Exploring profile and potential influencers of vaginal microbiome among asymptomatic pregnant Chinese women
- Published
- Accepted
- Received
- Academic Editor
- Elliot Lefkowitz
- Subject Areas
- Microbiology, Epidemiology, Gynecology and Obstetrics, Public Health, Women’s Health
- Keywords
- Vaginal microbiome, Pregnant women, Body mass index, Passive smoking, Vaginal cleanliness
- Copyright
- © 2019 He et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Exploring profile and potential influencers of vaginal microbiome among asymptomatic pregnant Chinese women. PeerJ 7:e8172 https://doi.org/10.7717/peerj.8172
Abstract
Background
This study was designed to explore the profile and potential influencers of the vaginal microbiome (VMB) among asymptomatic pregnant Chinese women and its possible association with pregnancy outcomes.
Methods
A prospective study was conducted among pregnant Chinese women receiving regular prenatal care at a hospital in Shanghai, China from March 2017 to March 2018. Vaginal swabs were obtained from 113 asymptomatic pregnant women in mid-pregnancy and sequenced by the V3–V4 region of 16S rRNA on an Ion S5™ XL platform. Demographic characteristics and major pregnancy outcomes were collected through questionnaires and electronic medical records.
Results
The predominant vaginal community state types (CSTs) were CST I (45.1%) and CST III (31.9%). Participants were divided into a lactobacilli-dominant group (LD, CST I/II/III/I–III/V, n = 100, 88.5%) and a less lactobacilli-dominant group (LLD, CST IV-A/B, n = 13, 11.5%). Women in the LLD group showed an increased alpha diversity [median (interquartile range, IQR): 2.41 (1.67, 2.49) vs. 0.30 (0.17, 0.59), P < 0.001], which was related to a lower pre-pregnancy body mass index (BMI) (P = 0.012), and a greater instance of passive smoking (P = 0.033). The relative abundance of Lactobacillus was correlated positively with the pre-pregnancy BMI (r = 0.177, P = 0.041), but negatively with passive smoking (r = − 0.204, P = 0.030).
Conclusion
The vaginal flora of asymptomatic pregnant Chinese women was mostly dominated by Lactobacillus crispatus and L. iners. A lower BMI and greater instance of passive smoking may contribute to a less lactobacilli-dominant VMB. However, a larger sample size is needed.
Introduction
The vaginal microbiome (VMB) is known to play an essential role in women’s reproductive health. Previous culture-based methods recognized the genus Lactobacillus to be the dominant bacterium in the vagina (Rogosa & Sharpe, 1960). These bacteria can maintain the balance of the vaginal microenvironment through the production of lactic acid from vaginal glycogen to keep a low pH value (usually < 4.5). They are also involved in the production of hydrogen peroxide (H2O2) and acidolin, which prevents the overgrowth of other intrinsic bacterial and the invasion of foreign pathogens (Aroutcheva et al., 2001; Miller et al., 2016; Mitchell et al., 2013).
The recently developing molecular technologies have enabled researchers to get a better understanding of the VMB (Fettweis et al., 2012; Mendz, Kaakoush & Quinlivan, 2016). With the help of the next generation of sequencing (NGS), Ravel et al. (2011) was able to cluster the VMB of asymptomatic child-bearing women into five vaginal community state types (CSTs). CST I, II, III, and V were dominated by L. crispatus, L. gasseri, L. iners and L. jensenii (with an abundance usually >90%) respectively, while CST IV represented a more diverse profile with a lesser abundance of Lactobacillus and could be further divided into two subtypes, CST IV-A (a coexistence of Lactobacillus and some anaerobes) and CST IV-B (mostly dominated by Gardnerella, Atopobium, Prevotella, and others) (Brotman et al., 2014b; Huang et al., 2015; Romero et al., 2014). Though the dominant bacteria in CST IV could also produce lactic acid, the pH value is slightly higher (Gajer et al., 2012; Ravel et al., 2011). Moreover, the VMB needs to maintain a lower diversity to obtain the microbial balance in the vagina, which is unlike other sites throughout the body, such as the gut and oral cavity (Huang et al., 2014).
The composition and abundance of the VMB could be affected by various factors, such as intrinsic genotypes, host behaviors, and health conditions (Greenbaum et al., 2019; Lewis, Bernstein & Aral, 2017; Zhang et al., 2018). Ethnicity may be the most influential of all of these factors. In Ravel’s study, White women were mostly dominated by CST I (45.4%), Asians by CST III (42.7%), and Blacks and Hispanics by CST IV (40.4% and 38.1%) (Ravel et al., 2011). Fettweis et al. (2014) further confirmed the lesser abundance of Lactobacillus in African Americans. In addition, the pH value of African descendants was higher than that of Whites and these women were more likely to develop bacterial vaginosis (BV).
Compared with non-pregnant women, the VMB of pregnant women exhibits a lower diversity and abundance of flora but a more stable community (Aagaard et al., 2012; Husain et al., 2014; Walther-Antonio et al., 2014). Since the maternal VMB during pregnancy could largely affect the next generation, researchers are interested in discovering how the VMB could affect pregnancy outcomes. The rich progress in the research of the VMB during pregnancy has been driven by the Human Microbiome Projects in recent years (Fettweis et al., 2019; Serrano et al., 2019). Researchers have found that the lesser abundance of Lactobacillus in the VMB was more likely to lead to the development of adverse pregnancy outcomes, such as preterm birth, still birth, and late miscarriage (Baqui et al., 2019; DiGiulio et al., 2015; Fettweis et al., 2019; Hyman et al., 2014; Tabatabaei et al., 2019). Results have also shown that race or ethnicity may modify the association of the VMB and preterm birth mostly among women of African and European ancestry (Callahan et al., 2017; Wheeler et al., 2018). However, the association among Asian populations remains unclear.
Studies involving local Asian women have been implemented in Japan, Korea, Thailand, and China (Chen et al., 2017; Hong et al., 2016; Matsumoto et al., 2018; Sirichoat et al., 2018), but few have well investigated the VMB of pregnant women using NGS (Kim et al., 2017; Matsumoto et al., 2018), especially among Chinese populations. Thus, in order to gain a more comprehensive understanding to VMB and to see if there was an association of VMB with pregnancy outcomes, we undertook this study to probe into the profile of the VMB among asymptomatic pregnant Chinese women.
Materials & Methods
Participants and procedures
A prospective study was conducted among pregnant Chinese women receiving regular prenatal care at Shanghai Punan Hospital of Pudong New District in Shanghai, China from March 2017 to March 2018. The study was reviewed and approved (IRB#2017-01-0608) by the ethical committee of the School of Public Health, Fudan University (IRB00002408 & FWA00002399).
Participants were recruited at their first prenatal visit. The inclusion criteria included: (a) aged 16 years or older with a singleton pregnancy, (b) gestational week earlier than 28 weeks, and (c) ability to complete the study procedure. The exclusion criteria included: (a) the use of antibiotics within four weeks prior to recruitment, (b) sexual intercourse 48 h prior to sampling, and (c) having a severe illness (such as liver or heart disease). After informed consents, participants were asked to complete a structured questionnaire on their sociodemographic characteristics, medical and reproductive history, and lifestyle data, and were then followed up for their major pregnancy outcomes including delivery (gestational weeks of delivery, delivery mode) and infant information (gender, birth weight) through hospital electronical records.
Sample collection and clinical examination
Vaginal samples were taken by skilled obstetricians from the posterior fornix using sterile swabs. Two swabs were obtained from each participant. One was placed in a tube without any buffer and stored at −80 °C before use and the other was used for clinical inspection with a wet mount. The results were reported by an experienced laboratory staff (Huang). The laboratory examination included the presence of vaginal inflammation and standardized I-IV grades of “vaginal cleanliness” were used by wet mount microscopy (Shang, Wang & Shen, 2014), which was a composite indicator based on grading bacillus, coccus, epithelial cells, and leukocytes per high power (HP). Higher grades imply a presence of vaginal inflammation (Table S1).
DNA extraction, 16S rRNA amplification and sequencing
The total genome DNA from samples was extracted using a mixture of cetyltrimethylammonium bromide (CTAB) and sodium dodecyl sulfate (SDS) methods. The hypervariable V3–V4 region of the 16S rRNA gene (Primer: 341F-CTAYGGGRBGCASCAG; 806R-GGACTACNNGGGTATCTAAT) was amplified with the sample-specific barcodes and then sequenced on an Ion S5™ XL platform (Novogene Co., Ltd., Beijing, China), generating results of 600 bp single-end reads.
Bioinformatic and statistical analyses
The upstream analysis of the sequencing data was conducted on the QIIME 2 software platform (Quantitative Insights into Microbial Ecology 2, Version 2018.11, http://qiime2.org) with its plugins DADA2, feature-table, feature-classifier and phylogeny. Single-end reads were assigned to samples based on their unique barcodes and truncated by cutting off the barcodes and primer sequences. Quality controls were performed and sequences were denoised with the DADA2. After filtering the low abundance of features (reads <10) and omitting chimeras, the representative sequences for amplicon sequence variants (ASVs) were screened for annotation using the SILVA-based training classifier database. The majority of the Lactobacillus genus could not reach the species level because of the short read lengths obtained by high-throughput sequencing, but it was essential for further analysis in studies of VMB (Van Der Pol et al., 2019).Therefore the BLAST (Basic Local Alignment Search Tool) database at the National Center for Biotechnology Information website (http://blast.ncbi.nlm.nih.gov) was utilized with a minimum support threshold of 80%, and the top hit was selected as the species level. If multiple top hits with exactly the same values are found, we expressed them as “Lactobacillus sp.” and numbered them in order.
After the ASV table was obtained, further analyses were performed using R 3.5.2 software for Windows (R Core Team, 2018) and the packages vegan, phyloseq, ggplot2, and pheatmap. Alpha diversity showing the richness and evenness within the subjects was computed using the Shannon index (SID). We also calculated the Bray–Curtis distance as beta diversity for clustering CSTs according to DiGiulio et al. (2015): extracting the most significant Principal Coordinates Analysis (PCoA) eigenvectors to form the distance matrix, applying the partitioning around medoids algorithm, and determining the number of clusters form the gap statistic (k = 7). A heatmap was displayed for visualizing. The linear discriminate analysis coupled with effect size measurements (LEfSe) was performed to identify differential species among different characteristic groups of participants with a linear discriminant analysis (LDA) score >2.0 considered as significant.
Continuous variables were presented using mean ± standard deviation (SD) or the median [interquartile range (IQR)], while discrete variables were presented using numbers and proportions. Referring to previous studies, the subjects were divided into two distinct subgroups according to the vaginal dominance of Lactobacillus. For univariate analyses of participant characteristics, alpha diversity, and relative abundance of taxa between groups, one-way ANOVA, the Mann–Whitney U test and Pearson χ2 test (or Fisher’s exact test) were used to compare the means, medians, and proportions, respectively. The relationships of two continuous variables, including the relative abundance of taxa, alpha diversity, pre-pregnancy body mass index (BMI), passive smoking (days per week), and gestational ages in weeks were investigated with Spearman’s rho. The level of a significant difference was set at a two-sided 0.05.
Results
Participant characteristics
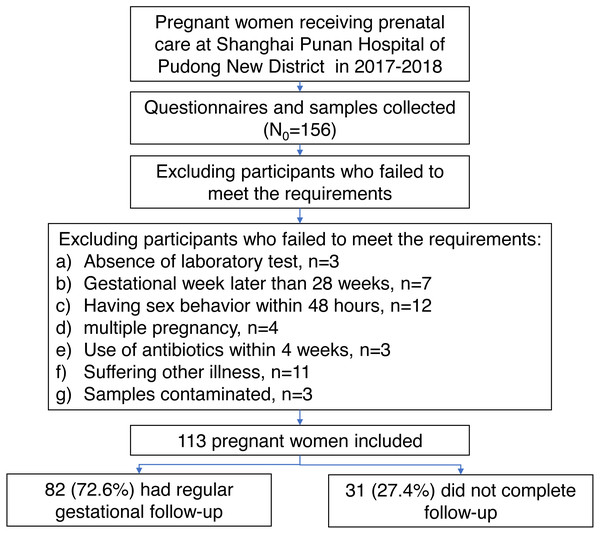
During the period from March 2017 to March 2018, a total of 156 women were recruited at their first prenatal visit in the Shanghai Punan Hospital of Pudong New District in Shanghai, China. After a careful screening, 113 pregnant women met the inclusion/exclusion criteria (Fig. 1). All subjects were Han Chinese in mid-pregnancy, with ages ranging from 17 to 34 years old (mean ± SD: 25.69 ± 3.69) and with gestational ages of 12.42 to 26.71 weeks (mean ± SD: 16.68 ± 2.76). After a regular gestational follow-up, the delivery data of 82 women (72.56%) was collected at Punan Hospital. The gestational age at birth ranged from 35.86 to 41.43 weeks (mean ± SD: 39.40 ± 1.23). The demographic characteristics of women with delivery data collected and not collected were comparable, except for the proportion of vaginal inflammation (Table S2).
Figure 1: The flowchart shows the selection of participants enrolled in this study with consent informed.
Vaginal microbiome profiles
A total of 113 vaginal samples were sequenced, generating 3,671,402 raw reads. Chimeric and low abundance of features (reads < 10) were filtered out, leaving 3,644,890 reads with an average of 32,255 reads per sample (ranging from 1,424 to 52,991 reads). Among annotated taxa, L. crispatus generated the most reads at 2,279,244, followed by L. iners and L. gasseri. Megasphaera, Dialister and Aerococcus were the top three genera following the Lactobacillus genus.
CSTs definition
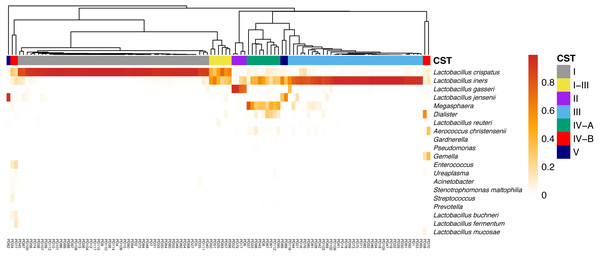
Seven CSTs were identified using the clustering Bray–Curtis distance matrix. Five of them were dominated by Lactobacillus. Of these, L. crispatus dominated CST I had the highest proportion (51/113, 45.1%), followed by L. iners, which dominated CST III (36/113, 31.9%). L. gasseri and L. jensenii dominated CST II and V took up only 3.5% (4/113) and 2.7% (3/113) of the subjects, respectively. A I-III type (6/113, 5.3%) contained almost equal abundance of L. crispatus and L. iners. The other two CSTs were divided into CST IV-A (coexistence of L. iners and Megasphaera, Dialister, etc., 9/113, 8.0%) and IV-B (diversity, 4/113, 3.5%) according to their lower abundance of Lactobacillus. The relative abundance of the top taxa in different CSTs was displayed in a heatmap for visualizing (Fig. 2).
Figure 2: The heatmap indicates the relative abundance of top taxa in all samples.
The CSTs were clustering using Bray–Curtis indices, and was annotated on the top of the heatmap. Notes. CST, community state types.LD and LLD groups
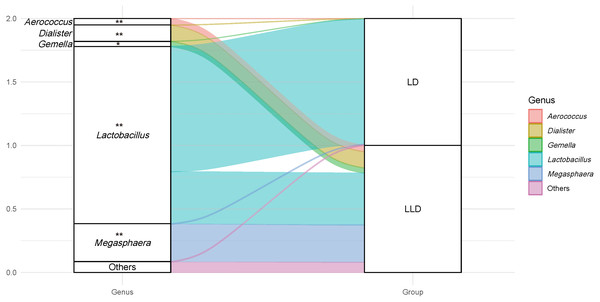
The relative abundance of the Lactobacillus genus in CST I/II/III/I–III/V were all higher than 90%, thus the participants were divided into two groups: The lactobacilli-dominant group (LD, CST I/II/III/I–III/V, n = 100, 88.5%), and the less lactobacilli-dominant group (LLD, CST IV-A/B, n = 13, 11.5%). More detailed bacterial abundance data from different CSTs are shown in Table S3. The relative abundance of other genera was also compared between groups. The genus of Megasphaera, Dialister, Aerococcus, Gardnerella, and Gemella were detected more frequently in the LLD group (P < 0.05 for all, Fig. 3). The sociodemographic, medical and reproductive history, lifestyle characteristics, and major pregnancy outcomes of subjects were compared between the two groups (Table 1).
Figure 3: An alluvial diagram showing the relative abundance of top taxa in LD and LLD groups.
The genus of Megasphaera, Dialister, Aerococcus, Gardnerella, and Gemella were detected more frequently in the LLD group (P < 0.05 for all). Genus with abundance less than 1% is not shown (Gardnerella). Notes. * indicates P value < 0.05, and ** indicates P value < 0.001. LD, lactobacilli-dominant group; LLD, less lactobacilli-dominant group.Results showed that women in the LLD group had a lower maternal pre-pregnancy BMI (especially the proportion of BMI lower than 18.5 kg/m2) (mean ± SD: 19.16 ± 2.60 vs. 21.16 ± 2.68, P = 0.012; 38.5% vs. 13.0%, P = 0.045) and participated in more passive smoking (>3 days per week) (38.5% vs. 15.0%, P = 0.033). There were no differences in the pregnancy outcomes between the groups (P > 0.05 for all). Nevertheless, women in the LLD group tended to have a higher proportion of vaginal inflammation (but not higher than CST I–III and III, Table S4), active smoking, and preterm birth in contrast with those in the LD group.
Comparative analysis of alpha diversity
A significantly increased SID was observed in the LLD group (median (IQR): 2.30 (1.69, 2.48) vs. 0.28 (0.17, 0.57), P < 0.001). To investigate the potential influential factors of alpha diversity within the subjects, SID was further compared among selected characteristics (Table 1). Results revealed that the presence of vaginal inflammation was correlated with a higher SID when compared with that of lower grades (median (IQR): 0.55 [0.29, 1.28] vs. 0.29 [0.18, 0.82], P = 0.017). Women with a lower BMI (P = 0.181) and who participated in more passive smoking (P = 0.262) also had a tendency toward higher diversity, in accordance with the result of CST distribution, although the difference did not achieve statistical significance at 0.05. With the growing gestational age of weeks at enrollment, the alpha diversity showed a declining trend (P = 0.120).
| Characteristics (N = 113) | LD Group (n = 100) | LLD Group (n = 13) | P | SID | P |
|---|---|---|---|---|---|
| CST I/II/III/I–III/V | CST IV-A/B | ||||
| Sociodemographic | |||||
| Age (mean ± SD) (years old) | 25.70 ± 3.78 | 25.62 ± 3.07 | 0.938 | – | – |
| <18 | 1(1.0) | 0(0.0) | >0.99* | 0.30 [0.30, 0.30] | 0.984 |
| 18–25 | 36(36.0) | 5(38.5) | 0.32 [0.21, 0.90] | ||
| 25–35 | 63(63.0) | 8(61.5) | 0.32 [0.18, 0.89] | ||
| Gestational age of weeks at enrollment (mean ± SD) (weeks) | 16.78 ± 2.86 | 15.92 ± 1.64 | 0.294 | – | – |
| 12–18 | 79(79.0) | 12(92.3) | 0.810* | 0.33 [0.21, 0.97] | 0.120 |
| 18–24 | 17(17.0) | 1(7.7) | 0.29 [0.17, 0.90] | ||
| 24–28 | 4(4.0) | 0(0.0) | 0.17 [0.13, 0.21] | ||
| Education status | 0.737* | 0.622 | |||
| Middle school and lower | 25(25.0) | 4(30.8) | 0.31 [0.21, 0.57] | ||
| High school and higher | 75(75.0) | 9(69.2) | 0.32 [0.19, 0.95] | ||
| Economic status (RMB per year) | 0.459 | 0.777 | |||
| <100,000 | 43(43.0) | 7(53.8) | 0.32 [0.21, 0.83] | ||
| ≥100,000 | 57(57.0) | 6(46.2) | 0.30 [0.18, 1.01] | ||
| Medical and reproductive history | |||||
| Maternal pre-pregnancy BMI (mean ± SD) (kg/m2) | 21.16 ± 2.68 | 19.16 ± 2.60 | 0.012 | – | – |
| Underweight (<18.5) | 13(13.0) | 5(38.5) | 0.045* | 0.49 [0.27, 1.24] | 0.181 |
| Normal weight (18.5–24.0) | 71(71.0) | 7(53.8) | 0.28 [0.18, 0.80] | ||
| Overweight and obese (>24.0) | 16(16.0) | 1(7.7) | 0.48 [0.21,0.84] | ||
| Unipara | 69(69.0) | 8(61.5) | 0.752* | 0.32 [0.21, 0.88] | 0.777 |
| No | 31(31.0) | 5(38.5) | 0.30 [0.16, 1.02] | ||
| Presence of inflammation (Grade III/IV) | 22(22.0) | 4(30.8) | 0.492* | 0.55 [0.29, 1.28] | 0.017 |
| No | 78(78.0) | 9(69.2) | 0.29 [0.18, 0.82] | ||
| Previous adverse pregnancy outcomes | 24(24.0) | 3(23.1) | >0.99* | 0.25 [0.15, 1.06] | 0.313 |
| No | 76(76.0) | 10(76.9) | 0.33 [0.21, 0.87] | ||
| Lifestyle | |||||
| Vaginal douching | 34(34.0) | 3(23.1) | 0.541* | 0.27 [0.20, 0.70] | 0.407 |
| Never | 66(66.0) | 10(76.9) | 0.35 [0.20, 1.01] | ||
| Active smoking | 6(6.0) | 2(15.4) | 0.230* | 0.33 [0.22, 1.15] | 0.538 |
| Never | 94(94.0) | 11(84.6) | 0.31 [0.19, 0.88] | ||
| Passive smoking (>3 days per week) | 15(15.0) | 5(38.5) | 0.033* | 0.58 [0.21, 1.31] | 0.262 |
| ≤3 days per week | 85(85.0) | 8(61.5) | 0.30 [0.19, 0.80] | ||
| Drinking | 8(8.0) | 1(7.7) | >0.99* | 0.21 [0.16, 0.33] | 0.109 |
| Never | 92(92.0) | 12(92.3) | 0.32 [0.21, 0.95] | ||
| Alpha diversity | |||||
| Shannon index (median [IQR]) | 0.28 [0.17, 0.57] | 2.30 [1.69, 2.48] | <0.001 | – | – |
| Pregnancy outcomes (N′ = 82) | 72(72.0) | 10(76.9) | >0.99* | – | – |
| Delivery mode | 0.742* | 0.258 | |||
| Cesarean | 31(43.1) | 5(50.0) | 0.28 [0.17, 0.66] | ||
| Vaginal delivery | 41(56.9) | 5(50.0) | 0.37 [0.19, 1.00] | ||
| Gestational age of weeks at birth | 0.327* | 0.212 | |||
| Preterm birth (<37 weeks) | 2(2.8) | 1(10.0) | 0.88 (0.59,1.57) | ||
| Term birth (≥37 weeks) | 70(97.2) | 9(90.0) | 0.31 [0.17, 0.89] | ||
| Fetus gender | 0.735* | 0.512 | |||
| Male fetus | 29(40.8) | 5(50.0) | 0.36 [0.20, 0.96] | ||
| Female fetus | 42(59.2) | 5(50.0) | 0.30 [0.18, 0.89] | ||
| Birth weight | >0.99* | 0.445 | |||
| Low birth weight | 4(5.6) | 0(0.0) | 0.52 [0.17, 0.89] | ||
| Normal birth weight | 62(87.3) | 100(100.0) | 0.32 [0.21, 1.04] | ||
| Large for birth weight | 5(7.0) | 0(0.0) | 0.28 [0.13, 0.46] |
Notes:
P-values were calculated using chi-squared or Fisher’s exact analysis (*) for assessment of association of frequency between groups and the Mann-Whitney U-Test for comparison of means and medians.
- LD
-
lactobacilli-dominant group
- LLD
-
less lactobacilli-dominant group
- SID
-
Shannon index
- CST
-
community state type
- SD
-
standard deviation
- BMI
-
Body Mass Index
- IQR
-
interquartile range
Bioinformatic trends analysis
To further explore the bioinformatic trends of alpha diversity (SID) and relative abundance of taxa with age, maternal pre-pregnancy BMI, passive smoking (days per week), and gestational age of weeks at enrollment, correlation calculations using Spearman’s rho were carried out. Results showed that the maternal pre-pregnancy BMI and the relative abundance of Lactobacillus correlated positively (r = 0.177, P = 0.041) at genus level, but not with any individual species. Moreover, as the days of passive smoking per weeks increased, the abundance of Aerococcus also increased (r = 0.309, P = 0.001), but Lactobacillus decreased (r = − 0.204, P = 0.030). No statistically significant correlations existed between other pairwise variables.
Differential abundance of taxa
LEfSe results distinguished the differential taxa among selected characteristics. The VMB of women with vaginal inflammation revealed more L. iners, Dialister, Enterococcus, and Aerococcus, and less L. crispatus. Smoking also affect the composition of VMB: active smokers have a higher abundance of Megasphaera and Prevotella, while passive smokers have more Aerococcus. BMI groups and selected pregnancy outcomes did not reveal any specific bacteria species.
Discussion
Overall, this prospective study revealed the predominance of CST I and III by investigating the profile of VMB among 113 asymptomatic pregnant Chinese women. Lower pre-pregnancy BMI and more passive smoking were correlated with a less lactobacilli-dominant VMB. No significant findings were associated with pregnancy outcomes.
Earlier studies have reported that the distribution of CSTs was largely affected by race, and the VMB of Asian women was generally dominated by CST III (Ravel et al., 2011; Zhou et al., 2010). Our study found that CST I was predominant among pregnant Chinese women, followed by CST III. This result, although slightly different, was in line with studies carried out among Korean and Japanese pregnant women (Kim et al., 2017; Matsumoto et al., 2018), implying the possibility of a diverse profile of VMB among Asian women due to genetic variations or other environmental factors such as socioeconomic status. We also identified a I–III type containing an equal abundance of L. crispatus and L. iners among this population, which was rarely reported in previous studies. Gajer et al. (2012) once divided this type into CST IV-A, but both the composition and the diversity (both alpha and beta) of CST I–III and IV-A were dissimilar. Perhaps the definition of CSTs should be on the basis of dissimilitude.
Apart from race, the composition of the VMB could be affected by other factors (Fettweis et al., 2014; Jasarevic et al., 2017). In Wen’s study, BMI was significantly correlated to the structure of the microbial community and negatively correlated with the presence of Mycoplasma and BV-associated bacterium-2 (BVAB2) (Wen et al., 2014). Our study noted a similar finding that women with a lower BMI (particularly <18.5 kg/m2) tended to harbor CST IV-A and IV-B, which are the less lactobacilli-dominant type of VMB. Moreover, a positive correlation was observed between BMI and the genus of Lactobacillus. This result was in accordance with the study by Mirmonsef et al. (2014), that BMI was positively associated with vaginal glycogen and a higher abundance of lactobacilli. Lower BMI may have lower concentrations of vaginal glycogen, leading to the lack of lactobacilli.
Smoking is also an important affecting factor. Women who were active smokers were reported as being prone to having higher proportions of BV-associated bacteria (Brotman et al., 2014a; Fettweis et al., 2014; Ryckman et al., 2009). A recent study by Nelson et al. (2018) reported an altered vaginal tract metabolomic profile among smokers, with higher agmatine, cadaverine, putrescine, tryptamine and tyramine, which were known to affect the virulence of infective pathogens and contribute to vaginal malodor. Notably, our study observed a different VMB composition among women who practiced more passive smoking (>3 days per week) instead of active smoking. Moreover, with the days of passive smoking per weeks increased, the abundance of Aerococcus (a BV-associated bacterium) also increased and Lactobacillus decreased. However, the differential taxa detected by LEfSe were not the same with women who actively smoked, leading to the issue of whether active and passive smoking had a similar mechanism that affected VMB. To achieve favorable pregnancy outcomes, pregnant women with either active or passive smoking exposures should remain under close scrutiny.
Although our study did not perform the clinical examination for bacterial vaginosis (BV) and aerobe vaginitis (AV), we used the index of “vaginal cleanliness” grades, which is used routinely as an indicator in Chinese hospitals to suggest the potential inflammation of the vagina. It is used as a pilot screening for BV and AV because of its low cost. Researchers have investigated its possible implications for BV and found that higher grades exhibited higher risks of BV (Lin, Wei & He, 2008). LEfSe results showed the relevance of higher grades with a greater abundance of Aerococcus, Enterococcus, and Dialister, which are AV/BV associated bacteria (Donders et al., 2017). Surprisingly, LEfSe results also revealed a higher abundance of L. iners and a lower abundance of L. crispatus in the higher grades group. This was consistent with our previous results that women with higher abundance of L. iners (CST III/I–III) had a higher proportion of vaginal inflammation, implying the greater risk of developing vaginal inflammation. Although a small sample size may be subject to some criticisms, the results still suggested the exclusive identity of L. iners (Petrova et al., 2017). Compared with other species of Lactobacillus (especially L. crispatus), L. iners possesses a poorer ability to produce the antibacterial product H2O2 and lactic acid (Kim et al., 2006; Petrova et al., 2017). Moreover, L. iners may even produce a toxin called Inerolysin similar to Gardnerella vaginalis (Rampersaud et al., 2018; Rampersaud et al., 2011). Therefore, in some cases, CST III has been described as a transitional state, sometimes associated with BV (Verstraelen et al., 2009; Walther-Antonio et al., 2014).
This study also explored the possible association of VMB and pregnancy outcomes. However, due to the limited sample size and a medium proportion of delivery data not collected, the results were uncertain. Since a lower BMI and the rate of passive smoking were also reportedly related to adverse pregnancy outcomes, these two may act as the modifications between VMB and adverse pregnancy outcomes (Wen et al., 2014). Moreover, CST III has been reported associated with preterm birth in some studies (Kindinger et al., 2017; Petricevic et al., 2014), further indicating the specialty of L. iners as we’ve mentioned previously. However, the proportion of CST III was high among Asian women, but the incidence rate of preterm birth was not higher than the western countries. We intend to expand our cohort in future research.
Our study had several limitations. Firstly, our study detected very little G. vaginalis and no BVAB1, which seemed different form other studies. However, due to the low proportion of G. vaginalis vagitypes among pregnant Asian women (3.6% in Korea by Kim et al. (2017), and 2/24 in Japan by Matsumoto et al. (2018)), we have to suppose that our participants in this study happened to have no vagitype of G. vaginalis. As for BVAB1, we did not really report it yet, probably due to the primer and reference database selection bias (Van Der Pol et al., 2019). Further researches may extend the reference database in advance. Moreover, our study only used a single sampling point, failing to explore the stability of the VMB throughout the pregnancy. Larger sample sizes and better study design are needed.
Conclusions
The vaginal flora of asymptomatic pregnant Chinese women was mostly dominated by L. crispatus and L. iners. A lower BMI and more passive smoking may contribute to a less lactobacilli-dominant VMB. The investigation of the profile and influencers among pregnant women provides a basis for a further probe into the association of VMB and pregnancy outcomes. However, larger sample sizes are needed.
Supplemental Information
Comparative analysis between women of delivery data collected and not collected
P-values were calculated using chi-squared or Fisher’s exact analysis (*) for assessment of association of frequency between groups and the Mann–Whitney U-Test for comparison of means and medians . SD = standard deviation; BMI = Body Mass Index.
Relative abundance of top taxa in different CSTs
CST = community state type; LD = lactobacilli-dominant group; LLD = less lactobacilli-dominant group.
Comparative analysis of relevant characteristics of subjects in different CSTs
P-values were calculated using chi-squared or Fisher’s exact analysis (*) for assessment of association of frequency between groups and the Mann–Whitney U-Test for comparison of means and medians. CST = community state type; LD = lactobacilli-dominant group; LLD = less lactobacilli-dominant group; SD = standard deviation; BMI = Body Mass Index; IQR = interquartile range.