Analysis of conserved miRNAs in cynomolgus macaque genome using small RNA sequencing and homology searching
- Published
- Accepted
- Received
- Academic Editor
- Yuriy Orlov
- Subject Areas
- Biochemistry, Genetics, Molecular Biology, Zoology
- Keywords
- microRNA, RNA sequencing, Cynomolgus macaque
- Copyright
- © 2020 Huang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Analysis of conserved miRNAs in cynomolgus macaque genome using small RNA sequencing and homology searching. PeerJ 8:e9347 https://doi.org/10.7717/peerj.9347
Abstract
MicroRNAs (miRNAs) are important regulators that fine-tune diverse cellular activities. Cynomolgus macaques (Macaca fascicularis) are used extensively in biomedical and pharmaceutical research; however, substantially fewer miRNAs have been identified in this species than in humans. Consequently, we investigated conserved miRNA profiles in cynomolgus macaques by homology searching and small RNA sequencing. In total, 1,455 high-confidence miRNA gene loci were identified, 408 of which were also confirmed by RNA sequencing, including 73 new miRNA loci reported in cynomolgus macaques for the first time. Comparing miRNA expression with age, we found a positive correlation between sequence conservation and expression levels during miRNA evolution. Additionally, we found that the miRNA gene locations in cynomolgus macaque genome were very flexible. Most were embedded in intergenic spaces or introns and clustered together. Several miRNAs were found in certain gene locations, including 64 exon-resident miRNAs, six splice-site-overlapping miRNAs (SO-miRNAs), and two pairs of distinct mirror miRNAs. We also identified 78 miRNA clusters, 68 of which were conserved in the human genome, including 10 large miRNA clusters predicted to regulate diverse developmental and cellular processes in cynomolgus macaque. Thus, this study not only expands the number of identified miRNAs in cynomolgus macaques but also provides clues for future research on the differences in miRNA repertoire between macaques and humans.
Introduction
MiRNAs are small (18–25 nucleotides long), non-coding RNAs that regulate the expression of target genes at the post-transcriptional level. Generally, miRNA genes are transcribed by RNA polymerase II and form primary miRNA hairpins (pri-miRNAs). A region of a pri-miRNA forming hairpin structure is recognized by a microprocessor complex consisting of Drosha and DGCR8 proteins, which produce a stem-loop precursor miRNA (pre-miRNA) (Lee et al., 2003). Pre-miRNAs are then exported to the cytoplasm and are further processed by the enzyme Dicer, which cleaves off the terminal loop, leaving a miRNA duplex about 22 nucleotides in length. Many hairpins produce functional mature miRNAs from both duplex arms (Kobayashi & Tomari, 2016; Rorbach, Unold & Konopka, 2018). Mature miRNAs assemble with Argonaute proteins into miRNA-induced silencing complexs (miRISCs) to mediate mRNA targeting, primarily through pairing between the miRNA seed region (nucleotides 2–8) and complementary site within the 3′ UTR of mRNA (Berezikov, 2011). In addition to the seed region, certain sites in the 3′ part of miRNAs can also contribute to target recognition (i.e., nucleotides 13–16 known as the ‘supplemental region’) (Gebert & MacRae, 2019). In animals, miRISCs induce gene silencing through translation repression and mRNA decay (Jonas & Izaurralde, 2015; Treiber, Treiber & Meister, 2019). A single miRNA usually regulates a large number of genes simultaneously, and one gene may be regulated by multiple miRNAs. In particular, transcription factors or RNA-binding proteins can be regulated by individual miRNAs and entire cellular pathways can be regulated by miRNA clusters. MiRNA binding of neighboring target sites on a target mRNA can result in cooperative repression (Gebert & MacRae, 2019; Uhlmann et al., 2012). It has been estimated that about 60% of mammalian protein-coding genes are conserved targets of miRNAs (Friedman et al., 2009). The comprehensive interactions between miRNAs and mRNAs often play significant roles in composing complex genetic networks and fine-tuning diverse biological functions, such as organism development, body patterning, tissue homeostasis, differentiation, and cell cycle regulation (Berezikov, 2011; Pinhal et al., 2018). Evolutionarily, many miRNAs are highly conserved due to their functional importance. However, excessive lineage-specific miRNAs have also emerged in various taxa (Heimberg et al., 2008; Bartel, 2018). It is worth noting that miRNA expansion appears to be associated with body-plan innovations and other phenotypic changes in bilaterians and vertebrates (Heimberg et al., 2008; Meunier et al., 2013; Christodoulou et al., 2010). Therefore, they contribute considerably to phenotypic evolution in animals.
Cynomolgus macaques (Macaca fascicularis), also known as crab-eating or long-tailed monkeys, are distributed widely in Southeast Asia, including Indonesia, Vietnam, Philippines, Cambodia, Myanmar, Malaysia, Brunei, and Thailand (Shiina et al., 2015). The species belongs to the Cercopithecinae subfamily of Old-world monkeys and has emerged as an alternative animal model after the banning of rhesus macaques (Macaca mulatta) from India. Cynomolgus macaques have been utilized extensively in biomedical and pharmaceutical research, including studies on aging, neurological diseases, diabetes, pregnancy, and drug evaluation (Theriault et al., 1999; Carter, 2011; Grigsby, 2016; Yang et al., 2014; Willard & Shively, 2012), thus prompting the sequencing of its genome (Ebeling et al., 2011; Yan et al., 2011). Based on comparative genomic analysis, cynomolgus macaques share 99.2% and 92.8% genomic identity with rhesus macaques and humans, respectively (Veeranagouda et al., 2015). Furthermore, recent animal study indicates a strong correlation between increasing metazoan morphological disparity and miRNA repertoires, although no correlation with protein domain diversity (Deline et al., 2018). Species-specific miRNAs are considered to be important contributors to cancers, autoimmune diseases, and dissimilar responses of species to toxicant exposure (Koufaris & Gooderham, 2013; Koufaris, 2016; Jimenez & Piera-Velazquez, 2013). Therefore, elucidating the differences in miRNA repertoires between monkeys and humans could have valuable implications for the use of nonhuman primate models in biomedical studies. So far, larger-scale annotations of miRNAs have only been conducted for a few species (Dannemann et al., 2012a; McCreight et al., 2017) and only 20 of the ∼300 known primate species have entries in miRBase (Kozomara, Birgaoanu & Griffiths-Jones, 2019; Perelman et al., 2011). Furthermore, the number of characterized human pre-miRNAs (n = 1, 917) is three times larger than that of rhesus macaques (n = 617). In addition, cynomolgus macaque miRNAs remain limited to several hundred (Yang et al., 2014; Veeranagouda et al., 2015; Yang et al., 2012). Thus, further studies are needed to extend the miRNA profile of this species. Recently, technological advances such as bioinformatics and high-throughput RNA sequencing allowed the identification of a great number of putative miRNAs in different organisms. The computational methods are better to discover conserved miRNAs comprehensively (Demirci, Baumbach & Allmer, 2017), while the RNA-seq method is more advantageous in identifying novel miRNAs with non-canonical structures. In this study, we used the two complementary approaches mentioned above to identify miRNAs in cynomolgus macaques. This study should not only expand the number of recognized miRNAs in cynomolgus macaques but also provide clues to elucidate the differences in miRNA repertoires between macaques and humans, and thus improve our understanding of the evolution and function of miRNAs.
Material and Methods
Sample collection
Peripheral whole blood samples were taken from four unrelated Vietnamese-origin cynomolgus macaques (2–4 years old, one male, three females). The monkeys were healthy and housed at Guangdong Landau Biotechnology Co., Ltd. (Guangzhou China). All experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Guangdong Landau Biotechnology Co., Ltd. (project number: IACUC-003). Peripheral whole blood samples (2 ml) were used for small RNA extraction. Firstly, red blood cells were selectively lysed using an EZNA Blood RNA Kit (Omega, USA) and white blood cells were collected by centrifugation (450 g, 10 min, 4 °C). To avoid interference of 18S and 28S rRNA, small RNAs (18–200 nt) were isolated from white blood cells using RNAiso (TaKaRa, China). The samples were dissolved in 30 µl of diethylpyrocarbonate-treated water and stored at −80 °C prior to small RNA sequencing at the Beijing Genomics Institute (BGI, Shenzhen, China).
Small RNA library construction and sequencing
Four small RNA libraries were prepared and sequenced using standard methods at BGI. Before library construction, the integrity and concentration of samples were checked using an Agilent 2100 Bioanalyzer (Agilent RNA 6000 Nano Kit, USA). The RNAs were size-fractionated on a 15% PAGE gel, and small fragments (range of 18–30 nt) were collected and then amplified by real-time polymerase chain reaction (RT-PCR) using adaptor primers for 17 cycles. The fragments (140–160 bp in length) were then fractionated with a 15% PAGE gel. The final library was quantified by determining average sequence length using the Agilent 2100 Bioanalyzer (Agilent DNA 1000 Reagents, USA) and quantifying the library using quantitative RT-PCR (StepOnePlus Real-Time PCR System, Applied Biosystems, Foster, USA). The fragment sizes of the four libraries were all between 140 bp and 160 bp and the concentration of each library was more than 8.5 nM, thus meeting the minimum requirements for the construction of a sequencing library. Finally, the libraries were used for sequencing analysis on an HiSeq 4000 System (Illumina, USA). The primary data were then subjected to low-quality filtering. Reads that satisfied any of the following parameters were removed: (1) reads aligned to adaptors or primers with less than three mismatches, (2) reads with >10% unknown bases (N bases), (3) reads with >50% low-quality bases in one read, and (4) reads <18 nt. The remaining clean reads were used in the following analyses. The clean reads of the four samples were submitted to the European Nucleotide Archive (ENA) under study accession number PRJEB38080.
Analysis of sequencing data
The cynomolgus macaque reference genome (v. macFas5) was downloaded from the UCSC Genome Browser (http://genome.ucsc.edu). Clean reads were aligned to the reference genome using BGI software SOAP2 (Li et al., 2009b). Sequences perfectly mapped to the genome along their entire length were further annotated by alignment against macaque genome annotations and known annotation documents from the NCBI/UCSC/Ensembl databases (Benson et al., 2013; Kalvari et al., 2018). Sequences matching rRNA, tRNA, snRNA, snoRNA, and repeat associated sequences or overlapping with exons were discarded, and the remaining sRNA tags were aligned to a reference set of all animal miRNAs from miRBase (release 22) using BLAST by allowing no more than two mismatches outside the seed region. To distinguish authentic miRNAs from false annotations, candidates corresponding to known miRNAs from miRBase were considered conserved miRNA orthologs in the cynomolgus macaques when they were supported by at least 10 reads of mature sequences from the four samples and detected in at least three individuals according to criteria described previously (Veeranagouda et al., 2015; Kozomara & Griffiths-Jones, 2014). The pre-miRNA sequences were predicted based on the presence of hairpin structures analyzed by MIREAP under default settings (https://sourceforge.net/projects/mireap/) (Tan et al., 2013).
Detection of miRNA genes by homology searching
BLAT and BLAST softwares were used for homology searching (Kent, 2002). All mammalian pre-miRNA sequences from miRBase (release 22) were aligned to the cynomolgus macaque genome. The candidates were assumed to be conserved pre-miRNA sequences in cynomolgus macaques only when they fulfilled three criteria: (1) similarity of full length pre-miRNA >93%; (2) gene location corresponding to only one miRNA (although can have multiple gene mapping information); and (3) in mature sequences, no more than two mismatches outside the seed region and no more than one mismatch in the seed region compared with existing miRNAs (Veeranagouda et al., 2015). Furthermore, 10 nt up and downstream of the conserved pre-miRNA sequences were analyzed by MIREAP (Tan et al., 2013) and RNAfold (Lorenz et al., 2011). Stem-loop hairpins were considered acceptable only when they fulfilled three criteria: (1) the mature miRNA present in one arm of the stem; (2) absent of large internal bulges (n < 6); and, (3) a minimal folding free energy (MFE) of predicted pre-miRNA secondary structures of no more than -15 kcal/mol. After mapping all pre-miRNA candidates with acceptable secondary structures to the genome, they were further annotated by alignment against known annotation documents from the NCBI/UCSC/Ensembl databases (Benson et al., 2013; Kalvari et al., 2018). Small RNAs were sorted by their chromosomal locations on the genome. Based on criteria described previously (Marco et al., 2013), if two neighboring miRNA loci were located within 10 kb and on the same strand, they were considered clustered miRNA genes. The miRNA orthologs were grouped into different miRNA families according to sequence similarity to known miRNA family members. ClustalW was used for the alignment of sequences. Data patch processing was implemented in python.
Target prediction
The target genes of miRNAs were predicted by TargetScan 7.2 (http://www.targetscan.org), miRanda (http://www.microrna.org), and PITA (https://tools4mirs.org/). Gene ontology (GO) was applied for functional annotation analysis of the predicted targets. Enriched pathways involved with the miRNAs were determined according to the Kyoto Encyclopedia of Genes and Genomes (KEGG). Both GO and KEGG analyses were performed using the clusterProfilers package in R (Yu et al., 2012), and an adjusted P-value (Benjamini–Hochberg false discovery rate correction) of less than 0.05 was considered significant.
Correlation between age and miRNA expression
Considering the variable RNA concentrations and biases in the RNA-seq procedures for each sample, the expression levels of mature miRNAs were normalized by transcripts per million, i.e., dividing the number of reads for each miRNA by the total number of clean reads for each sample and multiplying it by 106 (Xu et al., 2013). The ages of the miRNAs were assessed based on the set of homologous sequences identified in other vertebrate species in miRBase (Meunier et al., 2013). If the miRNA homologous sequences were shared among vertebrates or mammals, these miRNAs were considered old and grouped into vertebrate or mammalian categories, respectively. If the miRNAs were specific to primates or Cercopithecidae, they were considered young and grouped into primate or Cercopithecidae categories, respectively. The average expression levels of miRNAs in different age groups of the four cynomolgus macaques were statistically analyzed by GraphPad Prism 8. Spearman correlation coefficients (R values) between the age of miRNAs and average expression levels of mature miRNAs were computed by matrix analysis in GraphPad and P-values were derived at a confidence interval of 95%.
Results
Identification of miRNA genes in cynomolgus macaque genome by homology searching
To identify previously unknown miRNA sequences in macaques (Yang et al., 2014; Veeranagouda et al., 2015), we used all mammalian pre-miRNA sequences deposited in miRBase as a reference for homology searching. According to the criteria described above, a total of 1,697 homologous sequences were perfectly mapped to the cynomolgus macaque genome with nonoverlapping gene locations and were then used to analyze their secondary structures. 1,526 of the 1,697 homologous sequences were predicted to form acceptable stem-loop hairpins and were considered conserved miRNA candidates. Additionally, we annotated the 1,526 candidates in the cynomolgus macaque genome using the NCBI/UCSC/Ensembl databases and miRBase for screening of homologies in other species. A candidate identified by homology searching was deemed to be of high confidence if it met any of the following conditions: (1) annotated as miRNA in other species or homolog has experimental support; and, (2) reported as a Macaca fascicularis miRNA previously. In total, 1,455 of the 1,526 pre-miRNA candidates showed high confidence. The remaining 71 pre-miRNA candidates lacked support in the other species and were excluded from the following analysis. 672 of the 1,455 candidates were reported previously and the remaining 783 gene loci were firstly annotated in cynomolgus macaque genome (Yang et al., 2014; Veeranagouda et al., 2015). Compared the 1,455 pre-miRNAs with their homologs in other mammals, the stem-loop sequence similarities of 1,186 candidates (81.5% of 1,455) exceeded 95%. Furthermore, 1,074 candidates (73.8% of 1,455) contained 0 mismatches in the -5 p or -3 p mature sequences, with the remaining 299 and 82 having one and more than one mismatch, respectively. In comparison to human miRNAs, 1,083 of the 1,917 human pre-miRNAs deposited in miRBase had orthologs in the cynomolgus macaque genome. Thus, most pre-miRNA and mature miRNA sequences were highly conserved. Most of the 1,455 miRNA sequences in the cynomolgus macaque were single copies, although others had paralogs at several gene locations. For instance, mir-548, a poorly conserved primate-specific miRNA gene family, was represented by 53 paralogs distributed across 18 chromosomes, similar to that observed in humans (Liang, Guo & Liu, 2012). The names, stem-loop sequences, gene locations, homology similarities, mismatches in mature sequences, and MFE values of the predicted hairpin structures for the 1,455 pre-miRNA candidates are listed in Table S1.
Identification of miRNAs in cynomolgus macaque using small RNA sequencing
A major obstacle in research on primate miRNAs is the lack of experimentally verified miRNAs in nonhuman primates. To better characterize the identified miRNAs in cynomolgus macaques, small RNA-seq was performed using peripheral blood leucocytes from the four animals. On average, 14 million clean reads were obtained and about 9 million small RNAs (∼5,000 unique reads) were mapped to miRBase for each sample (Table 1). As described in the methods, sequences that matched against orthologs in miRBase were considered as known miRNAs, otherwise, they were considered novel. A total of 546 known mature miRNA sequences and 136 potentially novel mature miRNAs (Table S2) were identified. As the 136 potentially novel miRNAs need further confirmation, only the 546 known miRNAs were used in subsequent analyses. To understand whether these miRNAs resulted from one or both of arms of a given precursor, we assigned the 546 mature miRNAs based on pre-miRNA information. As shown in Table S3, 337 mature miRNAs were expressed from both the -5 p and -3 p arms of 170 pre-miRNAs, and the remaining 209 mature miRNAs were expressed from either the -5 p or -3 p arms of 213 pre-miRNAs. As seven mature miRNAs were derived from more than one pre-miRNA, the 546 mature miRNAs were processed from 383 stem-loop miRNA sequences. We identified 379 of the 383 pre-miRNAs by homology searching (as above), including 357 pre-miRNAs with mapping information for single gene and 22 with mapping information for 2–4 genes. We re-screened the remaining four pre-miRNAs, mir-383, mir-378c, mir-4536, and mir-5698, which were deposited in miRBase against the cynomolgus macaque genome. All showed sequence similarities below 60%, indicating that these four pre-miRNA sequences were distinct to cynomolgus macaques. Analysis of sequencing data indicated that 408 of the 1,455 miRNA loci were expressed in peripheral blood leucocytes. These 408 miRNA loci demonstrated high confidence as they were identified by both RNA sequencing and homology searching and showed homolog support in other species. In total, 335 have been reported in cynomolgus macaques previously (Yang et al., 2014; Veeranagouda et al., 2015), with the remaining 73 considered to be new pre-miRNA loci in cynomolgus macaques (Table 2).
| CE01 | CE02 | CE03 | CE04 | Average | |
|---|---|---|---|---|---|
| Clean reads | 11640333 | 10922961 | 11573957 | 20255682 | 13598233 |
| Unique sRNAs | 365682 | 469182 | 426731 | 219056 | 370163 |
| Clean reads for miRNAs | 7686565 | 6746516 | 6772031 | 15351472 | 9139146 |
| Unique sRNAs for miRNAs | 5845 | 4705 | 5319 | 4116 | 4996 |
Notes:
CE01, CE02, CE03, and CE04 represent four unrelated cynomolgus macaques. Clean reads are total sRNA reads after filtering noise. Unique sRNAs represent numbers of sRNA types. Clean reads for miRNAs mean clean reads mapped to known miRNAs in miRBase.
| pre-miRNAs | Accession numbers | Sequences | Average expression level | |
|---|---|---|---|---|
| -5p | -3p | |||
| mir-10399 | LR745789 | AATTACAGATTGTCTCAGAGAAAACAGATGAGTTACTCTCTCAGACAAGCTGTAGGTC | 14.04 | |
| mir-1246 | LR594712 | TGCATCCTTG AATGGATTTTTGGAGCAGGAGTGGACACCTGACCCAAAGGAAATCAATCCATAGGCTAGCAAT | 0.96 | |
| mir-1248 | LR594713 | TTT ACCTTCTTGTATAAGCACTGTGCTAAAATTGCAGACACTAGGACTATGTCTTGGTTTTTGCAATAAT GCTAGCAGAGTACACACAAGAAGAAAAGTAACAGCA | 2.55 | |
| mir-124-3 | LR745790 | TGAGGGCCCCTCTGCGTGTTCACAGCGGACCTTGATTTAATGTCTATACAATTAAGGCACGCGGTGAATGCCA AGAGAGGCGCCTCC | 0.22 | |
| mir-1294 | LR594714 | CACTTAATATGTGCCAAGATCTGTTCATTTATGATCTCACTGAGTCC TGTGAGGTTGGCATTGTTGTCT GGCGTTGTCTGATATACAACAGTGCCAACTTCACAGGACTCAGTGAAGTGAAGCTGAGGATTAGGAAGGTGTG | 1.04 | |
| mir-1301 | LR594715 | CTGCCAAGCGACCCCTAGAATGGGGATTGTGGGGGGTCGCTCTAGGCACCGCAGCACTGTGCTGGGGATG TTGCAGCTGCCTGGGAGTGACTTC ACACAGTCCTCTCTGCCT | 30.95 | |
| mir-1307 | LR594716 | CATCAAGACCCAGCTGAGTCACTGTCACTGCCTACCAATC TCGACCGGACCTCGACCGGCT CGTCTGTGTTGCCAATCG ACTCGGCGTGGCGTCGGTCGTGGTAGATAGGCGGTCATGCATACGAATTTTCAGCTCTTGTTCTGGTGAC | 1.02 | 543.87 |
| mir-1327 | LR745791 | GGGGACTGCTCTT TAAAAGGCTGTTAAATGGTGAT ACTATATTTTTTAACATCAGCATCATTTAGAAACCTTTTAGAGAACAGTTCTC | 1.08 | |
| mir-138-1 | LR745792 | AGCTGGTGTTGTGAATCAGGCCG TTGCCAATCAGAGAACGGCTACTTCACAACACCAGGGCC | 2.20 | |
| mir-1468 | LR745793 | GGCGGGCGGTTT CTCCGTTTGCCTGTTTTGCTGA TGTACATTCAACTCATTCTCAGCAAAATAAGCAAATGGAAAATTTGTCCATC | 3.87 | |
| mir-151b | LR594718 | ACCTCTGATGTATCAATCTCTCTTCGGGGCTCCCGAGACACAGAAACAGACACCTGCCC TCGAGGAGCTCACAGTCT AGACAAACAAACCCAGGGT | 3.50 | |
| mir-1839 | LR745794 | GAAAAGGTAGATAGAACAGGTCTTGTTTGCAAAATAAACTCAAGGCCTACTTATCTACCAACAG | 249.11 | 1.85 |
| mir-1843 | LR745796 | TATGGAGGTCTCTGTCTGGCTTAGGACAGCTGGCTAAG TCTGATCGTTCCCCTCCATACA | 33.89 | 51.60 |
| mir-1976 | LR745798 | TGGCCTCTGGGCACGGGGGTTGGGTGTGCAAAGGGTGGCAGCAAGGAAGGCAGGGTTCCTGAGGTGTGTC CTCCTGCCCTCCTTGCTGTAGACTTTGGCCTGAGCAAGGAG | 1.60 | |
| mir-199b | LR594719 | CCAGAGGACACCTCCACTCCGTCTA CCCAGTGTTTAGACTATCTGTTC AGGACTCCCAAATTGTACAGTAGTCTGCACATTGGTTAGGCTGGGCTGGGTTAGACCCTCGG | 230.72 | |
| mir-200b | LR745799 | CCAGCTCGGGCAGCCGTGGC CATCTTACTGGGCAGCATTGGA TGGAGTCAGGTCTC TAATACTGCCTGGTAATGATGA CGGCGGGGCCCTGCACG | 0.79 | 13.71 |
| mir-219b | LR594722 | GGAGCTCAGCCAC AGATGTCCAGCCACAATTCTCG GTTGGCCGCAGACTCGTACAAGAATTGCGTTTGGACAATCAGTGGCGAAGCCC | 1.33 | |
| mir-2355 | LR594723 | ATCCCCAGATACAGTGGACAATATGCTATTATAATTGTATGGC ATTGTCCTTGCTGTTTGGAGAT AA | 10.11 | |
| mir-3074 | LR594725 | GGGCTCGACTCCT GTTCCTGCTGAACTGAGCCAG TGTGTAAAATGAGAACTGATATCAGCTCAGTAGGCACCGGAGGGCGGGTCC | 11.20 | |
| mir-3074-2 | LR745800 | CAGGCTCCAAGGGGGCTTGACTCCT GTTCCTGCTGAACTGAGCCAG TGTGCACAAACCAACTGTGTTTCAGCTCAGTAGGCACGGGAGGCAGAGCCCAGGGAGGCCA | 11.20 | |
| mir-30e | LR745801 | TTCTGGGCAGTCTTTGCTAC TGTAAACATCCTTGACTGGAAG CTGTAAGGTGTTCAGAGGAGC TTTCAGTCGGATGTTTACAGC GGCAGGCTGCCACGGTCGTCCCCAGCTAC | 3875.85 | 1023.80 |
| mir-3120 | LR594726 | GGCTGGGTTGTCATGTGACTG CCTGTCTGTGCCTGCTGTACA GGTGAGCGGATGTTCTG CACAGCAAGTGTAGACAGGCA GACACATGACAACTCTGTCCAGCC | 4.30 | 9.52 |
| mir-3155a | LR745803 | CACTTTTGAGACGCCTGTTCCGGGCATCA GCTCCCACTGCAGAGGCTGG GGAGCCGGACAGCTCCCTTCC CAAGCTCTGCAGTGGGAACTGA TGCCTGGAACAGTTCCTGCA | 0.44 | 7.83 |
| mir-3158 | LR594729 | ATTCAGGCTGGTCCTGCAGAGAGGAAGCCCTTCTGCTTCCAGGTATTGG AAGGGCTTCCTCTCTGCAGGAC CAGCCTGAAT | 14.74 | |
| mir-3158-2 | LR745804 | ATTCAGGCTGGTCCTGCAGAGAGGAAGCCCTTCCAATACCTGGAAGCAG AAGGGCTTCCTCTCTGCAGGAC CAGCCTGAAT | 14.74 | |
| mir-3160-1 | LR594730 | GGACCTACCCTGGGCTTTCTAGTCTCAGCTCTCCTCCAGCTCAACTGGTCAGG AGAGCTGAGACTAGAAAGCCCA GGGCAGGTTC | 0.72 | |
| mir-3160-2 | LR745805 | ACCTGCCCTGGGCTTTCTAGTCTCAGCTCTCCTGACCAGTTGAGCTGGAGG AGAGCTGAGACTAGAAAGCCCA GGGTAGGT | 0.72 | |
| mir-3164 | LR745806 | CTTGGAAAC TGTGACTTTAAGGGAAATGACG CACAGCAGGCCCTGGAATCACG CCGTTTTGCTTGAAGTTGCAGT TTCCCAGG | 0.97 | 0.56 |
| mir-3174 | LR745808 | CCAGCATCAGCATTACCTGG TAGTGAGTTAGAAATGCAGAGC CCCGGGCTTCTCAGCAAACCTACTGGATCTG CATTTTAATTCACATGCATGGTAATGTCTGTAAAGCACT | 3.28 | |
| mir-3191 | LR745809 | GGGGTCACCTGTCTGGCCGTCTACCTTCCACACTGACAAGGGCCG TGGGGACGTAGCTGGCCAGACAG GTGACCCC | 1.24 | |
| mir-320b-1 | LR745810 | AATTAATCCCTCTCTTTCTAGTTCTTCCTAGAGTGAGG AAAAGCTGGGTTGAGAGGGCAA ACAAATTAACTAATTAATT | 119.04 | |
| mir-320c-1 | LR745811 | AAAAATGAGGCCTTATCTTCCCAGTTCTTCCCAGAGTCAGG AAAAGCTGGGTTGAGAGGGT AGAAAAAAAAT | 41.88 | |
| mir-320d-1 | LR745812 | TTCTCTTCCCAGTTCTTCCCAAAGTTGAG AAAAGCTGGGTTGAGAGGA | 25.86 | |
| mir-320d-3 | LR745813 | TCTCTTCCTGGTTCTTCCCGAAGTCAGG AAAAGCTGGGTTGAGAGGA | 25.86 | |
| mir-320e | LR745814 | CTCCATGGGGCTTTCTCTTCCCAGTTCTTCCTGGAGTCGGGG AAAAGCTGGGTTGAGAGGGTGA ACAGAAAAA | 2.08 | |
| mir-326 | LR594733 | CTCATCTGTCTGTTGGGCTGGAGGCAGGGCCTTTGTGAAGGCG GGTGGTGCTCAGATCG CCTCTGGGCCCTTCCTCCAG CCCCGAGGCGGATTCA | 7.38 | |
| mir-3609 | LR594734 | GTAACATTAACTTTTATTCTCGTTTTCCTTTTCTCTACCTTGTAGAGAAG CAAAGTGATGAGTAATACTGGCTG GAGCCC | 1.38 | |
| mir-3613 | LR745815 | TGGTTGGGTTTGGAT TGTTGTACTTTTTTTTTTGTTC GTTGCATTTTTAGGAACAAAAAAAAAAGCCCAACCCTTCA CACCACTTCA | 115.70 | |
| mir-3620 | LR745816 | GTGAGGTGGGGGCCAGCAGGGA GTGGGCTGGGCTGGGCTGGGCC AAGGTACAAGGCCTCACCCTGCATCCCGCACCCAG | 0.81 | |
| mir-3691 | LR594736 | TTGAGGCACTGGGT AGTGGATGATGGAGACTCGGTAC CCACTGCAGAGGGTGGGGACCAAGTCTGCATCAT CCACTCCTCAGTGCCTCAG | 0.89 | |
| mir-371b | LR745817 | GGTAAC ACTCAAAACATGGCGGCACTT TTTTCACCAGAGAGCAGAAAGTGCCCCCACAGTTTGAGTGCC | 1.38 | |
| mir-378g | LR745818 | C ACTGGGCTTGGAGTCAGAAG ACCTGACTCCAGCCCAGGTC | 0.40 | |
| mir-3913-1 | LR745819 | TTGTTTATAATAAACTGAAATA TTTGGGACTGATCTTGACACTCT TACATAAAATGTTTTGGCAGACATCAA GATCAGTCCCAAATATTTCAGTTTATTATAGACAG | 8.91 | |
| mir-4485 | LR594738 | AGAGGCACCGCCTGCCCAGTGACACATGTT TAACGGCCGCGGTACCCTGA CTGTGCA | 0.38 | |
| mir-4659a | LR745820 | GAAACTGATGAAGCTGCCATGTCTAAGAAGAAAACTTTGGAGAAAAAT TTTCTTCTTAGACATGGCAGCG TCAACAGTTTC | 0.93 | |
| mir-4661 | LR745821 | TTTACTCTG AACTAGCTCTGCGGATCCTGAT AGACAGCCTGATAGACAGTATCCACAGAGCTAGTCCAGAGTAAA | 0.75 | |
| mir-4676 | LR594739 | TGAACAAAA GAGCCAGTGGTGAGACAGTGA GTTGATTACTTCTCACTGTTTCACCACTGGCTCTTTGGTTCA | 16.80 | |
| mir-4684 | LR594740 | GCACCAGGGGTAC CTCTCTACTGACTTGCAACATA CATTTGTATTGGTG TGTTGCAAGTCAGTGGAGAGGT ACCCTTGGTGT | 0.71 | 0.56 |
| mir-4791 | LR594741 | TAAGAAC TGGATATGAAGACTGAAA TAAGCTCCATATCAATGAGAATTTCAATGGGATTATGTATAGTCAAT GTCCAGTAATTA | 0.63 | |
| mir-486-1 | LR745823 | TCTCCATCCTCCCTGGGGCA TCCTGTACTGAGCTGCCCCGAG GCCCTTCATGCTGCCCAGCT CGGGGCAGCTCAGTACAGGAT ACCTCGGGGTGGGAGTCAGCAGGAGGTGA | 7013.65 | 332.08 |
| mir-4999 | LR745825 | ATAGAAAATAAAACACATACT GCCGTATTGTCAGGTAGTGATA GGATTTA TCACTACCTGACAATACAATAT GTGTTTGTTTTATTTTATGT | 1.41 | 0.26 |
| mir-548ad | LR594743 | CTATTAGGTTGGTGC AAAAGTAATTGTGGTTTTTG AAAGTAACTTGGCGAAAACCACAATGACTTT GCACCAACCTAATAC | 2.20 | |
| mir-548at | LR745827 | TAGGTTGGTG CAAAAGTTGTTGCGGTTTTGGC CGCCAAAAGAAATGGCCAAAACCGCAATAACTTTT GTACCAACCTAA | 1.34 | |
| mir-548av | LR594747 | AAAAGTACTTGTGGATTTGCCATTACCTTTACCTTTAATGGC AAAACTGCAGTTACTTTTGC | 0.36 | |
| mir-548ay | LR594748 | AGAAGATGCTTACTACTAGGTTGGTGC AAAAGTAATTGTGGTTTTTGC ATTTAAAGTAATGGCCAAAACCGCG ATTACTCTTGCACGAACCTAACGGTAACACTTCT | 3.98 | |
| mir-548l | LR745828 | TATTAGGTTGGTGCAAAAGTATTTGCGGGTTTTGTCATTGAAAGTAATGG CAAAAACTGCAATTACTTCTGC ACCAACCTAATGC | 1.08 | |
| mir-5690 | LR594755 | CTTTTAATT TCAGCTACTACCTCTATTAGG ATTTGGGAATTATACTAATAGAGGTAGTAGTTGAAATTAAGAG | 4.21 | |
| mir-610 | LR594756 | TCTATTTGTCTTAGG TGAGCTAAATGTGTGCTAGGA CACATTTGAGCCAAATGTCCCAGCACACATTTAGC TCACATAAGAAAAATGAACTCTAGT | 8.26 | |
| mir-641 | LR594757 | CAGGCTGGGTGAAAGGAAGG AAAGACATAGGATAGAGTCACCTC TGTCCTCTGTCCTCCACCTATAGAGGTGA CTATCCTATGTCTTTCCTTCCTCTCACCCCTGAGTCTCA | 1.06 | |
| mir-6501 | LR594758 | GG AGTTGCCAGGGCTGCCTTTGGT GACAGCAGCAGTAGAGTTGCCAGAGCAGCCTGCGGTAACAGTA | 1.74 | |
| mir-6503 | LR745829 | AATGGTCCCCCAGGG AGGTCTGCGTTCTAATCCCCA GAAGCTAAGGATTAGG GGGACAAGGATGCAGACCTCC CTGGGGGACCGTT | 7.58 | 18.52 |
| mir-6503-2 | LR745831 | AATGGTCCCCGAG GGAGGTCTGCATTCTAATC CCCAGAAGCGAAGGATTAGG GGGACAAGGATGCAGACCTCC CTGGGGGACCATT | 0.17 | 12.61 |
| mir-6516 | LR745833 | TGGGTTTTGAA TTTGCAGTAACAGGTGTGAGCA TTCTAGCAGCAGTTTGGTGATCATGTATGATACTGCAAACAGGACCTA | 0.32 | |
| mir-655 | LR594759 | TTCGTTTCAGAACTATTCAAGGATATTTGAGGAGAGGTTATCCGT GTTATGTTCGCTTCATTCATCATGA ATAATACATGGTTAACCTCTTT TTGAATATCAGACTCT | 2.35 | |
| mir-6731 | LR594760 | ACAGG TGGGAGAGCAGGGTATTGTGGA AGCTCCAGGTGCCAACTGCCTGCCTCTATCCCCCACTCTCCCCAG | 1.20 | |
| mir-6735 | LR745834 | GCAGCC AGGGCAGAGAGCACAGGAATCTGA GGTGACTGGCACAGAAGACTC AGGCCTGTGGCTCCTCCCCCAG | 1.14 | 0.09 |
| mir-6813 | LR594761 | ACAGG CAGGGGCTGGGGTTTCAGGTTCT CAGTCAGAACCTTGGCCCCTCTCCCCAG | 0.69 | |
| mir-6816 | LR745836 | CCGAGTGGGGCGGGGTGGGTCCCTGCAGGGACTGTGACACT GAAGGACCTGCACCTCCGCCCACA G | 0.82 | |
| mir-6866 | LR745837 | CCATT TTAGAGGCTGGAATAGGGATT ATTGAGTCTGGAAGAGTAAGGATCCCTTTATCTGTCCTCTAG | 0.69 | |
| mir-7155 | LR594762 | TCTGGGGTCTTGGGCCATCTGGTTGTGACAGCCCCGA TGGCCCAAGACCTCAGACC | 0.68 | |
| mir-744 | LR594763 | TTGGGCAAGG TGCGGGGCTAGGGCTAACAGCA GTCTTACTGAAGGTTTCCTGGAAACCACGCACATG CTGTTGCCACTAACCTCAACCT TACTCGGTC | 1140.67 | 1.71 |
| mir-7848 | LR594765 | GCTGGAGCTGGGTGGGTGTGGCAGGCCCACCGTGGGTATGC AAAGCTCTGACAATGTTTTACTTG CTACCCTCGGTCTGCTTACCACA CTCCCAGTTCCAC | 18.23 | |
| mir-935 | LR745838 | GGCGGGGGCGCGGGCGGCAGTGGCGGGAGCGGCCCCTC GGCCATCCTCCGTCTGC CCAGTTACCGCTTCCGCTACCGC CGCCGCTCCCGCT | 1.44 | |
Notes:
Average expression levels for each mature miRNA were normalized by transcripts per million. Single or double underlines indicate mature miRNAs expressed from -5 p or -3 p arms of pre-miRNAs, respectively.
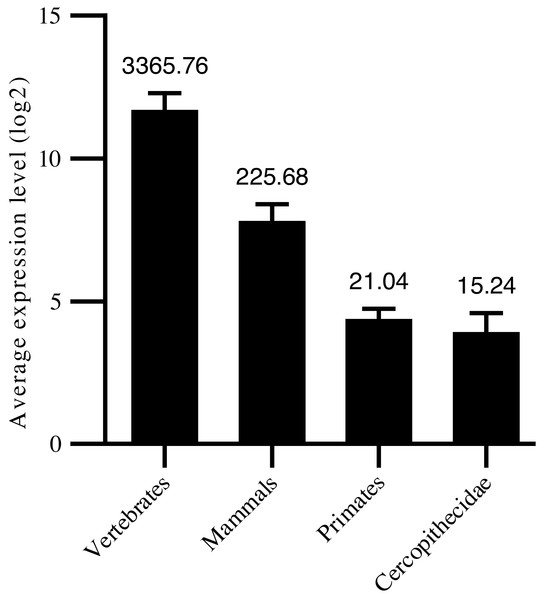
Thus, in the current study, we confirmed 1,455 high-confidence pre-miRNA sequences by homology screening and RNA sequencing. According to their sequence similarities (Dostie et al., 2003; Yue, Sheng & Orwig, 2008; Bartel, 2009), 915 were grouped into 491 families (Table S1). Another 540 were not clustered into currently known families, suggesting new targeting properties. To assess the age of the miRNAs, their precursor sequences were searched in miRBase. The age of miRNAs was based on the group of species in which miRNA homologous sequences were identified. We classified the 1,455 miRNAs into four groups: i.e., 267 conserved in vertebrates, 332 conserved in mammals, 806 primate-specific, and 50 Cercopithecidae-specific. Among the 408 high-confidence pre-miRNA sequences, 179 were conserved in vertebrates, 140 were conserved in mammals, 77 were conserved in primates, and 12 were conserved in Cercopithecidae. We then examined the relationship between average expression level of miRNAs and their age and found a positive correlation (Spearman R: 0.27 to 0.42, P < 10−4; Fig. 1). Primate-specific miRNAs demonstrated lower average expression levels (10-fold and 160-fold, respectively) than ancient miRNAs in mammals and vertebrates. In addition, Cercopithecidae-specific miRNAs showed the lowest expression level among the four groups.
Figure 1: Age of mature miRNAs relative to expression levels.
Primate-specific miRNAs showed approximately 10-fold and 160-fold lower average expression levels than ancient miRNAs from mammals and vertebrates, respectively.Characteristics of miRNA gene locations in cynomolgus macaques
According to the annotations of the 1,455 high-confidence pre-miRNA sequences (Table S1), the miRNA encoding genes in the cynomolgus macaque genome were also distributed in diverse locations, similar to that found in other mammals (Kim & Kim, 2007; Morin et al., 2008). Of the 1,455 pre-miRNAs, approximately 46% were located in intergenic regions, 49% were located in introns and 5% were located in RNA coding regions. It is worth noting that 64 pre-miRNA sequences were processed from exons of protein-coding transcripts or non-coding transcripts, including 46 derived from untranslated regions. Six pre-miRNA sequences were found overlapping intron-exon junctions, i.e., SO-miRNAs. Most miRNA gene is encoded by one strand of one locus as above, but in rare cases, two distinct pre-miRNAs are found transcribed and processed on both strands of one locus (Tyler et al., 2008; Picao-Osorio et al., 2015; Mohammed et al., 2018). Two pairs of these so-called antisense or mirror miRNAs were identified in our study, i.e., miR-3120/miR-214 and miR-24-1/miR-3074 (Table S1). Here, miR-3120 was located within an intron of the dynamin-3 gene and miR-214 was embedded on the antisense strand of the same locus. For the miR-24-1/miR-3074 pair, we found that miR-24-1 was embedded within an intron of the AOPEP gene and miR-3074 was processed from the antisense strand. The miR-24-1/miR-3074 mirror miRNAs were first reported in this study in cynomolgus macaques (Cordero et al., 2015).
MiRNA gene clusters in cynomolgus macaques
Previous studies have revealed that miRNAs are significantly enriched in clusters in introns or intergenic regions (Marco et al., 2013; Wang et al., 2016; Altuvia et al., 2005). To profile the clustering patterns of miRNAs in cynomolgus macaques, we grouped pre-miRNAs into distinct clusters following previous study (Marco et al., 2013). If two neighboring miRNA loci were located within 10 kb and on the same strand, they were considered to be clustered miRNA genes. Among the 1,455 pre-miRNAs, 285 miRNA genes were grouped into 78 distinct clusters. When comparing the 78 miRNA clusters to their human counterparts, if a human miRNA was not represented in the macaque clusters, it was screened cross the macaque genome in order to validate the existence of an ortholog in macaques (Wang et al., 2016). As such, 62 miRNA clusters were found to be similar to that in the human genome and six miRNA clusters showed minor changes (Table 3), indicating that most of the clustered miRNAs were evolutionarily conserved. Ten of the 78 clusters were newly emerged in the cynomolgus macaques. According to the similarity of seed regions of the miRNA sequences in each cluster, we grouped the 78 clusters into two sub-classes: 21 homo-seed clusters (miRNAs having identical sequences in the seed region) and 57 hetero-seed clusters (miRNAs having distinct sequences in the seed region). These results are consistent with previous studies suggesting that the composition of miRNA clusters is heterogeneous with a diversiform evolutionary mechanism (Wang et al., 2016). Homo-seed clusters are usually products of local duplications, and hetero-seed clusters are mainly shaped by a combination of de novo formations and local duplications (Wang et al., 2016).
| Cluster in Mafa | miRNA number in Mafa | miRNAs in Mafa | Type | Detected by RNA-seq | Cluster in humans | miRNA number in humans | miRNAs in humans |
|---|---|---|---|---|---|---|---|
| chr1:128814989-128815918+ | 2 | 137/2682 | hetero | no | chr1:98045270-98046113- | 2 | 2682/137 |
| chr1:186962061-186965108- | 2 | 30c-1/30e | homo | yes | chr1:40754370-40757360+ | 2 | 30e/30c-1 |
| chr1:227255265-227256419- | 2 | 429/200aa | homo | yes | chr1:1167123-1169076+ | 3 | 200b/200a/429 |
| chr1:30111736-30117576- | 2 | 214/199a-2 | hetero | yes | chr1:172138815-172144614- | 2 | 214/199a |
| chr1:57185008-57185289- | 2 | 181b-1/181a-1 | homo | yes | chr1:198858886-198859130- | 2 | 181b/181a |
| chr1:66367833-66368516- | 2 | 29c/29b-1 | homo | yes | chr1:207801864-207802513- | 2 | 29c/29b-2 |
| chr1:78854733-78855125- | 2 | 215/194-1 | hetero | yes | chr1:220117876-220118227- | 2 | 215/194 |
| chr10:30075091-30075493- | 2 | 130b/301b | homo | yes | chr22:21652989-21653375+ | 2 | 301b/130b |
| chr10:31357983-31358388- | 2 | 1306/3618 | hetero | yes | chr22:20085796-20086129+ | 2 | 3618/1306 |
| chr10:90709107-90709791- | 2 | 296/298 | hetero | yes | chr20:58817625-58818303- | 2 | 296/298 |
| chr11:112931512-112932150- | 2 | 7705/10527b | hetero | no | not detected | ||
| chr11:56821914-56822517- | 2 | 26a-2/9947b | hetero | yes | not detected | ||
| chr11:7274754-7275246+ | 2 | 200c/141 | homo | yes | chr12:6963702-6964176+ | 2 | 200c/141 |
| chr12:65115697-65116202+ | 2 | 7164/10bb | hetero | yes | not detected | ||
| chr13:54048315-54054337+ | 2 | 216a/217 | hetero | no | chr2:55983019-55989041- | 2 | 217/216a |
| chr14:107474425-107475005+ | 2 | 34b/34c | homo | no | chr11:111512949-111513505+ | 2 | 34b/34c |
| chr14:6660857-6661975- | 2 | 4691/7113 | hetero | no | chr11:68032864-68033971+ | 2 | 7113/4691 |
| chr15:108224172-108226742- | 2 | 3596d/3596bb | homo | no | not detected | ||
| chr15:10960721-10961048+ | 2 | 3154/199b | hetero | yes | chr9:128244744-128244977- | 2 | 199b/3154 |
| chr15:11398154-11399489- | 2 | 181b-2/181a-2 | homo | yes | chr1:198858886-198859130- | 2 | 181b/181a |
| chr16:18135796-18136249- | 2 | 6777/33b | hetero | no | chr17:17813479-17813916- | 2 | 6777/33b |
| chr16:1954993-1955463- | 2 | 132/212 | homo | yes | chr17:2049928-2050350- | 2 | 132/212 |
| chr16:33365362-33370038+ | 2 | 10226/148cb | hetero | no | not detected | ||
| chr16:43850674-43855488- | 2 | 142/4736 | hetero | yes | chr17:58331244-58336022- | 2 | 142/4736 |
| chr16:61839928-61840468- | 2 | 3064/5047 | hetero | yes | chr17:64500774-64501250- | 2 | 3064/5047 |
| chr16:7046633-7047042- | 2 | 195/497 | hetero | yes | chr17:7017627-7017999- | 2 | 195/497 |
| chr17:29263944-29264174- | 2 | 16-1/15a | homo | yes | chr3:160404606-160404818+ | 2 | 15b/16-2 |
| chr18:60473826-60477188+ | 2 | 1-2/133a-1 | hetero | no | chr18:21825711-21829036- | 2 | 133a/1 |
| chr19:14206540-14206853+ | 2 | 181c/181d | homo | yes | chr1:198858886-198859130- | 2 | 181b/181a |
| chr19:14522034-14522404+ | 2 | 8994/9307b | hetero | no | not detected | ||
| chr19:2102695-2104529- | 2 | 1227/6789 | hetero | no | chr19:2234061-2235922- | 2 | 1227/6789 |
| chr2:104189414-104189980- | 2 | 425/191 | hetero | yes | chr3:49020158-49020694- | 2 | 425/191 |
| chr2:62802629-62802867+ | 2 | 15b/16-2 | homo | yes | chr3:160404606-160404818+ | 2 | 15b/16-2 |
| chr20:14704691-14710146+ | 2 | 193b/365a | hetero | yes | chr16:14303979-14309361+ | 2 | 193b/365a |
| chr20:44876971-44882123+ | 2 | 138/7181b | hetero | yes | not detected | ||
| chr3:118484198-118485386+ | 2 | 489/653 | hetero | no | chr7:93482783-93484006- | 2 | 653/489 |
| chr3:13210687-13210931- | 2 | 6501/9922b | hetero | yes | not detected | ||
| chr3:163632511-163633319- | 2 | 29a/29b-2 | homo | yes | chr1:207801864-207802513- | 2 | 29c/29b-2 |
| chr3:30725019-30725854- | 2 | let-7c/99 | hetero | yes | chr21:16539100-16539904+ | 2 | 99a/let-7c |
| chr4:118507476-118512141- | 2 | 133b/206 | hetero | yes | chr6:52144400-52149009+ | 2 | 206/133b |
| chr6:148755825-148757619+ | 2 | 143/145 | hetero | yes | chr5:149428943-149430720+ | 2 | 143/145 |
| chr6:54606090-54606301- | 2 | 449a/449ba | homo | yes | chr5:55170585-55172337- | 3 | 449a/449b/ 449c |
| chr7:68810880-68814721+ | 2 | 1179∕7 − 2 | hetero | no | chr15:88608120-88611917+ | 2 | 1179∕7 − 2 |
| chr8:100682570-100682796- | 2 | 599/875 | hetero | yes | chr8:99536650-99536851- | 2 | 599/875 |
| chr8:136192174-136197663- | 2 | 30b/30d | homo | yes | chr8:134800531-134804940- | 2 | 30b/30d |
| chrun_ke145894:2386-3511- | 2 | 10396a/663-10b | hetero | no | not detected | ||
| chrx:111594304-111595504+ | 2 | 1912/1264 | hetero | no | chrx:114651588-114652718+ | 2 | 1912/1264 |
| chrx:144101311-144108532- | 2 | 513b-1/513b-2 | homo | no | chrx:147189716-147199114- | 2 | 513c/513b |
| chrx:148922686-148923813- | 2 | 224/452 | hetero | no | chrx:151958583-151959699- | 2 | 224/452 |
| chrx:44130132-44131103- | 2 | 221/222 | homo | yes | chrx:45746179-45747094- | 2 | 221/222 |
| chrx:52628444-52629475- | 2 | 98/let-7f-2 | homo | yes | chrx:53556240-53557267- | 2 | 98/let-7f-2 |
| chrx:71471342-71471593- | 2 | 421/374b | hetero | yes | chrx:74218391-74218608- | 2 | 421/374b |
| chrx:71540122-71540378- | 2 | 545/374a | hetero | yes | chrx:74287125-74287346- | 2 | 545/374a |
| chrx:71841808-71842408- | 2 | 7705/10527b | hetero | no | not detected | ||
| chr10:4843233-4844250- | 3 | let-7b/4763/let-7a | homo | yes | chr22:46112751-46113766+ | 3 | let-7a/4763/let-7b |
| chr14:118172242-118178007- | 3 | let-7a-2/ 10526 /100a | hetero | yes | chr11:122146522-122152296- | 2 | let-7a-2/100 |
| chr15:108223782-108226749+ | 3 | let-7a-2/let-7f/let-7d | homo | yes | chr9:94175961-94178916+ | 3 | let-7a/let-7f-1/let-7d |
| chr15:109228189-109229083+ | 3 | 23b/27b/24-2 | hetero | yes | chr9:95085226-95086085+ | 3 | 23b/27b/24-1 |
| chr16:25123023-25123392- | 3 | 451/144/4732 | hetero | yes | chr17:28861376-28861722- | 3 | 451a/144/4732 |
| chr16:78700471-78708445- | 3 | 657/338/1250 | hetero | yes | chr17:81125290-81133285- | 3 | 657/338/1250 |
| chr19:14166279-14166650- | 3 | 24-2/27a/23a | hetero | yes | chr9:95085226-95086085+ | 3 | 23b/27b/24-1 |
| chr19:52420893-52421627+ | 3 | 99b/let-7e/125a | hetero | yes | chr19:51692617-51693327+ | 3 | 99b/let-7e/125a |
| chr19:54627979-54628968+ | 3 | 371/372/373 | hetero | yes | chr19:53787677-53788770+ | 3 | 371a/372/373 |
| chr3:162480895-162485756- | 3 | 182/96/183 | hetero | yes | chr7:129770405-129774988- | 3 | 182/96/183 |
| chr3:44449530-44450041- | 3 | 25/93/106 | hetero | yes | chr7:100093570-100094063- | 3 | 25/93/106b |
| chr8:146049004-146055940- | 3 | 939/1234/ 6849 | hetero | yes | chr8:144394160-144400340- | 3 | 939/1234/6849 |
| chrx:149364083-149366356- | 3 | 105-1/767/ 105-2 | hetero | no | chrx:152392227-152394480- | 3 | 105−1/105−2/767 |
| chr14:9660229-9660537+ | 4 | 6750/6749/ 194-2/192 | hetero | yes | chr11:64891158-64902450- | 4 | 192/194−2/6750/ 6749 |
| chr5:111988697-111989381- | 5 | 367/302d/ 302a/302c/ 302b | hetero | no | chr4:112647876-112648547- | 5 | 367/302d/ 302a/302c/ 302b |
| chr17:74024859-74025648+ | 6 | 17/18a/19a/ 20a/19b-1/92a-1 | hetero | yes | chr13:91350617-91351382+ | 6 | 17/18a/19a/ 20a/19b-1/92a-1 |
| chrx:131080800-131081707- | 6 | 363-2/92a-1/ 19b-2/20b/ 18b/106a | hetero | yes | chrx:134169381-134170266- | 6 | 363/92a-2/ 19b-2/20b/ 18b/106a |
| chrx:131455304-131461841- | 6 | 450b/450a-1/ 450a-2/542/ 503/424 | hetero | yes | chrx:134540193-134546701- | 6 | 450b/450a-1/ 450a-2/542/ 503/424 |
| chrx:142887921-142895601- | 6 | 892c/890/ 888/ 892a/ 892b/891b | hetero | no | chrx:145992759-146001121- | 6 | 892c/890/ 888/892a/ 892b/891b |
| chrx:48324406-48334989+ | 7 | 532/188/ 500a/362/ 501/660/502 | hetero | yes | chrx:50003166-50014670+ | 8 | 532/188/ 500a/362/ 501/500b/ 660/502 |
| chr7:166087997-166103699+ | 8 | 493/337/665/ 431/433/127/ 432/136 | hetero | yes | chr14:100869074-100884771+ | 8 | 493/337/665/431/ 433/127/432/136 |
| chr7:166241788-166285152+ | 42 | 379/411/299/ 380/1197/ 323a/758/ 329-1/329-2/ 494/1193/543 /495/ 376a-3/ 376c/376a-2/ 654/376b /376a/1185-2/ 1185-1/381/487b/ 539/889/544a/ 655/487a/382/ 134/668/485 /323b/154/ 496/377/541/ 409/412/ 369/410/656a | hetero | yes | chr14:101022070-101066786+ | 42 | 379/411/ 299/380/ 1197/323a/ 758/329-1/ 329-2/494/ 1193/543/495/ 376c/376a-2/ 654/376b/ 376a-1/ 300/ 1185-1/1185-2/ 381/487b/539/889/ 544a/655/487a/ 382/134/668/485/323b/ 154/496/377/541/409/ 412/369/410/656 |
| chrx:144120330-144177313- | 11 | 513a-1/513a-2/ 513a-3/506/ 507/508/ 514b/509-1/ 509-2/510/514aa | hetero | no | chrX:147225859-147284712- | 11 | 513a-2/506/507/508/514b/ 509-2/509-3/509-1/510/514a-1/ 514a-2 |
| chr19:54493931-54598758+ | 40 | 512/512-2 /1323/498 /515-2/519e/ 519e/519e /519c/1283/ 520ak/526b/519a-2 /525/523a/518f/ 519a/518b /526a-1/518c/524/517a/ 519d/518a-1/520g/ 518d-1/ 518g /518d-2/ 523b /516b-1/518a-3/ 517c/520g/519b/ 521/518e/ 518a-4/516a-2/ 516a-1/517ba | hetero | no | chr19:53666691-53762418+ | 46 | 512-1/512-2/1323/498/ 520e /515-1/519e/ 520f/515-2/519c/1283-1/520a/ 526b/519b/525/ 523/518f/ 520b /518b/526a-1/ 520c/518c/524/517a/519d/521-2/ 520d/517b/520g/516b-2/ 526a-2/518e/518a-1/518d/ 516b-1/518a-2/517c/ 520h /521-1/ 522/519a-1/ 527/516a-1/1283-2/ 516a-2/519a-2 |
Notes:
- hetero
-
hetero-seed, miRNAs with distinct sequences in seed region
- homo
-
homo-seed, miRNAs with identical sequences in seed region
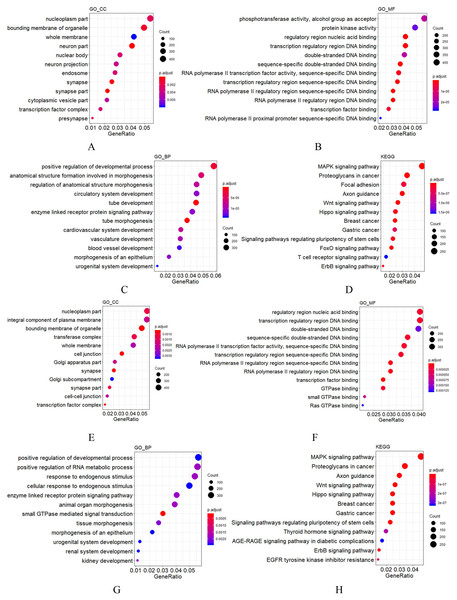
Figure 2: GO and KEGG analysis of mir-379/mir-656 (A–D) and mir-512/mir-517 (E–H) gene clusters.
Vertical axis: GO terms and KEGG pathway names. CC, MF and BP represent cellular component, molecular function and biological process, respectively. The larger the point, the higher the degree of enrichment. The greater the number of candidate target genes in this GO term and KEGG pathway, the color of the points corresponds to different p-adjust ranges.For most miRNA clusters revealed in this study, the number of pre-miRNAs ranged from two to three. Nonetheless, 10 large clusters with no less than five miRNA precursors were also observed (Table 3), which were composed of members from different miRNA families but with the same expression state. Among the 10 large clusters, most miRNAs in the six of them were detected by RNA-seq in this study, but none was detected by RNA-seq in the rest of the four clusters (Table 3). These results indicate that each of the 10 clusters was transcribed as a polycistronic element (Ryazansky, Gvozdev & Berezikov, 2011; Saini, Griffiths-Jones & Enright, 2007). Comparing the compositions of the 10 miRNA-clusters in cynomolgus macaques to their orthologs in humans, eight showed identical composition, whereas the mir-379/mir-656 and mir-512/mir-517 clusters showed minor changes. The mir-379/mir-656 cluster in macaques was positioned on chromosome 7 and contained 42 miRNA genes. Compared to humans, 40 out of the 42 miRNA genes were the same as humans. Simultaneously, a mir-376a copy was added and a mir-300 ortholog was lost. In the macaques, the mir-512/mir-517 cluster was located on chromosome 19 and contained 40 miRNA genes. Most shared common seed regions of the miR-515 family and likely originated from a common ancestor (Rodriguez et al., 2004; Zhang, Wang & Su, 2008). Comparing the miRNA composition of the mir-512/mir-517 cluster to the C19MC in humans, 38 of the 40 miRNA genes had orthologs in humans, two (mir-518g and mir-523b) were unique to macaques, and eight, including mir-522, mir-527, and six mir-520 variants, were not found in the macaque genome. These data show that although most C19MC members are conserved throughout the primate kingdom, frequent miRNA gains and losses, such as the gain of mir-518 and mir-523 copies and loss of mir-520 copies in macaques, have occurred (Berezikov et al., 2006; Iwama et al., 2013; Awan et al., 2017). These findings are consistent with previous results suggesting that the C19MC region is under rapid evolution (Iwama et al., 2013; Bentwich et al., 2005). Specifically, this region is enriched by dispersed Alu elements, which are believed to have facilitated the expansion of C19MC (Zhang, Wang & Su, 2008; Lehnert et al., 2009). To understand the functions of miRNA clusters, the potential targets of the two very large miRNA clusters were predicted using TargetScan, followed by GO functional annotation. Among the target genes of the mir-379/mir-656 cluster, those relevant to cellular components (CC) were enriched in synapse, membrane, and cell projection; those relevant to molecular function (MF) were mainly clustered in binding and catalytic activity; those relevant to biological process (BP) were mainly enriched in cellular process, biological regulation, metabolic process, development process, and response to stimulus (Fig. 2). For the mir-512/mir-517 cluster, the functional annotations of their target genes were similar to those of the mir-379/mir-656 cluster. KEGG analysis of the two very large miRNA clusters suggested that both regulate normal growth, development, and pathological processes through many pathways, such as MAPK signaling and Axon guidance (Fig. 2), consistent with previous studies on humans (Haller et al., 2010; Lavon et al., 2010; Cortez & Calin, 2009; Grün et al., 2005; Donker et al., 2012; Noguer-Dance et al., 2010; Tsai et al., 2009; Li et al., 2009a; Kleinman et al., 2014; Ward et al., 2014). The other eight large miRNA clusters in macaques are with conserved compositions to that in humans, indicating that their functions are also evolutionarily conserved.
Discussion
The majority of reported cynomolgus macaque miRNAs are predicted based on homology to the rhesus genome (Veeranagouda et al., 2015). In this study, we firstly identified conserved miRNAs in cynomolgus macaques based on homology to all mammalian pre-miRNA sequences deposited in miRBase. To prevent mis-annotation of homologous sequences as miRNAs, we proposed a more stringent analysis process than previously reported (Veeranagouda et al., 2015). First, it requires that the full-length sequence similarity of homologous sequences exceed 93%. Secondly, it requires the homologous sequences to form a stable hairpin-like secondary structure with the mature sequences present in the stem. Next it also requires another supporting information: experimental supports for homolog in other species, or annotated as miRNA in other species, or reported in macaques previously. Using these criteria, 1,455 of the 1,697 homologous sequences were considered pre-miRNA gene loci of high confidence, more than half of which were firstly annotated in cynomolgus macaque genome (Yang et al., 2014; Veeranagouda et al., 2015). The data indicated that homology searching is a useful tool for high-throughput identification of conserved miRNAs (McCreight et al., 2017). Using these criteria, the false-positive annotations were reduced, but also some real pre-miRNA sequences were missed, such as poorly conserved miRNAs, or miRNAs that were not expressed at sufficient levels in the sequenced samples, or miRNAs that did not form acceptable hairpins. Thus, we just predicted a subset of conserved miRNAs in cynomolgus macaque. Using small RNA sequencing, 408 of the 1,455 genes were detected in peripheral blood leucocytes, which aided the confirmation of -5 p and -3 p mature miRNA sequences. The remaining 1,047 not supported with reads may be expressed in other tissues or under other circumstances, because large number of miRNAs exhibit noticeable spatiotemporal and tissue-specific expression. In addition, environmental stress or physiological disorders will significantly alter miRNA expression profiles (Pinhal et al., 2018). Therefore, we just experimentally verified a subset of miRNAs and further experimental confirmations of many miRNAs are needed in cynomolgus macaques.
When contrasting expression levels of miRNAs with their age, our results were in good agreement with previous reports that a positive correlation was found between sequence conservation and expression levels during miRNA evolution (Dannemann et al., 2012b). Newly emerged miRNAs are often expressed at lower levels compared with broadly conserved miRNAs (Wang et al., 2016), which suggest that miRNAs with low levels of conservation and expression may still be under evolutionary selection. Furthermore, the number and expression of miRNAs increased simultaneously during evolution, consistent with that observed in previous studies (Meunier et al., 2013; Berezikov et al., 2011; Lee, Risom & Strauss, 2007). The miRNA expansion may correlate with organismal complexity and body-plan innovation (Meunier et al., 2013; Christodoulou et al., 2010; Mohammed et al., 2018; Grimson et al., 2008).
We also systematically investigated the miRNA gene locations in the cynomolgus macaque genome. Unlike protein-coding genes, the locations of miRNA genes were highly polytropic. Most miRNAs resided in intergenic spaces or within introns of the hosts. Very few of miRNAs were found embedded in exons, overlapping-splice-sites, or antisense regions. Such unusual genomic arrangements of the miRNAs intensively raise our attention to explore their possible regulatory functions. For example, the MIR1306 gene overlaps the DGCR8 coding region and the MIR675 gene is embedded in the first exon of H19 lncRNA, both of which are conserved in primates (Morin et al., 2008; Friedländer et al., 2008; Keniry et al., 2012; Wang et al., 2018). It has been reported that the mir-1306 hairpin is cleaved by the Drosha-DGCR8 complex, which plays an important negative-feedback role in controlling DGCR8 expression (Morin et al., 2008; Friedländer et al., 2008; Keniry et al., 2012; Wang et al., 2018). H19 is an important imprinted gene involved in placental development prior to birth, which limits the growth of the placenta by regulating miR-675 processes (Keniry et al., 2012; Wang et al., 2018; Han et al., 2009). Thus, it can be inferred that there is an expressive and functional interrelationship between miRNAs and host genes. For example, host genes can act as miRNA reservoirs and miRNA can negatively regulate its host. Among the exon-resident miRNAs identified in this study, it would be interesting to investigate whether such exonic miRNA hairpins serve dual roles as miRNA precursors as well as RNA instability elements as miRNA hairpin processing could destabilize host transcripts, as mentioned above. We also found some pre-miRNAs overlapping intron-exon junctions. These SO-miRNAs may be involved in regulating gene expression in cynomolgus macaques, whereby microprocessor complex-dependent cleavage of SO-miRNA exons could result in premature transcriptional termination of coding genes, as described in humans (Pianigiani et al., 2018). Two pairs of distinct antisense miRNAs were found in this study. The sequences and locations of miR-3120/miR-214 pair were conserved in mammals. Using miRNA target prediction software, we found that miR-3120-3p was predicted to interact with the 3′ UTR of heat shock protein family A member 5 (HSPA5) and miR-214-3p was predicted to target the 3′ UTR of phosphatase and tensin homolog (PTEN), consistent with previous research in humans (Scott et al., 2012). HSPA5 is a chaperone for protein folding in the endoplasmic reticulum and PTEN interacts with the mir-3120 host gene dynamin-3 to regulate synaptic proteins involved in receptor cycling and synaptic plasticity (Yang et al., 2008). The data suggest that the miR-3120/miR-214 pair represent a genetic unit in cynomolgus macaques that evolved to regulate the complex neuronal pathways associated with synaptic vesicle function and neuronal plasticity (Scott et al., 2012). For the miR-24-1/miR-3074 antisense miRNAs, although they have different seed sequences, KEGG analysis indicated that the targets of miR-24-3p and miR-3074-5p contribute to similar biological pathways, including Axon guidance and EGFR tyrosine kinase inhibitor resistance (Table S4). The miR-24-1/miR-3074 antisense miRNAs may regulate essential aspects of cellular function in coordination (Cordero et al., 2015). These overlapping regulatory functions indicate that direct or indirect coordinated regulation exist between these antisense miRNA pairs. Future research may identify other miRNAs resident in unusual genomic regions, such as exon-resident miRNAs, SO-miRNAs, and antisense miRNAs, which work in coordination with their host genes to regulate diverse and complex cellular functions.
Another characterization of miRNA genomic locations is that many are clustered together. In total, 78 miRNA clusters were found in this study, 68 of which were conserved in the human genome, including 10 large clusters with no less than five miRNA precursors. It was reported that these 10 large miRNA clusters have significant functions in humans. For example, miRNAs in the mir-17/92 cluster are potential human oncogenes (He et al., 2005) and critical regulators of normal development and disease, such as body height and cardiomyocyte proliferation (Chen et al., 2013; Bai et al., 2019). The X-linked mir-506/514 cluster has regulatory roles in testis development and spermatogenesis (Zhang et al., 2007). Furthermore, the very large mir-379/mir-656 and mir-512/mir-517 clusters both participate in development. The former, known as the chromosome 14 miRNA cluster (C14MC), is comprised of 42 miRNA genes in humans and is located at the DLK-DIO3 imprinted domain (Seitz et al., 2004; Morales-Prieto et al., 2013; Schmidt, Block & Golos, 2018; Sadovsky et al., 2015; Cavaillé et al., 2002; Glazov et al., 2008). The latter, known as the chromosome 19 miRNA cluster (C19MC), is comprised of 46 miRNA genes in humans and is imprinted in the placenta (Noguer-Dance et al., 2010). C14MC is reported to not only play important roles in the control of neurogenesis, embryonic development, and pregnancy, but is also associated with progression of hematopoietic and solid tumors (Haller et al., 2010; Lavon et al., 2010; Cortez & Calin, 2009; Grün et al., 2005; Donker et al., 2012; Morales-Prieto et al., 2013). In addition, variations in the expression of miRNAs in C19MC are associated with embryogenesis and tumorigenesis, suggesting a critical role of these miRNAs in development and cancer (Noguer-Dance et al., 2010; Tsai et al., 2009; Li et al., 2009a; Kleinman et al., 2014; Ward et al., 2014). Eight of the 10 miRNA-clusters in macaques are with conserved compositions to that in humans, indicating that their functions are evolutionarily conserved. The other two miRNA-clusters, the mir-379/mir-656 and mir-512/mir-517, showed minor changes to that in humans and then were used for functional prediction. The results indicated that both of the large miRNA-clusters regulate normal growth, development, and pathological processes through many pathways, consistent with previous studies on humans. Therefore, the miRNA clusters are important as they are involved in vital biological functions, including physiological and pathological processes, by targeting overlapping sets of genes cooperatively (Marco et al., 2013; Wang et al., 2016; Kim & Nam, 2006).
Conclusions
In summary, 1,455 high-confidence miRNA gene loci were identified in the genome of cynomolgus macaques, 408 of which were also confirmed by RNA-seq. Due to the stringent annotation criteria and limited sequenced samples, we just obtained a subset of miRNAs in macaque. The miRNA genes in cynomolgus macaque were distributed in diverse locations. Very few of miRNAs, such as exon-resident miRNAs, SO-miRNAs, and antisense miRNAs, were predicated to regulate diverse functions in coordination with their host genes. Simultaneously, miRNAs processed from the large clusters were predicted to have essential cellular and developmental functions. This study not only expands the number of identified miRNAs in cynomolgus macaques but also provides clues for future research to improve our understanding of the evolution and function of miRNAs in primate. In the future, a great number of macaque miRNAs need to be verified by experiments. Further investigations on the functional role of primate-specific miRNAs are also needed because evolutionarily young miRNAs may correlate with organismal complexity and body-plan innovation and have the potential for development as novel biomarkers or therapeutic targets in complex diseases, especially in neuropsychiatric disorders.
Supplemental Information
M. fascicularis pre-miRNA gene loci identified by homology searching
°: underlined names indicate antisense miRNAs; MFE: minimal folding free energy; Category of age: The ages of the miRNAs were assessed based on the set of homologous sequences identified in other vertebrate species in miRBase. Vertebrates, Mammals, Primates and Cercopithecidae mean the miRNA homologous sequences were shared among Vertebrates, Mammals, Primates and Cercopithecidae, respectively. † : a: reported in cynomolgus macaques previously; b: annotated as miRNAs in other species; c: miRNA homologies in other species having experimental support.
136 potentially novel mature miRNAs
Average expression levels for each mature miRNA were normalized by transcripts per million; MFE: minimal folding free energy.
383 M. fascicularis pre-miRNAs identified by small RNA sequencing
°: single underlines means that the sRNA read perfectly matched the mature miRNA sequence deposied in miRBase, while the relavent pre-miRNA sequence did not found in cynomolgus macaque genome; Average expression levels for each mature miRNA were normalized by transcripts per million.
KEGG analysis of mirror miRNA pair, miR-24-1/miR-3074 in cynomolgus macaque
°: The same pathways that targets of miR-24-1 and miR-3074 contributed to are marked by underscore.