Abstract
Cancer is a collective name for a variety of diseases that can begin in virtually every organ or body tissue as abnormal cells develop uncontrollably and ten million new cancer cases are diagnosed all over the world at present. Whereas HIV is a virus that makes people susceptible to infection and contributes to the condition of acquired immune deficiency syndrome (AIDS). Almost 37 million people are currently diagnosed with HIV and 1 million people die every year, which is the worst-case scenario. Potential medicinal compounds have played a crucial role in the production of certain clinically beneficial novel anti-cancer and anti-HIV agents that are produced from natural sources especially from plants. These include Taxol, Vinblastine, Podophyllotoxin, Betulinic acid, Camptothecin, and Vincristine, etc. In the past decades, bioactive compounds were extracted directly from the plant sources which was more time consuming, led to low yield productivity, high cost, and bad impact on biodiversity. Endophytes, the microorganisms that reside inside the host plant by not causing any kind of harm to them and have potential applications in agriculture, medicine, pollution, and food industries. Therefore, by isolating and characterizing novel endophytes from medicinal plants and extracting their secondary metabolites to produce useful bioactive compounds can be beneficial for well-being and society as a future therapeutics. This approach is not harmful to biodiversity economical, timesaving, low cost, and can lead to the discovery of various industrial and commercially important novel anti-tumor and anti-HIV agents in the future. The Himalayas are home to several medicinal plants and the endophytic microbial biodiversity of the Himalayan region is also not much explored yet. However, the effect of compounds from these endophytes on anticancer and antiviral activity, especially anti-HIV has been largely unexplored. Hence, the present review is designed to the exploration of endophytic microbial diversity that can give rise to the discovery of various novel potential industrially valuable bioactive compounds that can lessen the rate of such type of pandemic diseases in the future by providing low-cost future therapeutics in future.
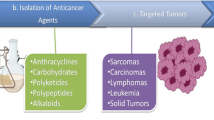
Graphic Abstract




Similar content being viewed by others
Abbreviations
- HIV:
-
Human immunodeficiency virus
- AIDS:
-
Acquired immune deficiency syndrome
- WHO:
-
World Health Organization
- ART:
-
Antiretroviral therapy
- UNAIDS:
-
United Nations Programme on HIV and AIDS
- IHR:
-
Indian Himalayan region
- cART:
-
Combination antiretroviral therapy
- DNA:
-
Deoxyribose nucleic acid
- RT:
-
Reverse transcriptase
- CD4:
-
Cluster of differentiation 4
- LTR:
-
Long terminal repeats
- ROS:
-
Reactive oxygen species
- μg/L:
-
Microgram per litre
- μg/g:
-
Microgram per gram
- g/L:
-
Gram per litre
- kg:
-
Kilogram
- %:
-
Percent
References
Li S-J, Zhang X, Wang X-H, Zhao C-Q (2018) Novel natural compounds from endophytic fungi with anticancer activity. Eur J Med Chem 156:316–343. https://doi.org/10.1016/j.ejmech.2018.07.015
Wink M et al (2005) Sustainable bioproduction of phytochemicals by plant in vitro cultures: anticancer agents. Plant Genet Resour 3(2):90–100. https://doi.org/10.1079/pgr200575
Roy A, Jauhari N, Bharadvaja N (2018) Medicinal plants as a potential source of chemopreventive agents. Anticancer Plants 2:109–139
Majumder A, Jha S (2009) Biotechnological approaches for the production of potential anticancer leads podophyllotoxin and paclitaxel: an overview. J Biol Sci 1(1):46–69
Xu XY et al (2019) Anti-HIV lignans from Justicia procumbens. Chin J Nat Med 17(12):945–952. https://doi.org/10.1016/S1875-5364(19)30117-7
Fabian L et al (2020) Design, synthesis and biological evaluation of quinoxaline compounds as anti-HIV agents targeting reverse transcriptase enzyme. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2019.111987
Zuo X et al (2018) Current insights into anti-HIV drug discovery and development: a review of recent patent literature (2014–2017). Expert Opin Ther Pat 28(4):299–316. https://doi.org/10.1080/13543776.2018.1438410
Bedi A, Adholeya A, Deshmukh SK (2018) Novel Anticancer compounds from Endophytic fungi. Curr Biotechnol 7(3):168–184. https://doi.org/10.2174/2211550105666160622080354
Joseph B, Mini Priya R (2011) Bioactive compounds from endophytes and their potential in pharmaceutical effect: a review. Am J Biochem Mol Biol 1(3):291–309. https://doi.org/10.3923/ajbmb.2011.291.309
Rampelotto PH, de Siqueira Ferreira A, Barboza ADM, Roesch LFW (2013) Changes in diversity, abundance, and structure of soil bacterial communities in Brazilian Savanna under different land use systems. Microb Ecol 66(3):593–607. https://doi.org/10.1007/s00248-013-0235-y
Chitale VS, Behera MD, Roy PS (2014) Future of endemic flora of biodiversity hotspots in India. PLoS ONE 9(12):e115264. https://doi.org/10.1371/journal.pone.0115264
Hanson T et al (2009) Warfare in biodiversity hotspots. Conserv Biol 23(3):578–587. https://doi.org/10.1111/j.1523-1739.2009.01166.x
Rinu K, Sati P, Pandey A (2014) Trichoderma gamsii (NFCCI 2177): a newly isolated endophytic, psychrotolerant, plant growth promoting, and antagonistic fungal strain. J Basic Microbiol 54(5):408–417. https://doi.org/10.1002/jobm.201200579
Sheikha AF (2015) Rumex nervosus: an overview. Int J Innov Hortic 4(2):87–95
Pan SY et al (2010) New perspectives on innovative drug discovery: an overview. J Pharm Pharm Sci 13(3):450–471
Adefa Seid M, Tsegay BA (2011) Ethnobotanical survey of traditional medicinal plants in Tehuledere district, South Wollo, Ethiopia. J Med Plants Res 5(26):6233–6242. https://doi.org/10.5897/JMPR11.1070
El-Sheikha AF (2017) Medicinal plants: ethno-uses to biotechnology era. In: Malik S (ed) Biotechnology and production of anti-cancer compounds. Springer International Publishing, Cham, pp 1–38
Gordaliza M (2007) Natural products as leads to anticancer drugs. Clin Transl Oncol 9(12):767–776. https://doi.org/10.1007/s12094-007-0138-9
Clardy J, Walsh C (2004) Lessons from natural molecules. Nature 432(7019):829–837. https://doi.org/10.1038/nature03194
Wani MC, Taylor HL, Wall ME, Coggon P, Mcphail AT (1971) Plant antitumor agents.VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia2. J Am Chem Soc 93(9):2325–2327. https://doi.org/10.1021/ja00738a045
Seca AML, Pinto DCGA (2018) Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int J Mol Sci. https://doi.org/10.3390/ijms19010263
Schiff PB, Horwitz SB (1980) Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA 77(3):1561–1565. https://doi.org/10.1073/pnas.77.3.1561
Torres K, Horwitz SB (1998) Mechanisms of Taxol-induced cell death are concentration dependent,". Cancer Res 58(16):3620–3626
Danishefsky SJ et al (1996) Total synthesis of baccatin III and taxol. J Am Chem Soc 118(12):2843–2859. https://doi.org/10.1021/ja952692a
Holton RA et al (1994) First total synthesis of taxol. 2. Completion of the C and D rings. J Am Chem Soc 116(4):1599–1600. https://doi.org/10.1021/ja00083a067
Nicolaou KC et al (1994) Total synthesis of taxol. Nature 367(6464):630–634. https://doi.org/10.1038/367630a0
Itokawa H, Kuo Hsiung L (2003) Taxus, the genus Taxus. In: Itokawa H, Kuo Hsiung L (eds) Taxus. CRC Press, Boca Raton
Wheeler NC et al (1992) Effects of genetic, epigenetic, and environmental factors on taxol content in taxus brevifolia and related species. J Nat Prod 55(4):432–440. https://doi.org/10.1021/np50082a005
Nadeem M, Rikhari HC, Kumar A, Palni LMS, Nandi SK (2002) Taxol content in the bark of Himalayan Yew in relation to tree age and sex. Phytochemistry 60(6):627–631. https://doi.org/10.1016/S0031-9422(02)00115-2
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67(4):491–502. https://doi.org/10.1128/mmbr.67.4.491-502.2003
Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67(2):257–268. https://doi.org/10.1021/np030397v
Malik S, Cusidó RM, Mirjalili MH, Moyano E, Palazón J, Bonfill M (2011) Production of the anticancer drug taxol in Taxus baccata suspension cultures: a review. Process Biochem 46(1):23–34. https://doi.org/10.1016/j.procbio.2010.09.004
Elavarasi A, Rathna GS, Kalaiselvam M (2012) Taxol producing mangrove endophytic fungi Fusarium oxysporum from Rhizophora annamalayana. Asian Pac J Trop Biomed 2(2):S1081–S1085. https://doi.org/10.1016/S2221-1691(12)60365-7
Wang J, Li G, Lu H, Zheng Z, Huang Y, Su W (2000) Taxol from Tubercularia sp. strain TF5, an endophytic fungus of Taxus mairei. FEMS Microbiol Lett 193(2):249–253. https://doi.org/10.1111/j.1574-6968.2000.tb09432.x
Sun D, Ran X, Wang J (2008) “Isolation and identification of a taxol-producing endophytic fungus from Podocarpus. Acta Microbiol Sin 48(5):589–595
Kumar G, Chandra P, Choudhary M (2017) Chemical science review and letters endophytic fungi: a potential source of bioactive compounds. Chem Sci Rev Lett 6(24):2373–2381
Kumar P et al (2019) Hyper-production of taxol from Aspergillus fumigatus, an endophytic fungus isolated from Taxus sp. of the Northern Himalayan region. Biotechnol Rep 24:e00395. https://doi.org/10.1016/j.btre.2019.e00395
Ueda JY et al (2002) Antiproliferative activity of Vietnamese medicinal plants. Biol Pharm Bull 25(6):753–760. https://doi.org/10.1248/bpb.25.753
Retna AM, Ethalsa P (2013) A review of the taxonomy, ethnobotany, chemistry and pharmacology of Catharanthus roseus (Apocyanaceae). IJERT 2(10):3899–3912
Deshmukh SK et al (2009) Anti-inflammatory and anticancer activity of ergoflavin isolated from an endophytic fungus. Chem Biodivers 6(5):784–789. https://doi.org/10.1002/cbdv.200800103
Omeje EO et al (2017) Endophytic fungi as alternative and reliable sources for potent anticancer agents. In: Badria FA (ed) Natural products and cancer drug discovery. InTech, New York
Senthil Kumaran R, Muthumary J, Hur BK (2008) Production of taxol from Phyllosticta spinarum, an endophytic fungus of Cupressus sp. Eng. Life Sci 8(4):438–446. https://doi.org/10.1002/elsc.200800019
Kim SU, Strobel G, Ford E (1999) Screening of taxol-producing endophytic fungi from Ginko biloba and Taxus cuspidata in Korea. Agric Chem Biotechnol 42(2):97–99
Gangadevi V, Muthumary J (2008) Taxol, an anticancer drug produced by an endophytic fungus Bartalinia robillardoides Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. World J Microbiol Biotechnol 24(5):717–724. https://doi.org/10.1007/s11274-007-9530-4
Rehman S et al (2008) An endophytic Neurospora sp. from Nothapodytes foetida producing camptothecin. Prikl Biokhim Mikrobiol 44(2):225–231. https://doi.org/10.1134/s0003683808020130
Amna T et al (2006) Bioreactor studies on the endophytic fungus Entrophospora infrequens for the production of an anticancer alkaloid camptothecin. Can J Microbiol 52(3):189–196. https://doi.org/10.1139/w05-122
Shweta S, Gurumurthy BR, Ravikanth G, Ramanan US, Shivanna MB (2013) Endophytic fungi from Miquelia dentata Bedd., produce the anti-cancer alkaloid, camptothecine. Phytomedicine 20(3–4):337–342. https://doi.org/10.1016/j.phymed.2012.11.015
Chithra S, Jasim B, Sachidanandan P, Jyothis M, Radhakrishnan EK (2014) Piperine production by endophytic fungus Colletotrichum gloeosporioides isolated from Piper nigrum. Phytomedicine 21(4):534–540. https://doi.org/10.1016/j.phymed.2013.10.020
Arora J, Ramawat KG (2017) “An introduction to endophytes. Springer, Cham, pp 1–23
Kumar A, Patil D, Rajamohanan PR, Ahmad A (2013) Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS ONE 8(9):e71805. https://doi.org/10.1371/journal.pone.0071805
Parthasarathy R, Shanmuganathan R, Pugazhendhi A (2020) Vinblastine production by the endophytic fungus Curvularia verruculosa from the leaves of Catharanthus roseus and its in vitro cytotoxicity against HeLa cell line. Anal Biochem. https://doi.org/10.1016/j.ab.2019.113530
Kumara PM, Soujanya KN, Ravikanth G, Vasudeva R, Ganeshaiah KN, Shaanker RU (2014) Rohitukine, a chromone alkaloid and a precursor of flavopiridol, is produced by endophytic fungi isolated from Dysoxylum binectariferum Hook.f and Amoora rohituka (Roxb). Wight & Arn. Phytomedicine 21(4):541–546. https://doi.org/10.1016/j.phymed.2013.09.019
Jouda JB et al (2016) Antibacterial and cytotoxic cytochalasins from the endophytic fungus Phomopsis sp. harbored in Garcinia kola (Heckel) nut. BMC Complement. Altern. Med. 16(1):462. https://doi.org/10.1186/s12906-016-1454-9
Goutam J et al (2017) Isolation and characterization of ‘Terrein’ an antimicrobial and antitumor compound from endophytic fungus Aspergillus terreus (JAS-2) associated from Achyranthus aspera Varanasi, India. Front Microbiol. https://doi.org/10.3389/fmicb.2017.01334
Taware R et al (2014) Isolation, purification and characterization of Trichothecinol-A produced by endophytic fungus Trichothecium sp. and its antifungal, anticancer and antimetastatic activities. Sustain Chem Process 2(1):8. https://doi.org/10.1186/2043-7129-2-8
Khan MIH, Sohrab MH, Rony SR, Tareq FS, Hasan CM, Mazid MA (2016) Cytotoxic and antibacterial naphthoquinones from an endophytic fungus, Cladosporium sp. Toxicol Rep 3:861–865. https://doi.org/10.1016/j.toxrep.2016.10.005
Li X, Guo Z, Deng Z, Yang J, Zou K (2015) A new α-pyrone derivative from endophytic fungus Pestalotiopsis microspora. Rec Nat Prod 9(4):503–508
Verekar SA et al (2014) Anticancer activity of new depsipeptide compound isolated from an endophytic fungus. J Antibiot (Tokyo) 67(10):697–701. https://doi.org/10.1038/ja.2014.58
Mishra PD et al (2013) Anti-inflammatory and anti-diabetic naphthaquinones from an endophytic fungus Dendryphion nanum (Nees) S. Hughes. Indian J Chem 52B:565–567
Sharma N et al (2018) New cytochalasin from Rosellinia sanctae-cruciana, an endophytic fungus of Albizia lebbeck. J Appl Microbiol 125(1):111–120. https://doi.org/10.1111/jam.13764
Arora D et al (2016) Isolation and characterization of bioactive metabolites from Xylaria psidii, an endophytic fungus of the medicinal plant Aegle marmelos and their role in mitochondrial dependent apoptosis against pancreatic cancer cells. Phytomedicine 23(12):1312–1320. https://doi.org/10.1016/j.phymed.2016.07.004
Kusari S, Zühlke S, Košuth J, Čellárová E, Spiteller M (2009) Light-independent metabolomics of endophytic Thielavia subthermophila provides insight into microbial hypericin biosynthesis. J Nat Prod 72(10):1825–1835. https://doi.org/10.1021/np9002977
Bunyapaiboonsri T, Yoiprommarat S, Srikitikulchai P, Srichomthong K, Lumyong S (2010) Oblongolides from the endophytic fungus Phomopsis sp. BCC 9789. J Nat Prod 73(1):55–59. https://doi.org/10.1021/np900650c
Das S, Krishi Viswavidyalaya C, Sharangi AB (2017) Madagascar periwinkle (Catharanthus roseus L): diverse medicinal and therapeutic benefits to humankind. J Pharmacogn Phytochem 6(5):1695–1701
Kumar A (2016) 3. IJMPS - vincristine and vinblastine a review.
Zhao J et al Endophytic fungi for producing bioactive compounds originally from their host plants Gamma-ray burst polarimeter POLAR view project biology of secondary metabolism in plant-microbe interactions (BSMPMI) view project endophytic fungi for producing bioactive compounds originally from their host plants
Sharma V, Kaur H, Kumar T, Mishra T (2016) Traditional indian herb Cathranthus roseus used as cancer treatment: a review. Int J Pharmacogn Phytochem Res 8(12):1926–1928
Bo G, Haiyan L, Lingqi Z (1998) Isolation of an fungus producting vinbrastine. J Yunnan Univ 20(3):214–215
Lingqi Z et al (2000) Preliminary study on the isolation of endophytic fungus of Catharanthus roseus and its fermentation to produce products of therapeutic value. Chin Tradit Herb Drugs 31(11):805–807
Xianzhi Y, Lingqi Z, Bo G, Shiping G (2004) Preliminary study of a vincristine-proudcing endophytic fungus isolated from leaves of Catharanthus roseus. Chin Tradit Herb Drugs 35(1):79–81
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83(3):770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Feher M, Schmidt JM (2003) Property distributions: differences between drugs, natural products, and molecules from combinatorial chemistry. J Chem Inf Comput Sci 43(1):218–227. https://doi.org/10.1021/ci0200467
Weiss SG, Tin-Wa M, Perdue RE, Farnsworth NR (1975) Potential anticancer agents II: antitumor and cytotoxic lignans from Linum album (Linaceae). J Pharm Sci 64(1):95–98. https://doi.org/10.1002/jps.2600640119
Konuklugil B (1996) Aryltetralin lignans from genus Linum. Fitoter 67(4):379–381
Ayres DC, Loike JD (1990) Lignans: chemical, biological, and clinical properties. Cambridge University Press, Cambridge
Schacter L (1996) Etoposide phosphate: what, why, where, and how? Semin Oncol 23:1–7
Guerram M, Jiang ZZ, Zhang LY (2012) Podophyllotoxin, a medicinal agent of plant origin: past, present and future. Chin J Nat Med 10(3):161–169. https://doi.org/10.3724/SP.J.1009.2012.00161
Gordaliza M, García PA, Miguel Del Corral JM, Castro MA, Gómez-Zurita MA (2004) Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives. Toxicon 44(4):441–459. https://doi.org/10.1016/j.toxicon.2004.05.008
Xianzhi Y, Shiping G, Lingqi Z, Hua S (2003) Select of producing podophyllotoxin endophytic fungi from podophyllin plant. Nat Prod Res Dev 15(5):419–422
Nadeem M (2012) Fusarium solani, P1, a new endophytic podophyllotoxin-producing fungus from roots of Podophyllum hexandrum. Afr J Microbiol Res. https://doi.org/10.5897/ajmr11.1596
Yousefzadi M et al (2010) Podophyllotoxin: current approaches to its biotechnological production and future challenges. Eng Life Sci 10(4):281–292. https://doi.org/10.1002/elsc.201000027
Chandra S (2012) Endophytic fungi: novel sources of anticancer lead molecules. Appl Microbiol Biotechnol 95(1):47–59. https://doi.org/10.1007/s00253-012-4128-7
Liu LF, Desai SD, Li TK, Mao Y, Sun M, Sim SP (2000) Mechanism of action of camptothecin. Ann N Y Acad Sci 922:1–10. https://doi.org/10.1111/j.1749-6632.2000.tb07020.x
Clarance P, Khusro A, Lalitha J, Sales J, Paul A (2019) Optimization of camptothecin production and biomass yield from endophytic fungus Fusarium solani strain ATLOY-8 ARTICLE INFO. J Appl Pharm Sci 9(10):35–046. https://doi.org/10.7324/JAPS.2019.91005
Zheng J, Zhou Y, Li Y, Xu DP, Li S, Bin Li H (2016) Spices for prevention and treatment of cancers. Nutrients 8:495. https://doi.org/10.3390/nu8080495
Manayi A, Nabavi SM, Setzer WN, Jafari S (2019) Piperine as a potential anti-cancer agent: a review on preclinical studies. Curr Med Chem 25(37):4918–4928. https://doi.org/10.2174/0929867324666170523120656
Hwang YP et al (2011) Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by piperine via the inhibition of PKCα/ERK1/2-dependent matrix metalloproteinase-9 expression. Toxicol Lett 203(1):9–19. https://doi.org/10.1016/j.toxlet.2011.02.013
Mérino D, Lalaoui N, Morizot A, Solary E, Micheau O (2007) TRAIL in cancer therapy: present and future challenges. Expert Opin Therap Targets 11(10):1299–1314. https://doi.org/10.1517/14728222.11.10.1299
Abdelhamed S et al (2014) Piperine enhances the efficacy of TRAIL-based therapy for triple-negative breast cancer cells. Anticancer Res 34(4):1893–1899
yun Ouyang D et al (2013) Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem. Toxicol. 60:424–430. https://doi.org/10.1016/j.fct.2013.08.007
Karar J, Maity A (2011) PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 4:51. https://doi.org/10.3389/fnmol.2011.00051
Doucette CD, Hilchie AL, Liwski R, Hoskin DW (2013) Piperine, a dietary phytochemical, inhibits angiogenesis. J Nutr Biochem 24(1):231–239. https://doi.org/10.1016/j.jnutbio.2012.05.009
Kim YS, Farrar W, Colburn NH, Milner JA (2012) Cancer stem cells: potential target for bioactive food components. J Nutr Biochem 23(7):691–698. https://doi.org/10.1016/j.jnutbio.2012.03.002
Li S, Lei Y, Jia Y, Li N, Wink M, Ma Y (2011) Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine 19(1):83–87. https://doi.org/10.1016/j.phymed.2011.06.031
Wagenaar MM, Corwin J, Strobel G, Clardy J (2000) Three new cytochalasins produced by an endophytic fungus in the genus Rhinocladiella. J Nat Prod 63(12):1692–1695. https://doi.org/10.1021/np0002942
Guo B, Wang Y, Sun X, Tang K (2008) Bioactive natural products from endophytes: a review. Appl Biochem Microbiol 44(2):136–142. https://doi.org/10.1134/S0003683808020026
Kaul S, Gupta S, Ahmed M, Dhar MK (2012) Endophytic fungi from medicinal plants: a treasure hunt for bioactive metabolites. Phytochem Rev 11(4):487–505. https://doi.org/10.1007/s11101-012-9260-6
Al-Sokari SS (2015) In vitro antimicrobial activity of crude extracts of some medicinal plants from Al-Baha region in Saudi Arabia. J. Food Nutr. Sci. 3(1):74
Wang, et al (2014) An endophytic fungus in Ficus carica and its secondary metabolites. Mycosystema. https://doi.org/10.13346/j.mycosystema.130182.
Ek-Ramos MJ, Gomez-Flores R, Orozco-Flores AA, Rodríguez-Padilla C, González-Ochoa G, Tamez-Guerra P (2019) Bioactive products from plant-endophytic Gram-positive bacteria. Front Microbiol 10:463. https://doi.org/10.3389/fmicb.2019.00463
Taechowisan T, Lu C, Shen Y, Lumyong S (2007) Antitumor activity of 4-Arylcoumarins from endophytic Streptomyces aureofaciens CMUAc130. J Cancer Res Ther 3(2):86–91. https://doi.org/10.4103/0973-1482.34685
Vu HNT et al (2018) Antimicrobial and cytotoxic properties of bioactive metabolites produced by Streptomyces cavourensis YBQ59 Isolated from Cinnamomum cassia Prels in Yen Bai Province of Vietnam. Curr Microbiol 75(10):1247–1255. https://doi.org/10.1007/s00284-018-1517-x
Pang X et al (2017) Metabolites from the plant endophytic fungus Aspergillus sp. CPCC 400735 and their anti-HIV activities. J Nat Prod 80(10):2595–2601. https://doi.org/10.1021/acs.jnatprod.6b00878
Ornano L, Feroci M, Guarcini L, Venditti A, Bianco A (2018) Anti-HIV agents from nature: natural compounds from Hypericum hircinum and carbocyclic nucleosides from iridoids. Stud Nat Prod Chem 56:173–228
Kashiwada Y et al (1998) Anti-AIDS agents, 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J Nat Prod 61(9):1090–1095. https://doi.org/10.1021/np9800710
Fujioka T et al (1994) Anti-aids agents, 11. betulinic acid and platanic acid as anti-HIV principles from syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod 57(2):243–247. https://doi.org/10.1021/np50104a008
Moghaddam MG, Ahmad FBH, Samzadeh-Kermani A (2012) Biological activity of betulinic acid: a review. Pharmacology Pharmacy 3(2):119–123. https://doi.org/10.4236/pp.2012.32018
Zhao H, Holmes SS, Baker GA, Challa S, Bose HS, Song Z (2012) Ionic derivatives of betulinic acid as novel HIV-1 protease inhibitors. J Enzyme Inhib Med Chem 27(5):715–721. https://doi.org/10.3109/14756366.2011.611134
Wang Y, Dai CC (2011) Endophytes: a potential resource for biosynthesis, biotransformation, and biodegradation. Ann Microbiol 61(2):207–215. https://doi.org/10.1007/s13213-010-0120-6
Morandini LMB et al (2016) Lanostane-type triterpenes from the fungal endophyte Scleroderma UFSMSc1 (Persoon) Fries. Bioorg Med Chem Lett 26:1173–1176. https://doi.org/10.1016/j.bmcl.2016.01.044
Li G, Kusari S, Kusari P, Kayser O, Spiteller M (2015) Endophytic Diaporthe sp. LG23 produces a potent antibacterial tetracyclic triterpenoid. J Nat Prod 78(8):2128–2132. https://doi.org/10.1021/acs.jnatprod.5b00170
Peyrat LA, Eparvier V, Eydoux C, Guillemot JC, Litaudon M, Stien D (2017) Betulinic acid, the first lupane-type triterpenoid isolated from both a Phomopsis sp. and its host plant diospyros carbonaria benoist. Chem Biodivers 14(1):e1600171. https://doi.org/10.1002/cbdv.201600171
Rang L, Chang-Hui C, Cheng AIX, Jian-Ping Z (2010) Structural origins of the differential antioxidative activities between baicalein and baicalin. Chin J Magn Reson 27:132–140
Li BQ, Fu T, Dongyan Y, Mikovits JA, Ruscetti FW, Wang JM (2000) Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem Biophys Res Commun 276(2):534–538. https://doi.org/10.1006/bbrc.2000.3485
Baylor NW, Fu T, Yan YD, Ruscetti FW (1992) Inhibition of human T cell leukemia virus by the plant flavonoid baicalin (7-glucuronic acid, 5, 6-dihydroxyflavone). J Infect Dis 165(3):433–437. https://doi.org/10.1093/infdis/165.3.433
Li BQ, Fu T, Yan YD, Baylor NW, Ruscetti FW, Kung HF (1993) Inhibition of HIV infection by baicalin—a flavonoid compound purified from Chinese herbal medicine. Cell Mol Biol Res 39(2):119–124
Ng TB, Huang B, Fong WP, Yeung HW (1997) Anti-human immunodeficiency virus (anti-HIV) natural products with special emphasis on HIV reverse transcriptase inhibitors. Life Sci. 61(10):933–949. https://doi.org/10.1016/S0024-3205(97)00245-2
Ferdous KJ, Afroz F, Islam MR, Mazid MA, Sohrab MH (2019) Isolated endophytic fungi from the plant Curcuma longa and their potential bioactivity—a review. Pharmacol Pharm 10(05):244–270. https://doi.org/10.4236/pp.2019.105021
Xiuyun J, Youjian F, Fengmei C, Jihong J (2014) Volatile constituents and their fibrinolytic activity of endophytic fungus Fusarium sp. GI024 from Ginkgo biloba. Wei Sheng Wu Xue Tong Bao 33(6):8–11
Zhang D et al (2015) Pericoannosin A, a polyketide synthase-nonribosomal peptide synthetase hybrid metabolite with new carbon skeleton from the endophytic fungus Periconia sp. Org Lett 17(17):4304–4307. https://doi.org/10.1021/acs.orglett.5b02123
Ding L et al (2010) Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorganic Med Chem Lett 20(22):6685–6687. https://doi.org/10.1016/j.bmcl.2010.09.010
Umashankar T, Govindappa M, Ramachandra Y (2012) In vitro antioxidant and anti-HIV activity of endophytic coumarin from Crotalaria pallida Aiton. Planta Med. https://doi.org/10.1055/s-0032-1307610
Liu L et al (2009) Chloropestolide A, an antitumor metabolite with an unprecedented spiroketal skeleton from Pestalotiopsis fici. Org Lett 11(13):2836–2839. https://doi.org/10.1021/ol901039m
Prasad S, Tyagi AK (2015) Curcumin and its analogues: a potential natural compound against HIV infection and AIDS. Food Funct 6(11):3412–3419. https://doi.org/10.1039/c5fo00485c
Hewlings S, Kalman D (2017) Curcumin: a review of its effects on human health. Foods 6(10):92. https://doi.org/10.3390/foods6100092
Kumari N et al (2015) Inhibition of HIV-1 by curcumin A, a novel curcumin analog. Drug Des Devel Ther 9:5051–5060. https://doi.org/10.2147/DDDT.S86558
Emerman M, Malim MH (1998) HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science 280(5371):1880–1884. https://doi.org/10.1126/science.280.5371.1880
Ali A, Banerjea AC (2016) Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci Rep. https://doi.org/10.1038/srep27539
Wiggers HJ, Zaioncz S, Cheleski J, Mainardes RM, Khalil NM (2017) Curcumin, a multitarget phytochemical. Stud Nat Prod Chem 53:243–276
Zorofchian Moghadamtousi S, Abdul Kadir H, Hassandarvish P, Tajik H, Abubakar S, Zandi K (2014) A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res Int. https://doi.org/10.1155/2014/186864
Ding L, Maier A, Fiebig HH, Lin WH, Hertweck C (2011) A family of multicyclic indolosesquiterpenes from a bacterial endophyte. Org Biomol Chem 9(11):4029–4031. https://doi.org/10.1039/c1ob05283g
Zhang Q et al (2012) N-N-coupled indolo-sesquiterpene atropo-diastereomers from a marine-derived actinomycete. European J Org Chem 2012(27):5256–5262. https://doi.org/10.1002/ejoc.201200599
Rosen BR, Werner EW, O’Brien AG, Baran PS (2014) Total synthesis of dixiamycin B by electrochemical oxidation. J Am Chem Soc 136(15):5571–5574. https://doi.org/10.1021/ja5013323
Srivastava V, Negi AS, Kumar JK, Gupta MM, Khanuja SPS (2005) Plant-based anticancer molecules: a chemical and biological profile of some important leads. Bioorg Med Chem 13(21):5892–5908. https://doi.org/10.1016/j.bmc.2005.05.066
Kala CP (2000) Status and conservation of rare and endangered medicinal plants in the Indian trans-Himalaya. Biol Conserv 93(3):371–379. https://doi.org/10.1016/S0006-3207(99)00128-7
Okami Y (1986) Marine microorganisms as a source of bioactive agents. Microb Ecol 12(1):65–78. https://doi.org/10.1007/BF02153223
Pimentel MR, Molina G, Dionísio AP, Maróstica-Junior MR, Pastore GM (2011) The use of endophytes to obtain bioactive compounds and their application in biotransformation process. Biotechnol Res Int 2011:1–11. https://doi.org/10.4061/2011/576286
Patil RH, Patil MP, Maheshwari VL (2016) Bioactive secondary metabolites from endophytic fungi: a review of biotechnological production and their potential applications. Stud Nat Prod Chem 49:189–205
Zhao J, Shan T, Mou Y, Zhou L (2011) Plant-derived bioactive compounds produced by endophytic fungi. Mini Rev Med Chem 11(2):159–168. https://doi.org/10.2174/138955711794519492
Balandrin MF, Klocke JA (1988) Medicinal, aromatic, and industrial materials from plants. Springer, Berlin, pp 3–36
Nadeem M, Palni LMS, Kumar A, Nandi SK (2007) Podophyllotoxin content, above- and belowground biomass in relation to altitude in Podophyllum hexandrum populations from Kumaun region of the Indian Central Himalaya. Planta Med 73(4):388–391. https://doi.org/10.1055/s-2007-967154
Acknowledgement
The authors would like to thank Prof. P. K. Khosla, Hon’ble Vice-Chancellor, Shoolini University of Biotechnology and Management Sciences, Solan and Foundation for Life Sciences and Business Management (FLSBM), Solan (H.P.)-India for providing financial support and necessary facilities. We would also like to thank Dr. Vikram Thakur, Senior Research Fellow, Department of Virology, PGIMER, Chandigarh for finalizing the content of the review. All authors would also like to thanks Prof. Saurabh Kulshrestha, Dean Research, and development, Shoolini University, Solan (H.P.), India for finalizing the overall content of the manuscript.
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Banyal, A., Thakur, V., Thakur, R. et al. Endophytic Microbial Diversity: A New Hope for the Production of Novel Anti-tumor and Anti-HIV Agents as Future Therapeutics. Curr Microbiol 78, 1699–1717 (2021). https://doi.org/10.1007/s00284-021-02359-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02359-2