, 14–20. [Google Scholar] [CrossRef]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance generated by plant/fungal symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.-H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef]
- Akello, J.; Dubois, T.; Gold, C.S.; Coyne, D.; Nakavuma, J.; Paparu, P. Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp.). J. Invertebr. Pathol. 2007, 96, 34–42. [Google Scholar] [CrossRef]
- Giordano, L.; Gonthier, P.; Varese, G.C.; Miserere, L.; Nicolotti, G. Mycobiota inhabiting sapwood of healthy and declining Scots pine (Pinus sylvestris L.) trees in the Alps. Fungal Divers. 2009, 38, 69–83. [Google Scholar]
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal Endophytes: A Continuum of Interactions with Host Plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Verma, S.K.; Lal, M.; Das, M.D. Structural Elucidation of Bioactive Secondary Metabolites from Endophytic Fungus. Asian J. Pharm. Clin. Res. 2017, 10, 395–400. [Google Scholar] [CrossRef]
- Tuntiwachwuttikul, P.; Taechowisan, T.; Wanbanjob, A.; Thadaniti, S.; Taylor, W.C. Lansai A–D, secondary metabolites from Streptomyces sp. SUC1. Tetrahedron 2008, 64, 7583–7586. [Google Scholar] [CrossRef]
- Castillo, U.F.; Strobel, G.A.; Ford, E.J.; Hess, W.M.; Porter, H.; Jensen, J.B.; Albert, H.; Robison, R.; Condron, M.A.M.; Teplow, D.B.; et al. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microbiology 2002, 148, 2675–2685. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Doyle, E.; Gegechkori, V.; Morton, D.W. High-performance thin-layer chromatography linked with (bio)assays and FTIR-ATR spectroscopy as a method for discovery and quantification of bioactive components in native Australian plants. J. Pharm. Biomed. Anal. 2020, 184, 113208. [Google Scholar] [CrossRef]
- Biva, I.J.; Ndi, C.P.; Griesser, H.J.; Semple, S.J. Antibacterial constituents of Eremophila alternifolia: An Australian aboriginal traditional medicinal plant. J. Ethnopharmacol. 2016, 182, 1–9. [Google Scholar] [CrossRef]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef]
- Xing, Y.-M.; Chen, J.; Cui, J.-L.; Chen, X.-M.; Guo, S.-X. Antimicrobial Activity and Biodiversity of Endophytic Fungi in Dendrobiumdevonianum and Dendrobium thyrsiflorum from Vietman. Curr. Microbiol. 2011, 62, 1218–1224. [Google Scholar] [CrossRef]
- Dang, L.; Li, G.; Yang, Z.; Luo, S.; Zheng, X.; Zhang, K. Chemical constituents from the endophytic fungus Trichoderma ovalisporum isolated from Panax notoginseng. Ann. Microbiol. 2010, 60, 317–320. [Google Scholar] [CrossRef]
- Soares, R.D.F.; Campos, M.G.N.; Ribeiro, G.P.; Salles, B.C.C.; Cardoso, N.S.; Ribeiro, J.R.; Souza, R.M.; Leme, K.C.; Soares, C.B.; de Oliveira, C.M. Development of a chitosan hydrogel containing flavonoids extracted from Passiflora edulis leaves and the evaluation of its antioxidant and wound healing properties for the treatment of skin lesions in diabetic mice. J. Biomed. Mater. Res. Part A 2020, 108, 654–662. [Google Scholar] [CrossRef]
- Azzazy, H.M.; Fahmy, S.A.; Mahdy, N.K.; Meselhy, M.R.; Bakowsky, U. Chitosan-Coated PLGA Nanoparticles Loaded with Peganum harmala Alkaloids with Promising Antibacterial and Wound Healing Activities. Nanomaterials 2021, 11, 2438. [Google Scholar] [CrossRef]











| Variety | Description | Advantages | Disadvantages | Wound Type Application | Polymer | Ref. |
|---|---|---|---|---|---|---|
| Hydrogels | Water-absorbent cross-linked polymeric networks resulting from the reaction of monomers | Efficient flexibility, good ability in swelling and sustaining a significant amount of water, moisturizing, removal of necrotic tissue, good porosity, and monitoring the wound without removing the dressing | Inability to absorb enough exudates leading to bacterial proliferation, and low mechanical strength | Chemotherapy peels | Polyethylene oxide, polyvinyl pyrrolidine, Polyvinyl alcohol | [10,11,12,13,14] |
| Ulcers | ||||||
| Laser resurfacing | ||||||
| Average thickness wounds | ||||||
| Donor graft sites and artificial organ wounds | ||||||
| Hydrocolloids | Colloidal material (gel) constituted with elastomers and adhesives in the form of films or sheets | Excellent exudate absorption properties, transparency, enhanced angiogenesis, and formation of granulation tissue | Not permeable to gas, vapor, water, and bacteria, their debriding capability, skin maceration, and producing a foul smell | Chronic ulcers | Pectin, carboxymethylcellulose, gelatin, and cellulose | [10,13,15,16] |
| Burns | ||||||
| Average thickness wounds | ||||||
| Donor graft sites | ||||||
| Foams | A porous structure using capillary action as its mechanism to absorb fluids | Exudate absorbance, preventing bacteria invasion, maintaining sufficient moisture at the wound surface, being removed easily, protecting the skin around the wound, maintaining an efficient temperature, mechanical protection, being nontoxic, being cost-effective with a long shelf life | Drying out the wound in case of minimal or no exudate presence and maceration of the surrounding skin in case of exudate saturation in dressing | Chronic wounds | Polyurethane, silicone, silk fibroin | [13,17,18,19,20,21] |
| Burns | ||||||
| Mohs surgery and wounds | ||||||
| Laser resurfacing wounds | ||||||
| Films | Consists of adhesives, porous, and thin transparent polymers | The possibility of having a high mechanical strength, high water transmission rate, protecting the wound against bacterial infection | The possibility of having a low mechanical strength | Superficial wounds | Soy protein isolates, chitosan, polyvinyl alcohol | [13,22,23,24] |
| Laser wounds | ||||||
| Surgery defect sites | ||||||
| Skin tears | ||||||
| Dermal patches | Dressings consisted of a multilayered structure with an impermeable excipient-loaded film, drugs, and a release liner | Suitable for skin adhesion, not having a liquid reservoir, controlling the drug delivery rate | Needing flux moderation in case of loading with highly soluble drugs, and decrease in drug release rate with wear time, not suitable for most of the drugs | Hypertension | Poly(vinyl pyrrolidones), poly(vinyl alcohol) | [25,26,27,28,29,30] |
| Topical wounds | ||||||
| Fibers and nanofibers | Polymeric fibers produced with electrospinning process | Excellent mechanical properties, thermal stability, antimicrobial activity, biodegradability, control in water vapor transmission rate, oxygen permeability, fluid drainage ability, high porosity, and high surface area | Higher cost of production in some cases, hard to produce fibers with diameters less than 10 nm | Partial thickness burns | Polyurethane, collagen, silk fibroin, polycaprolactone, poly (lactic-co-glycolic acid), polyethylene oxide, etc. | [31,32,33,34,35,36,37,38,39,40] |
| Diabetic ulcers | ||||||
| Bone bleeding | ||||||
| Chronic infected wounds | ||||||
| Acute wounds | ||||||
| Venous ulcers | ||||||
| Pressure ulcers | ||||||
| Membranes | A thin semi-permeable barrier | Porous structure, transparency, excessive loss of water, the ability to contain an occlusive layer to impede microbial invasion | Cytotoxicity in some cases | Superficial wounds | Pectin, collagen, chitosan, chitin, alginate, zein, polycaprolactone, polyvinyl acetate, polyvinyl alcohol, polytetrafluoroethylene, cellulose, etc. | [41,42,43,44,45,46,47,48,49,50,51,52] |
| Frictional wounds | ||||||
| Skin-scratching wounds | ||||||
| Skin donor sites | ||||||
| Skin with external contamination | ||||||
| Polymer-drug conjugates | Polymer-based water-soluble nanocarriers conjugated with bioactive agents | Improving the water solubility of the hydrophobic drugs, enhancing the pharmacokinetic profile of the conjugated drug, extending the volume of distribution, and protecting the conjugated drug against degradation | Limitations to be applied on a large scale, low stability in vivo, short half-life, and immunogenicity | Diabetic wounds such as venous leg and lower limb ulcers | N-(2-hydroxypropyl) methacrylamide copolymer, polyglutamic acid, Poly(ethylene glycol), Polyamidoamine, hyaluronic acid, poly (vinyl ether-co-maleic anhydride), poly (vinyl pyrrolidone), etc. | [53,54,55,56,57,58,59,60,61] |
| Scientific Name | Classes of Chemical Compounds | Common Medical Uses | Solvents Used for Extraction | Activity against Infected Wound Bacteria | References |
|---|---|---|---|---|---|
| Acalipha alinifolia/fruticosa | Phenolic compounds, cardiac glycosides, tannins, flavonoids, and phytosterols | Anti-bacterial, antifungal, antioxidant, and anthelmintic properties | Aqueous, acetone, and methanol | S. aureus | [157,158,159,160] |
| asthma, pneumonia, scabies, and skin diseases | P. aeruginosa | ||||
| Delonix elata | Saponins, tannins, flavonoids, and steroids | anti-inflammatory, anti-arthritic, and antioxidant | Aqueous, ethanol, chloroform, acetone, petroleum ether, and methanol | S. aureus | [161,162,163,164,165] |
| E. coli | |||||
| P. aeruginosa | |||||
| Delonix regia | Flavonoids, alkaloids, terpenoids, steroids, and phenolic acids | Anti-diarrhoeal, anti-inflammatory, antidiabetic, antioxidant, hepatoprotective, antimicrobial, anthelmintic, wound healing, gastroprotective | Aqueous, Methanol | E. coli | [166,167,168,169,170,171,172,173,174,175,176,177,178,179] |
| P. aeruginosa | |||||
| S. aureus | |||||
| Klebsiella pneumoniae | |||||
| Digera muricata | Phenol, flavonoids, alkaloids, terpenes, sterols, tannins, glycosides, and lignins | Antibacterial, antifungal, diuretic, laxative, free radical scavenger activity, anthelmintic | Petroleum ether, chloroform, ethanol, distilled water | E. coli | [180,181,182] |
| S. aureus | |||||
| Hygrophilia auriculata | Alkaloids, terpenoids, tannins, flavonoids, and fatty acids | Medicinal usage in Indian Ayurveda | Distilled water, 50% aqueous ethanol, methanol, petroleum ether, chloroform, diethyl ether | P. aeruginosa | [183,184,185,186,187,188,189,190,191,192] |
| S. aureus | |||||
| E. coli | |||||
| K. pneumoniae | |||||
| Maerua oblongifolia | Alkaloids, terpenoids carbohydrates, glycosides, phytosterols, saponins, proteins, and amino acids | Wound healing activity, treating toothache, the roots of this plant possess alternative, tonic, and medicinal properties | Petrol-Et2-MeOH (1:1:1), Dichloromethane/methanol, aqueous | E. coli | [193,194,195,196] |
| S. aureus | |||||
| K. pneumoniae | |||||
| Pterocarpus santalinus | Alkaloids, phenols, saponins, glycosides, flavonoids, triterpenoids, sterols, and tannins | Antipyretic, anti-inflammatory, anthelmintic, tonic, haemorrhage, dysentery, aphrodisiac, anti-hyperglycaemic and diaphoretic | 70% methanol | S. aureus | [197,198,199] |
| P. aeruginosa | |||||
| E. coli | |||||
| Syzygium cumini | Flavonoids, glucoside derivatives, and phenols | Diabetes, sores and ulcers, leucorrhoea, and antidote in opium poisoning | Methanol, aqueous | P. aeruginosa | [200,201,202,203] |
| E. coli | |||||
| S. aureus | |||||
| Gyrocarpus americanus | Alkaloids | Unknown medicinal values | Ethanol, methanol, water | NA | [204,205,206] |
| Punica granatum | Polyphenols, sterols, triterpenoids, flavonoids, fatty acids, and tannins | Anti-inflammatory, anti-cancer, antioxidant, and antibacterial activity | Methanol, petroleum ether, chloroform, aqueous, chloramphenicol | S. aureus | [207,208,209,210,211,212,213] |
| E. coli | |||||
| K. pneumoniae | |||||
| Euphorbia heterophilla | Flavonoids, saponins, diterpenes, and phorbol esters | Wound healing activity, used for the treatment of constipation, bronchitis, and asthma, anti-inflammatory activity | Aqueous, petroleum ether, Butanol, ethanol | S. aureus | [214,215,216,217,218,219,220] |
| E. coli | |||||
| K. pneumoniae | |||||
| P. aeruginosa |
| Plant | Chemical Constituents | Activity and Use | Activity against Infected Wound Bacteria | References |
|---|---|---|---|---|
| Clove (Syzygium aromaticum L.) | Eugenol, acetyleugenol, thymol, cinnamaldehyde, etc. | Anti-inflammatory, antibacterial, antifungal, anti-allergic, anti-carcinogenic, anti-mutagenic | P. aeruginosa | [230,231,232,233,234,235] |
| S. aureus | ||||
| K. pneumoniae | ||||
| Rosemary (Rosmarinus officinalis L.) | A-pinene, myrcene, 1,8-cineole, borneol, and camphor | Antioxidant, antimicrobial | S. aureus | [236,237,238] |
| K. pneumoniae | ||||
| E. coli | ||||
| Fennel (Foeniculum vulgare Mill.) | Trans-anethole, estragole | Hepatoprotective, antioxidant, anti-inflammatory, antidiabetic, antitumor, and acaricidal | S. aureus | [239,240,241,242,243,244,245,246] |
| E. coli | ||||
| P. aeruginosa | ||||
| Tea tree (Melaleuca alternifolia) | Terpinen-4-ol, 1,8-Cineol, α-Pinene, α-Terpineol, Sabinene, | Antibacterial, antifungal, used for skin treatment, airway treatment, oral treatment, and vaginal infections | S. aureus | [247,248,249] |
| E. coli | ||||
| P. aeruginosa | ||||
| Cinnamon (Cinnamomum cassia) | Cinnamaldehyde, (−)-α-Pinene/Ylangene, terpenes, aldehydes, etc. | Antibacterial, antioxidant, wound healing applications | S. aureus | [250,251,252] |
| E. coli | ||||
| P. aeruginosa | ||||
| Thyme (Thymus vulgaris L.) | 1R-α-pinene, o-cymol, 4-carene, β-linalool, Camphor, Thymol, Carvacrol | Antioxidant, antibacterial | S. aureus | [253,254] |
| E. coli | ||||
| Oregano | Linalool, Thymol, Carvacrol, Ethyl caprate, etc. | Antioxidant, antibacterial, anti-inflammatory | S. aureus | [253,255,256,257] |
| E. coli | ||||
| Basil (Ocimum basilicum L.) | Linalool, 1,8-cineole, aromadendrene, and transcaryophyllene | Antibacterial and antioxidant | S. aureus | [258,259,260] |
| K. pneumoniae | ||||
| E. coli | ||||
| Shigella flexneri | ||||
| Orange (Citrus sinensis) | Limonene, alcohol compounds, carvone, β-myrcene | Antibacterial | E. coli | [261,262] |
| P. aeruginosa | ||||
| S. aureus | ||||
| Peppermint (Mentha × piperita L.) | Menthol, Menthone, Limonene, β-pinene, α-pinene, Menthyl acetate etc. | Antibacterial, antifungal | S. aureus | [263,264] |
| E. coli | ||||
| Juniperus chinensis L. | Bornyl acetate, sabinene, trans-sabinyl acetate, carotol, elemol | Air cleaning effect and antibacterial activity (mainly against Propionibacterium acnes), used for skincare and cleansing | E. coli | [265] |
| P. aeruginosa | ||||
| S. aureus | ||||
| Camellia oil | Triterpenes, Sesquiterpenes, tocopherols, phthalate esters, and cannabinoid | Skin-moisturizing effect, skin-soothing effect; effective against atopic or allergic skin conditions (effects due to high amount of oleic acid); prevention of skin dryness and alleviating itching (due to the gamma-linolenic acid content) | E. coli | [266,267] |
| Bacillus cereus | ||||
| C. albicans | ||||
| Dendropanax species | ɤ-elemene, tetramethyltricyclo hydrocarbons, β-Selinene and β-Zingiberene | Antioxidant and antimicrobial or antibacterial activities | S. aureus | [268,269] |
| Bacillus. cereus | ||||
| Portulaca oleracea L. | Phenolic compounds, α-linolenic acid (ω3) | Alleviate skin irritation and allergic responses, antibacterial and anti-inflammatory effects/it is used mainly in acne care products and cosmetics for sensitive skin | S. aureus | [221,270] |
| Klebsiella oxytoca | ||||
| Houttuynia cordata Thunb. | 3-oxododecanal (Hou), 2- undecanone, pinene, camphene, myrcene, limonene | Effective in treating skin inflammation and atopic diseases | S. aureus | [271,272] |
| Glycyrrhiza glabra L. | α-pinene, β-pinene, octanol, γ-terpinene, stragole, isofenchon, β-caryophyllene, citronellyl acetate, caryophyllene oxide, and geranyl hexanolate | Detoxifying and anti-inflammatory effects, effective in alleviating skin diseases such as acne, atopy, eczema, urticarial | E. coli | [273,274] |
| S. aureus | ||||
| Ziziphus jujuba Mill. | Triterpenoid acids, alkaloids, saponins, flavonoids, and their glycosides | Effective in moisturizing the skin and keeping the skin healthy | E. coli | [275,276] |
| S. aureus | ||||
| P. aeruginosa | ||||
| Chamaecyparis obtuse (Siebold & Zucc.) Endl. | δ-cadinene, α-pinene, γ-cadinene, α-cedrol, α-muurolene, γ-eudesmol, γ-muurolene, α-elemene and α-copaene | Sterilising effect (due to the high content of phytoncide) | S. aureus | [277,278] |
| Types of Endophytic Compounds | Endophyte | Host Plant | Main Bioactivity | Activity against Wound Infection Bacteria | References |
|---|---|---|---|---|---|
| Aliphatic compounds | Chaetomium globosum | Ginkgo biloba | Antifungal, antibacterial | S. aureus | [296,301] |
| Cladosporium sp. | Quercus variabilis | Antifungal, antimicrobial | NA | [302] | |
| Fungal endophytes | Chinese herbs | Antimicrobial, antibacterial | Klebsiella pneumonia | [300,303,304,305] | |
| S. aureus | |||||
| P. aeruginosa | |||||
| Phomopsis sp. | Excoecaria agallocha | Antifungal, antimicrobial | S. aureus | [306] | |
| E. coli | |||||
| Alkaloids | Acremonium zeae | Maize | Antifungal, antibacterial | Pseudomonas fluorescens | [307,308] |
| Enterobacter agglomerans | |||||
| Phomopsis sp. | Garcinia dulcis | Antibacterial | S. aureus | [309,310] | |
| Flavonoids | Nodulisporium sp. | Juniperus cedre | Antifungal, Antibacterial | NA | [298] |
| Peptides | Cryptosporiopsis sp., Pezicula sp. | Pinus sylvestris and Fagus sylvatica | Antifungal, antibacterial | E. coli | [311,312] |
| Fusarium tricinctum | Rhododendron tomentosum | Antimicrobial | S. aureus | [313] | |
| Penicillium sp. | Acrostichum aureurm | Antifungal, Antibacterial | S. aureus | [314] | |
| Phenols | Alternaria sp. | Sonneratia alba | Antibacterial | S. aureus | [315] |
| Phoma species | Saurauia scaberrinae | Antibacterial | S. aureus | [299] | |
| Penicillium sp. | Cerbera manghas | Antibacterial | S. aureus | [299] | |
| Quinones | Ampelomyces sp. | Urospermum picroides | Antibacterial | S. aureus | [316] |
| Steroids | Colletotrichum sp. | Artemisia annua | Antifungal, antimicrobial | S. aureus | [300] |
| P. aeruginosa | |||||
| Nodulisporium sp. | Juniperus cedre | Antifungal, Antibacterial | NA | [298] | |
| Fungal endophytes | Daphnopsis americana | Antibacterial | S. aureus | [317,318] | |
| Periconia sp. | Taxus cuspidate | Antibacterial | S. aureus | [297] | |
| K. pneumoniae | |||||
| Fungal endophytes | NA | Antimicrobial | S. aureus | [319] | |
| P. aeruginosa | |||||
| E. coli |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firoozbahr, M.; Kingshott, P.; Palombo, E.A.; Zaferanloo, B. Recent Advances in Using Natural Antibacterial Additives in Bioactive Wound Dressings. Pharmaceutics 2023, 15, 644. https://doi.org/10.3390/pharmaceutics15020644
Firoozbahr M, Kingshott P, Palombo EA, Zaferanloo B. Recent Advances in Using Natural Antibacterial Additives in Bioactive Wound Dressings. Pharmaceutics. 2023; 15(2):644. https://doi.org/10.3390/pharmaceutics15020644
Chicago/Turabian StyleFiroozbahr, Meysam, Peter Kingshott, Enzo A. Palombo, and Bita Zaferanloo. 2023. "Recent Advances in Using Natural Antibacterial Additives in Bioactive Wound Dressings" Pharmaceutics 15, no. 2: 644. https://doi.org/10.3390/pharmaceutics15020644