Abstract
In this study, we have investigated the application of the non-precious β-PbO2/MWNTs nanocomposites, which were developed via a simple wet-chemistry procedure, toward electrochemical oxidation of lignin. Structural characterization of the synthesized electrocatalysts was performed through X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), and BET surface area analysis. Cyclic voltammetry (CV), linear sweep voltammetry (LSV), and chronoamperometry (ChA) measurements were carried out to evaluate and compare the performance of different electrocatalysts toward lignin oxidation. The 33.3 wt% β-PbO2 nanocomposite possessed the highest electro-catalytic activity and stability for depolymerization of lignin. Several oxidation products such as vanillin and methyl salicylate were also identified by gas chromatography-mass spectroscopy (GS-MS) analysis. The enhanced electrochemical surfaces areas of the low-cost β-PbO2/MWNTs electrocatalyst combined with its good stabilities offer a promising route for developing an energy-efficient lignin depolymerization process.
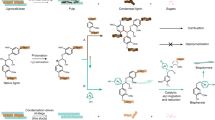
Graphic abstract
Electrochemical conversion of lignin-rich waste streams to functionalized compounds is appropriate for production of targeted biofuel and platform chemicals.









Similar content being viewed by others
References
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) The Catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599. https://doi.org/10.1021/cr900354u
Laskar DD, Yang B, Wang H, Lee J (2013) Pathways for biomass-derived lignin to hydrocarbon fuels. Biofuels Bioprod Biorefin 7:602–626. https://doi.org/10.1002/bbb.1422
Yang H, Yan R, Chen H et al (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Ma Z, Troussard E, van Bokhoven JA (2012) Controlling the selectivity to chemicals from lignin via catalytic fast pyrolysis. Appl Catal A 423–424:130–136. https://doi.org/10.1016/j.apcata.2012.02.027
Kang S, Li X, Fan J, Chang J (2013) Hydrothermal conversion of lignin: a review. Renew Sustain Energy Rev 27:546–558. https://doi.org/10.1016/j.rser.2013.07.013
Van Dyk JS, Pletschke BI (2012) A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol Adv 30:1458–1480. https://doi.org/10.1016/j.biotechadv.2012.03.002
Yan N, Zhao C, Dyson PJ et al (2008) Selective degradation of wood lignin over noble-metal catalysts in a two-step process. Chemsuschem 1:626–629. https://doi.org/10.1002/cssc.200800080
Zhu H, Wang L, Chen Y et al (2014) Electrochemical depolymerization of lignin into renewable aromatic compounds in a non-diaphragm electrolytic cell. RSC Adv 4:29917. https://doi.org/10.1039/C4RA03793F
Song Q, Wang F, Cai J et al (2013) Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ Sci 6:994. https://doi.org/10.1039/c2ee23741e
Toledano A, Serrano L, Pineda A et al (2014) Microwave-assisted depolymerisation of organosolv lignin via mild hydrogen-free hydrogenolysis: catalyst screening. Appl Catal B 145:43–55. https://doi.org/10.1016/j.apcatb.2012.10.015
Movil O, Garlock M, Staser JA (2015) Non-precious metal nanoparticle electrocatalysts for electrochemical modification of lignin for low-energy and cost-effective production of hydrogen. Int J Hydrog Energy 40:4519–4530. https://doi.org/10.1016/j.ijhydene.2015.02.023
Bailey A, Brooks HM (1946) Electrolytic oxidation of lignin. J Am Chem Soc 68:445–446. https://doi.org/10.1021/ja01207a029
Pardini VL, Smith CZ, Utley JH et al (1991) Electroorganic reactions. 38. Mechanism of electrooxidative cleavage of lignin model dimers. J Org Chem 56:7305–7313. https://doi.org/10.1021/jo00026a022
Parpot P, Bettencourt AP, Carvalho AM, Belgsir EM (2000) Biomass conversion: attempted electrooxidation of lignin for vanillin production. J Appl Electrochem 30:727–731. https://doi.org/10.1023/A:1004003613883
Lu Z, Tu B, Chen F (2003) Electro-degradation of sodium lignosulfonate. J Wood Chem Technol 23:261–277. https://doi.org/10.1081/WCT-120026933
Tolba R, Tian M, Wen J et al (2010) Electrochemical oxidation of lignin at IrO2-based oxide electrodes. J Electroanal Chem 649:9–15. https://doi.org/10.1016/j.jelechem.2009.12.013
Zhou M, Dai Q, Lei L et al (2005) Long life modified lead dioxide anode for organic wastewater treatment: electrochemical characteristics and degradation mechanism. Environ Sci Technol 39:363–370. https://doi.org/10.1021/es049313a
Tong S-P, Ma C-A, Feng H (2008) A novel PbO2 electrode preparation and its application in organic degradation. Electrochim Acta 53:3002–3006. https://doi.org/10.1016/j.electacta.2007.11.011
Aquino JM, Rocha-Filho RC, Ruotolo LAM et al (2014) Electrochemical degradation of a real textile wastewater using β-PbO2 and DSA® anodes. Chem Eng J 251:138–145. https://doi.org/10.1016/j.cej.2014.04.032
Zhao G, Zhang Y, Lei Y et al (2010) Fabrication and electrochemical treatment application of a novel lead dioxide anode with superhydrophobic surfaces, high oxygen evolution potential, and oxidation capability. Environ Sci Technol 44:1754–1759. https://doi.org/10.1021/es902336d
Oury A, Kirchev A, Bultel Y, Chainet E (2012) PbO2/Pb2+ cycling in methanesulfonic acid and mechanisms associated for soluble lead-acid flow battery applications. Electrochim Acta 71:140–149. https://doi.org/10.1016/j.electacta.2012.03.116
Carr JP, Hampson NA (1972) Lead dioxide electrode. Chem Rev 72:679–703. https://doi.org/10.1021/cr60280a003
Schümann U, Gründler P (1998) Electrochemical degradation of organic substances at PbO2 anodes: monitoring by continuous CO2 measurements. Water Res 32:2835–2842. https://doi.org/10.1016/S0043-1354(98)00046-3
Amadelli R, Samiolo L, De Battisti A, Velichenko AB (2011) Electro-oxidation of some phenolic compounds by electrogenerated O3 and by direct electrolysis at PbO2 anodes. J Electrochem Soc 158:P87–P92. https://doi.org/10.1149/1.3589913
Santos JEL, de Moura DC, da Silva DR et al (2019) Application of TiO2-nanotubes/PbO2 as an anode for the electrochemical elimination of Acid Red 1 dye. J Solid State Electrochem 23:351–360. https://doi.org/10.1007/s10008-018-4134-5
Chianca de Moura D, Cerro-López M, Quiroz MA et al (2015) Large disk electrodes of Ti/TiO2-nanotubes/PbO2 for environmental applications. RSC Adv 5:31454–31459. https://doi.org/10.1039/C4RA16723F
Pan K, Tian M, Jiang Z-H et al (2012) Electrochemical oxidation of lignin at lead dioxide nanoparticles photoelectrodeposited on TiO2 nanotube arrays. Electrochim Acta 60:147–153. https://doi.org/10.1016/j.electacta.2011.11.025
Zhang Y, Peng Y, Yin X et al (2014) Degradation of lignin to BHT by electrochemical catalysis on Pb/PbO2 anode in alkaline solution. J Chem Technol Biotechnol 89:1954–1960. https://doi.org/10.1002/jctb.4282
Shao D, Liang J, Cui X et al (2014) Electrochemical oxidation of lignin by two typical electrodes: Ti/SbSnO2 and Ti/PbO2. Chem Eng J 244:288–295. https://doi.org/10.1016/j.cej.2014.01.074
Wang Y, Yang F, Liu Z et al (2015) Electrocatalytic degradation of aspen lignin over Pb/PbO2 electrode in alkali solution. Catal Commun 67:49–53. https://doi.org/10.1016/j.catcom.2015.03.033
Movil-Cabrera O, Rodriguez-Silva A, Arroyo-Torres C, Staser JA (2016) Electrochemical conversion of lignin to useful chemicals. Biomass Bioenerg 88:89–96. https://doi.org/10.1016/j.biombioe.2016.03.014
Ghahremani R, Staser JA (2018) Electrochemical oxidation of lignin for the production of value-added chemicals on Ni–Co bimetallic electrocatalysts. Holzforschung 72:951–960. https://doi.org/10.1515/hf-2018-0041
Singh S, Ghatak HR (2018) Process optimization of lignin conversion into value added chemicals by thermochemical pretreatment and electrooxidation on a stainless steel anode. Holzforschung 72:187–199. https://doi.org/10.1515/hf-2017-0108
Singh S, Ghatak HR (2017) Vanillin formation by electrooxidation of lignin on stainless steel anode: kinetics and by-products. J Wood Chem Technol 37:407–422. https://doi.org/10.1080/02773813.2017.1310899
Schmitt D, Regenbrecht C, Schubert M et al (2017) Treatment of black liquor (BL) by adsorption on AE resins and a subsequent electrochemical degradation of BL to obtain vanillin. Holzforschung 71:35–41. https://doi.org/10.1515/hf-2015-0210
Sirés I, Low CTJ, Ponce-de-León C, Walsh FC (2010) The characterisation of PbO2-coated electrodes prepared from aqueous methanesulfonic acid under controlled deposition conditions. Electrochim Acta 55:2163–2172. https://doi.org/10.1016/j.electacta.2009.11.051
Dai H (2002) Carbon nanotubes: synthesis, integration, and properties. Acc Chem Res 35:1035–1044. https://doi.org/10.1021/ar0101640
Baglio V, Di Blasi A, D’Urso C et al (2008) Development of Pt and Pt–Fe catalysts supported on multiwalled carbon nanotubes for oxygen reduction in direct methanol fuel cells. J Electrochem Soc 155:B829. https://doi.org/10.1149/1.2938368
Shao Y, Liu J, Wang Y, Lin Y (2009) Novel catalyst support materials for PEM fuel cells: current status and future prospects. J Mater Chem 19:46–59. https://doi.org/10.1039/B808370C
Prabhuram J, Zhao TS, Tang ZK et al (2006) Multiwalled carbon nanotube supported ptru for the anode of direct methanol fuel cells. J Phys Chem B 110:5245–5252. https://doi.org/10.1021/jp0567063
Yang W-H, Wang H-H, Chen D-H et al (2012) Facile synthesis of a platinum–lead oxide nanocomposite catalyst with high activity and durability for ethanol electrooxidation. Phys Chem Chem Phys 14:16424–16432. https://doi.org/10.1039/C2CP41944K
Morales J, Petkova G, Cruz M, Caballero A (2004) Nanostructured lead dioxide thin electrode. Electrochem Solid-State Lett 7:A75. https://doi.org/10.1149/1.1646831
Wang S, Wang X, Jiang SP (2008) PtRu nanoparticles supported on 1-aminopyrene-functionalized multiwalled carbon nanotubes and their electrocatalytic activity for methanol oxidation. Langmuir 24:10505–10512. https://doi.org/10.1021/la800925t
Schilling T, Bron M (2008) Oxygen reduction at Fe–N-modified multi-walled carbon nanotubes in acidic electrolyte. Electrochim Acta 53:5379–5385. https://doi.org/10.1016/j.electacta.2008.02.062
Yang W-H, Zhang Q-H, Wang H-H et al (2017) Preparation and utilization of a sub-5 nm PbO2 colloid as an excellent co-catalyst for Pt-based catalysts toward ethanol electro-oxidation. New J Chem 41:12123–12130. https://doi.org/10.1039/C7NJ02620J
Li W, Liang C, Zhou W et al (2003) Preparation and characterization of multiwalled carbon nanotube-supported platinum for cathode catalysts of direct methanol fuel cells. J Phys Chem B 107:6292–6299. https://doi.org/10.1021/jp022505c
Wan J, Cai W, Feng J et al (2007) In situ decoration of carbon nanotubes with nearly monodisperse magnetite nanoparticles in liquid polyols. J Mater Chem 17:1188. https://doi.org/10.1039/b615527h
Belin T, Epron F (2005) Characterization methods of carbon nanotubes: a review. Mater Sci Eng B 119:105–118. https://doi.org/10.1016/j.mseb.2005.02.046
Bagshaw NE, Clarke RL, Halliwell B (2007) The preparation of lead dioxide for X-ray diffraction studies. J Appl Chem 16:180–184. https://doi.org/10.1002/jctb.5010160604
Yeh J-W, Chang S-Y, Hong Y-D et al (2007) Anomalous decrease in X-ray diffraction intensities of Cu–Ni–Al–Co–Cr–Fe–Si alloy systems with multi-principal elements. Mater Chem Phys 103:41–46. https://doi.org/10.1016/j.matchemphys.2007.01.003
Centeno B, Tascón ML, Vázquez MD, Batanero PS (1991) Electrochemical study of PbO2 at a carbon paste electrode with electrolytic binder. Electrochim Acta 36(2):277–282. https://doi.org/10.1016/0013-4686(91)85249-7
Delahay P, Pourbaix M, Rysselberghe PV (1951) Potential-pH diagram of lead and its applications to the study of lead corrosion and to the lead storage battery. J Electrochem Soc 98:57. https://doi.org/10.1149/1.2778106
Comninellis C (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim Acta 39:1857–1862. https://doi.org/10.1016/0013-4686(94)85175-1
Martínez-Huitle CA, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc Rev 35:1324–1340. https://doi.org/10.1039/B517632H
Liu M, Wen Y, Qi J et al (2017) Fine chemicals prepared by bamboo lignin degradation through electrocatalytic redox between Cu cathode and Pb/PbO2 anode in alkali solution. ChemistrySelect 2:4956–4962. https://doi.org/10.1002/slct.201700881
Acknowledgements
The work was performed at the Center for Electrochemical Engineering Research (CEER) at Ohio University. We are also grateful to acknowledge Benjamin Sheets for his valuable suggestions and discussions. The authors would like to thank the Integrated Biorefinery Research Facility operated by the National Renewable Energy Laboratory for biorefinery lignin. The authors would like to thank the Russ Vision Fund for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bateni, F., Ghahremani, R. & Staser, J.A. Electrochemical oxidative valorization of lignin by the nanostructured PbO2/MWNTs electrocatalyst in a low-energy depolymerization process. J Appl Electrochem 51, 65–78 (2021). https://doi.org/10.1007/s10800-020-01451-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-020-01451-y