A new species of the Asian music frog genus Nidirana (Amphibia, Anura, Ranidae) from Southwestern China
- Published
- Accepted
- Received
- Academic Editor
- Jia-Yong Zhang
- Subject Areas
- Taxonomy, Zoology
- Keywords
- Taxonomy, New species, Molecular phylogenetic analysis, Morphology, China
- Copyright
- © 2019 Li et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. A new species of the Asian music frog genus Nidirana (Amphibia, Anura, Ranidae) from Southwestern China. PeerJ 7:e7157 https://doi.org/10.7717/peerj.7157
Abstract
The Asian music frog genus Nidirana is widely distributed in East and Southeastern Asia. Systematic profiles of the group remain on debate, and cryptic species are expected especially in the species with wide distributional range. Here, we describe a new species of the genus from Southwestern China. Phylogenetic analyses based on mitochondrial DNA and nuclear DNA supported the new species as an independent clade nested into the Nidirana clade and sister to N. hainanensis. Morphologically, the new species could be distinguished from its congeners by a combination of the following characters: a large body size in males (SVL > 49 mm); the presence of lateroventral grooves both on fingers and toes; relative finger lengths: II < IV < I < III; tibiotarsal articulation reaching the level between eye and nostril when leg stretched forward; a pair of subgular internal vocal sacs at corners of throat in males; nuptial pad present on the inner side of base of fingers I and II in males in breading season; webbing formula: I 2 –21/3 II 2 –22/3 III 31/2 –32/3 IV 32/3 –3V. The findings provided a better knowledge on phylogenetic assignments of the genus Nidirana, and indicated future deeper investigations necessarily for exploring systematic settings of the group.
Introduction
The Asian music frog genus Nidirana Dubois, 1992 is widely distributed in East and Southeast Asia, from Japan, westwards to southern China, southwards to northern Thailand and northern Vietnam and Laos (Frost, 2019). Systematic assignments of the genus have been much controversial. Thompson (1912) established the genus Babina, separating from the genus Rana Linnaeus, 1758, and transferred two species (Rana holsti Boulenger, 1892 and Rana subaspera Barbour, 1908 to the new genus. Dubois (1992) established the subgenus Nidirana under the genus Rana, which contained six species: Rana psaltes Kuramoto, 1985, Rana adenopleura Boulenger, 1909, Rana caldwelli Schmidt, 1925, Rana chapaensis Bourret, 1937, Rana daunchina Chang, 1933 and Rana pleuraden Boulenger, 1904. However, “Rana psaltes” was synonymized with “R. okinavana” (Matsui, 2007), and “Rana caldwelli” was considered as the subjective junior synonym of “R. adenopleura” (Lyu et al., 2017). Although Frost et al. (2006) merged the “Nidirana” group into the genus Babina, most systematic studies suggested that “Nidirana” group was distinct from the genus Babina seusu stricto (e.g., Chen et al., 2005; Chuaynkern et al., 2010; Fei et al., 2009; Fei, Ye & Jiang, 2010; Fei, Ye & Jiang, 2012). As note, all the previous studies did not include all species of Babina seusu lato. Therefore, Lyu et al. (2017) performed a most-species-comprehensive analysis based on molecular phylogenetics, morphology and bioacoustics of Babina seusu lato, and confirmed that Nidirana was a distinct genus containing eight species: Nidirana okinavana (Boettger, 1895), Nidirana adenopleura (Boulenger, 1909), Nidirana hainanensis (Fei, Ye & Jiang, 2007), Nidirana chapaensis (Boulenger, 1909), Nidirana daunchina (Chang, 1933), Nidirana lini (Chou, 1999), Nidirana pleuraden (Boulenger, 1904) and Nidirana nankunensis Lyu et al., 2017.
In the genus Nidirana, N. adenopleura was reported to have the widest distributional range across the Iriomote and Ishigaki Isands and Yaeyama group of Ryukyu Island in Japan and Taiwan, Guizhou, Anhui, Zhejiang, Jiangxi, Hunan, Fujian, Guangdong and Guangxi provinces of China (Frost, 2019; Fei et al., 2009; Fei, Ye & Jiang, 2012). The species is heavily dependent on the still water environment (Fei et al., 2009). It is expected that the wide distributional range and habitat requirements of the species might promote population divergence even speciation in it. Two new species were described in the populations of N. adenopleura: N. hainanensis found by Fei, Ye & Jiang (2007) from Diaoluo Mountain of Hainan Island and N. nankunensis found by Lyu et al. (2017) from Longmen County, Guangdong Province, China. Noticeably, neither of the two species were found to be the sister clade of N. adenopleura (Lyu et al., 2017). Even so, distinct populations of N. adenopleura were still noted to encompass differences on morphology, breeding habit and calls (Fei et al., 2009). For instance, Wu, Dong & Xu (1986) found that there were obvious morphological differences between populations from Leigong Mountain and Fanjing Mountain of Guizhou Province and other populations of N. adenopleura in China. This indicated that the populations probably represented cryptic species, and thus deeper investigations especially with molecular phylogenetic methods were necessary to evaluate the taxonomic status of them.
In recent years, we carried out a series of biodiversity surveys in Leigong Mountain, Leishan County, Guizhou Province, China, and collected some specimens superficially resembling N. adenopleura on morphology. Phylogenetic analyses based on mitochondrial DNA and nuclear DNA, morphological comparisons and bioacoustics comparisons consistently indicated these specimens as a new taxon of Nidirana. Herein we describe it as a new species.
Methods
Sampling
Four adult females and six adult males of the new taxon of Nidirana were collected from Leigong Mountain, Leishan County, Guizhou Province, China between June 2015 and July 2017 (for voucher information see Table S1; Fig. 1). Seven tadpoles of the new taxon (for voucher information see Table S2) were also collected at the same site as the adult specimens on 26 August 2018. For tadpole matching, we compare the genetic divergence between the collected tadpoles and adult specimens. The stages of tadpoles were identified following Gosner (1960). After taking photographs, they were euthanized using isoflurane, and then the specimens were fixed in 10% buffered formalin. Tissue samples were taken and preserved separately in 95% ethanol prior to fixation (Li et al., 2018). Specimens were deposited in Chengdu Institute of Biology, Chinese Academy of Sciences (CIB, CAS).
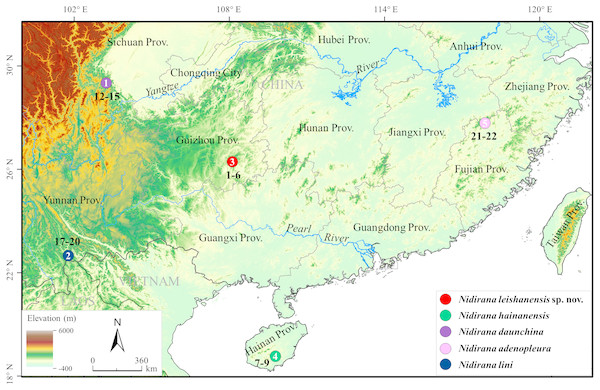
Figure 1: Sampling localities in this study.
Localities 1–5 were all in China: 1, Emei Mountain, Sichuan Province (Prov.); 2, Jiangcheng County (Co.), Yunnan Prov.; 3, Leigong Mountain, Leishan Co., Guizhou Prov.; 4, Diaoluo Mountain, Lingshui Co., Hainan Prov.; 5, Wuyi Mountain, Fujian Prov. Different species were denoted as different colors. Sample numbers (for information see Table 1) were labeled beside localities.| Sample no. | Species | Locality | Voucher number | GenBank accession number | ||||
|---|---|---|---|---|---|---|---|---|
| 12S | 16S | COI | Tyr1 | CXCR4 | ||||
| 1 | Nidirana leishanensis sp. nov. | *Leigong Mountain, Leishan County, Guizhou Province, China | CIBLS20150627003 | MK293792 | MK293810 | MK293828 | MK293859 | MK293845 |
| 2 | Nidirana leishanensis sp. nov. | *Leigong Mountain, Leishan County, Guizhou Province, China | CIBLS20150628007 | MK293793 | MK293811 | MK293829 | MK293860 | MK293846 |
| 3 | Nidirana leishanensis sp. nov. | *Leigong Mountain, Leishan County, Guizhou Province, China | CIBLS20150628002 | MK293794 | MK293812 | MK293830 | MK293861 | MK293847 |
| 4 | Nidirana leishanensis sp. nov. | *Leigong Mountain, Leishan County, Guizhou Province, China | CIBLS20150628005 | MK293795 | MK293813 | MK293831 | MK293862 | MK293848 |
| 5 | Nidirana leishanensis sp. nov. | *Leigong Mountain, Leishan County, Guizhou Province, China | CIBLS20180628001 | MK293796 | MK293814 | MK293832 | MK293863 | MK293849 |
| 6 | Nidirana leishanensis sp. nov. | *Leigong Mountain, Leishan County, Guizhou Province, China | CIBLS20180628002 | MK293797 | MK293815 | MK293833 | MK293864 | MK293850 |
| 7 | Nidirana hainanensis | *Diaoluo Mountain, Lingshui County, Hainan Province, China | CIB20110629003 | MK293789 | MK293807 | MK293825 | / | MK293843 |
| 8 | Nidirana hainanensis | *Diaoluo Mountain, Lingshui County, Hainan Province, China | CIB20110629004 | MK293790 | MK293808 | MK293826 | / | / |
| 9 | Nidirana hainanensis | *Diaoluo Mountain, Lingshui County, Hainan Province, China | CIB20110629005 | MK293791 | MK293809 | MK293827 | MK293858 | MK293844 |
| 10 | Nidirana hainanensis | *Diaoluo Mountain, Lingshui County, Hainan Province, China | SYS a003741 | # MF807899 | # MF807821 | # MF807860 | / | / |
| 11 | Nidirana daunchina | *Emei Mountain, Sichuan Province, China | SYS a004594 | # MF807900 | # MF807822 | # MF807861 | / | / |
| 12 | Nidirana daunchina | *Emei Mountain, Sichuan Province, China | CIB2011081601 | MK293801 | MK293819 | MK293837 | MK293868 | / |
| 13 | Nidirana daunchina | *Emei Mountain, Sichuan Province, China | CIB2011081602 | MK293802 | MK293820 | MK293838 | MK293869 | / |
| 14 | Nidirana daunchina | *Emei Mountain, Sichuan Province, China | CIB2011081603 | MK293803 | MK293821 | MK293839 | MK293870 | MK293854 |
| 15 | Nidirana daunchina | *Emei Mountain, Sichuan Province, China | CIB20110629001 | MK293804 | MK293822 | MK293840 | MK293871 | MK293855 |
| 16 | Nidirana chapaensis | *Sa pa, Lao Cai, Vietnam | T2483/2000.4850 | / | # KR827711 | # KR087625 | / | / |
| 17 | Nidirana lini | *Jiangcheng County, Yunnan Province, China | SYS a003967 | # MF807896 | # MF807818 | # MF807857 | / | / |
| 18 | Nidirana lini | *Jiangcheng County, Yunnan Province, China | CIB20110629011 | MK293798 | MK293816 | MK293834 | MK293865 | MK293851 |
| 19 | Nidirana lini | *Jiangcheng County, Yunnan Province, China | CIB20110629010 | MK293799 | MK293817 | MK293835 | MK293866 | MK293852 |
| 20 | Nidirana lini | *Jiangcheng County, Yunnan Province, China | CIB20110629008 | MK293800 | MK293818 | MK293836 | MK293867 | MK293853 |
| 21 | Nidirana adenopleura | Wuyi Mountain, Fujian Province, China | CIB20180827001 | MK293787 | MK293805 | MK293823 | MK293856 | MK293841 |
| 22 | Nidirana adenopleura | Wuyi Mountain, Fujian Province, China | CIB20180827002 | MK293788 | MK293806 | MK293824 | MK293857 | MK293842 |
| 23 | Nidirana adenopleura | Yanping District, Nanping City, Fujian Province, China | SYS a005911 | # MF807922 | # MF807844 | # MF807883 | / | / |
| 24 | Nidirana adenopleura | New Taipei City, Taiwan Province, China | UMMZ 189963 | # DQ283117 | # DQ283117 | / | / | / |
| 25 | Nidirana okinavana | *Okinawa, Japan | / | # AB761266 | # AB761266 | # AB761266 | / | / |
| 26 | Nidirana nankunensis | *Nankun Mountain, Guangdong Province, China | SYS a003618 | # MF807906 | # MF807828 | # MF807867 | / | / |
| 27 | Nidirana pleuraden | Gaoligong Mountain, Yunnan Province, China | SYS a003775 | # MF807894 | # MF807816 | # MF807855 | / | / |
| 28 | Babina subaspera | *Amami Island, Kagoshima, Japan | / | # AB761265 | # AB761265 | # AB761265 | / | / |
| 29 | Babina holsti | *Okinawa, Japan | / | # AB761264 | # AB761264 | # AB761264 | / | / |
| 30 | Amolops mantzorum | Xiling Mountain, Sichuan Province, China | / | # NC024180 | # NC024180 | # NC024180 | / | / |
| 31 | Odorrana margaretae | China | HNNU1207003 | # NC024603 | # NC024603 | # NC024603 | / | / |
| 32 | Nidirana daunchina | Sichuan Province, China | CIB-WU37990 | / | / | / | # DQ360041 | / |
| 33 | Nidirana chapaensis | Xieng Khouang Province, Laos, Vietnam | FMNH 256531 | / | / | / | # EU076792 | / |
| 34 | Nidirana chapaensis | Xieng Khouang Province, Laos, Vietnam | FMNH 256531 | / | / | / | / | # KR264317 |
| 35 | Nidirana pleuraden | Yunnan Province, China | SCUM0405185CJ | / | / | / | # DQ360042 | / |
Notes:
* denotes the locality being the type locality of the species.
Morphological analysis
The terminology and methods were followed Fei & Ye (2005). Measurements were taken with a dial caliper to the nearest 0.1 mm. Seventeen morphometric characters of adult specimens were measured: snout-vent length (SVL; distance from the tip of the snout to the posterior edge of the vent), head length (HDL; distance from the tip of the snout to the articulation of jaw), head width (HDW; greatest width between the left and right articulations of jaw), snout length (SL; distance from the tip of the snout to the anterior corner of the eye), eye diameter (ED; distance from the anterior corner to the posterior corner of the eye), interorbital distance (IOD; minimum distance between the inner edges of the upper eyelids), internasal distance (IND; minimum distance between the inner margins of the external nares), upper eyelid width (UEW; greatest width of the upper eyelid margins measured perpendicular to the anterior-posterior axis), maximal tympanum diameter (TYD), length of lower arm and hand (LAL; distance from the elbow to the distal end of the Finger IV), lower arm width (LW; maximum width of the lower arm), hand length (HAL; distance from the posterior end of the inner metacarpal tubercle to the distal tip of Finger IV), hindlimb length (HLL; maximum length from the vent to the distal tip of the Toe IV), tibia length (TL; distance from knee to tarsus), maximal tibia width (TW), length of foot and tarsus (TFL; distance from the tibiotarsal articulation to the distal end of the toe IV) and foot length (FL; distance from tarsus to the tip of the fourth toe). Ten morphometric characters of tadpoles were measured: total length (TOL), SVL, maximum body height (BH), maximum body width (BW), SL, snout to spiraculum (SS; distance from spiraculum to the tip of the snout), mouth width (MW; distance between two corners of mouth), maximum width of tail base (TBW), tail length (TAL; distance from base of vent to the tip of tail) and tail height (TAH; maximum height between upper and lower edges of tail).
Molecular data and phylogenetic analyses
Three male specimens, one female specimen and two tadpoles of the new taxon were included in the molecular analyses (for voucher information see Table 1). For phylogenetic analyses, three samples of N. hainanensis from Diaoluo Mountain, Hainan Province, China (type locality of the species), four samples of N. daunchina from Emei Mountain, Sichuan Province, China (type locality of the species), three samples of N. lini from Jiangcheng County, Yunnan Province, China (type locality of the species) and two samples of N. adenopleura from Wuyi Mountain, Fujian Province, China were also sequenced (for voucher information see Table 1; Fig. 1).
Total DNA was extracted using a standard phenol-chloroform extraction protocol (Sambrook, Fritsch & Maniatis, 1989). Three fragments of the mitochondrial genes 12S rRNA,16S rRNA and cytochrome oxidase subunit I (COI) were amplified using the primers in Kocher et al. (1989), Simon et al. (1994) and Che et al. (2012), respectively. They were amplified under the following conditions: an initial denaturing step at 95 °C for 4 min; 36 cycles of denaturing at 95 °C for 30 s, annealing at 52 °C (for 12S and 16S)/47 °C (for COI) for 40 s and extending at 72 °C for 70 s, and a final extending step of 72 °C for 10 min. The nuclear gene fragments C-X-C chemokine receptor type 4 (CXCR4) and Tyrosinase exon 1 (Tyr1) were amplified using the primers and protocols in Biju & Bossuyt (2003) and Bossuyt & Milinkovitch (2000), respectively. All primers were presented in the Table S3. PCR products were purified with spin columns and then were sequenced with both forward and reverse primers same as used in PCR. Sequencing was conducted using an ABI3730 automated DNA sequencer in Shanghai DNA BioTechnologies Co., Ltd. (Shanghai, China). All sequences were deposited in GenBank (for GenBank accession numbers see Table 1).
For phylogenetic analyses, the available sequence data for all related species of the genus Nidirana were downloaded from GenBank (for GenBank accession numbers of all sequences see Table 1). Corresponding sequences of one Amolops mantzorum and one Odorrana margaretae were used as outgroups according to Lyu et al. (2017).
Sequences were assembled and aligned using the Clustalw module in BioEdit v. 7.0.9.0 (Hall, 1999) with default settings. Alignments were checked by eye and revised manually if necessary. To avoid bias in alignments, GBLOCKS v. 0.91.b (Castresana, 2000) with default settings was used to extract regions of defined sequence conservation from the length-variable 12S and 16S gene fragments. Non-sequenced fragments were defined as missing loci. Finally, for phylogenetic analyses, the dataset concatenated with 12S (for 30 samples) + 16S (for 31 samples) + COI (for 30 samples) + Tyr1 (for 16 samples) + CXCR4 (for 15 samples) gene sequences.
Gene trees were reconstructed for the mitochondrial gene concatenated dataset and the concatenated dataset of all five genes, respectively. Phylogenetic analyses were conducted using maximum likelihood (ML) and Bayesian Inference (BI) methods, implemented in PhyML v. 3.0 (Guindon et al., 2010) and MrBayes v. 3.12 (Ronquist & Huelsenbeck, 2003), respectively. To avoid under- or over-parameterization (Lemmon & Moriarty, 2004; McGuire et al., 2007), the best partition scheme and the best evolutionary model for each partition were chosen for the phylogenetic analyses using PARTITIONFINDER v. 1.1.1 (Robert et al., 2012). In this kind of analyses, 12S, 16S genes and each codon position of each protein-coding gene were defined and Bayesian Inference Criteria was used. ML and BI analyses were based on the PARTITIONFINDER results (Table S4). For the ML tree, branch supports were drawn from 10,000 nonparametric bootstrap replicates. In BI analyses, the parameters for each partition were unlinked, and branch lengths were allowed to vary proportionately across partitions. Two runs each with four Markov chains were simultaneously run for 50 million generations with sampling every 1,000 generations. The first 25% trees were removed as the “burn-in” stage followed by calculations of Bayesian posterior probabilities and the 50% majority-rule consensus of the post burn-in trees sampled at stationarity.
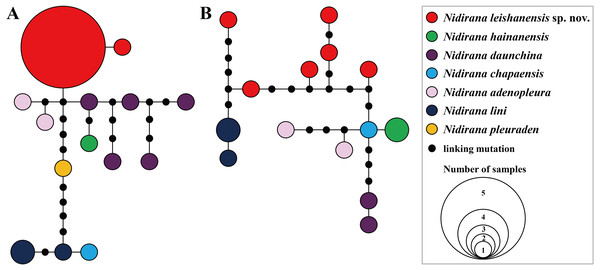
On each nuclear gene, there was no enough number of variable sites for reconstructing reliable phylogenetic relationships between Nidirana species. Therefore, we just examine haplotype relationships of the new taxon and its related species based on Tyr1 and CXCR4 gene datasets, respectively, by constructing haplotype networks using the maximum parsimony method in TCS v. 1.21 (Clement, Posada & Crandall, 2000). Genetic distance between Nidirana species based on uncorrected p- distance model was estimated on COI, Tyr1 and CXCR4 genes, respectively, using MEGA v. 6.06 (Tamura et al., 2011).
Morphological comparisons
All ten adult specimens (for voucher information see Table S1) and seven tadpole specimens (for voucher information see Table S2) of the new taxon were measured. For comparisons, measurements of the holotype and three paratypes of N. hainanensis (for voucher information see Table S1) were retrieved from Fei, Ye & Jiang (2007).
In order to reduce the impact of allometry, the correct value from the ratio of each character to SVL was calculated and then was log-transformed for the following morphometric analyses. One-way analysis of variance (ANOVA) was used to test the significance of differences on morphometric characters between males and females and between different species. The significance level was set at 0.05. To show the spatial distribution of each species on the morphometric characters, principal component analyses (PCA) were performed. These analyses were carried out in R (R Development Core Team, 2008).
The new species was also morphologically compared with all other Nidirana species. Comparative morphological data were obtained from literature for N. adenopleura (Boulenger, 1909), N. chapaensis (Bourret, 1937), N. daunchina (Chang & Hsu, 1932), N. hainanensis (Fei, Ye & Jiang, 2007), N. lini (Chou, 1999), N. nankunensis (Lyu et al., 2017), N. okinavana (Boettger, 1895) and N. pleuraden (Boulenger, 1904).
Bioacoustics analyses
The advertisement call of the new species from Leigong Mountain, Leishan County, Guizhou Province, China was recorded from the specimen CIBLS20170727002 at ambient air temperature of 23 °C and air humidity of 87% in the ridge of paddy field on 27 June 2017. For comparisons, the advertisement call of N. hainanensis from Diaoluo Mountain, Lingshui County, Hainan Province, China were recorded from the specimen CIB20110629003 at ambient air temperature of 24 °C and air humidity of 85% in the ridge of paddy field on 10 May 2012. SONY PCM-D50 digital sound recorder was used to record within 20 cm of the calling individual. The sound files in wave format were resampled at 48 kHz with sampling depth 24 bits. Calls were recoded and examined as described by Wijayathilaka & Meegaskumbura (2016). Call recordings were visualized and edited with SoundRuler 0.9.6.0 (Gridi-Papp 2003–2007) and Raven Pro 1.5 software (Cornell Laboratory of Ornithology, Ithaca, NY, USA). Ambient temperature of the type locality was taken by a digital hygrothermograph.
Skull scanning
Skulls of three male specimens (voucher numbers: CIBLS20170727002, CIBLS20150628001 and CIBLS20150628002) and two female specimens (voucher numbers: CIBLS20150627001 and CIBLS20150627002) of the new taxon were scanned in the high-resolution X-ray scanner (Quantum GX micro-CT Imaging System; PerkinElmer, Boston, MA, USA). The specimens were scanned along the coronal axis at an image resolution of 2,000 × 2,000. Segmentation and three-dimensional reconstruction of the CT images were made using VG57 Studio Max 2.2 (Volume Graphics, Heidelberg, Germany). Raw data of skull from CT scanning were deposited in the database figshare with doi: 10.6084/m9.figshare.7980158.
The Animal Care and Use Committee of Chengdu Institute of Biology, CAS provided full approval for this purely observational research (Number: CIB2011032201). Field work was approved by the Management Office of the Leigong Mountain National Nature Reserve (project number: LGS-201304006).
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:08105A58-4826-475D-BF07-A0C5C16D6BDD. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Results
Phylogenetic analyses and genetic divergence
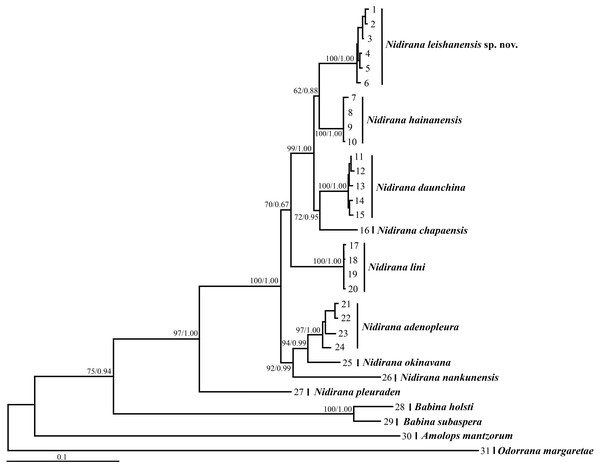
Aligned sequence matrix of 12S + 16S + COI, Tyr1and CXCR4 genes contained 1,488 bp, 519 bp and 642 bp, respectively. ML and BI analyses based on the 12S + 16S + COI matrix and all-five-genes matrix resulted in essentially identical topologies (Fig. 2 and Fig. S1). All samples of the new taxon occurring from Leishan Mountain of Guizhou Province, China were strongly clustered into a monophyly, which was nested into the Nidirana clade and sister to the N. hainanensis clade. Two haplotypes were found for all samples of the new taxon in Tyr1 gene and six haplotypes in CXCR4 gene, respectively, and there was no common haplotype between the new taxon and its related species (Fig. 3). The genetic distance base on COI gene between the new taxon and its sister species N. hainanensis was mean 0.057 (range 0.055–0.060; Table 2) higher than the interspecific genetic distance between other sister species, i.e., that between N. hainanensis and N. daunchina (0.054), N. daunchina and N. chapaensis (0.048) and N. adenopleura and N. okinavana (0.049). The mean genetic distance on Tyr1 gene between the new taxon and its congeners ranged from 0.009 to 0.024, and that on CXCR4 gene ranged from 0.005 to 0.009.
Figure 2: Maximum likelihood (ML) tree of Nidirana leishanensis sp. nov. and the related species reconstructed based on the concatenated sequences of the mitochondrial 12S, 16S and COI genes and the nuclear Tyr1 and CXCR4 genes.
Bootstrap supports from ML analyses/Bayesian posterior probabilities from BI analyses were labeled beside nodes. See information of samples 1–31 in Table 1.Figure 3: Haplotype networks of Nidirana leishanensis sp. nov. and its related species constructed based on the nuclear gene sequences.
(A) Tyr1 gene. (B) CXCR4 gene. Different species were denoted as different colors.| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Nidirana leishanensis sp. nov. | ||||||||||
| 2. Nidirana hainanensis | 0.057 | |||||||||
| 3. Nidirana daunchina | 0.063 | 0.054 | ||||||||
| 4. Nidirana chapaensis | 0.057 | 0.043 | 0.048 | |||||||
| 5. Nidirana lini | 0.110 | 0.095 | 0.092 | 0.085 | ||||||
| 6. Nidirana adenopleura | 0.105 | 0.092 | 0.095 | 0.087 | 0.102 | |||||
| 7. Nidirana okinavana | 0.107 | 0.095 | 0.095 | 0.085 | 0.103 | 0.049 | ||||
| 8. Nidirana nankunensis | 0.098 | 0.105 | 0.109 | 0.096 | 0.093 | 0.075 | 0.071 | |||
| 9. Nidirana pleuraden | 0.133 | 0.118 | 0.112 | 0.110 | 0.114 | 0.113 | 0.114 | 0.125 | ||
| 10. Babina subaspera | 0.186 | 0.180 | 0.179 | 0.185 | 0.157 | 0.166 | 0.173 | 0.165 | 0.162 | |
| 11. Babina holsti | 0.190 | 0.177 | 0.176 | 0.185 | 0.164 | 0.163 | 0.169 | 0.169 | 0.167 | 0.043 |
Morphological comparisons
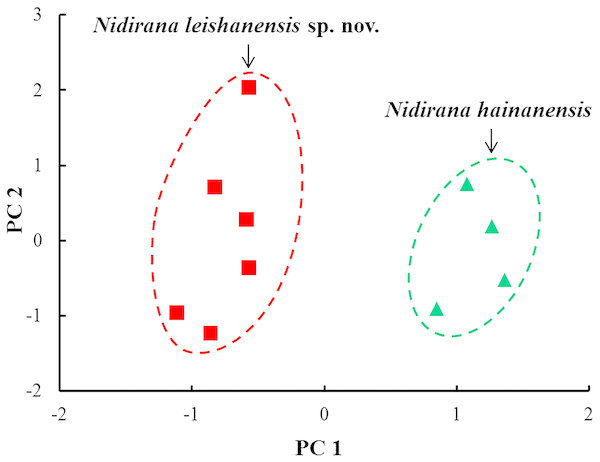
The results of one-way ANOVA showed that in the new taxon, the male group was significantly different from the female group on the ratio of IND to SVL (p-value < 0.05; Table 3). Therefore, morphometric analyses between the new taxon and N. hainanensis were conducted only in males. In PCA for males, the total variation of the first two principal components was 69.3%. In males on the two-dimensional plots of PC1 vs. PC2, the new taxon could be distinctly separated from N. hainanensis (Fig. 4). The results of one-way ANOVA indicated that in males, the new taxon was significantly different from N. hainanensis on many morphometric characters (all p-values < 0.05; Table 3). More detailed descriptions of results from morphological comparisons between the new taxon and its congeners were presented in the following sections for describing the new species.
| Male | Female | p-value from ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|
| NL(n = 6) | NH(n = 4) | NL(n = 4) | NLvs.NHin male | Male vs. Female inNL | ||||
| Ranging | Mean ± SD | Ranging | Mean ± SD | Ranging | Mean ± SD | |||
| SVL | 49.5–56.4 | 53.3 ± 3.2 | 32.8–33.5 | 33.3 ± 0.3 | 43.7–55.3 | 50.2 ± 5.3 | 0.000 | 0.343 |
| HDL | 18.4–23.7 | 20.5 ± 2.0 | 12.7–12.9 | 12.8 ± 0.1 | 17.0–19.3 | 18.1 ± 1.1 | 0.971 | 0.254 |
| HDW | 17.5–22.2 | 19.5 ± 1.7 | 11.5–12.5 | 12.0 ± 0.4 | 16.5–19.3 | 17.6 ± 1.2 | 0.710 | 0.490 |
| SL | 7.0–8.5 | 7.7 ± 0.6 | 5.3–5.7 | 5.5 ± 0.2 | 6.7–8.4 | 7.5 ± 0.7 | 0.001 | 0.398 |
| IND | 5.6–6.7 | 6.2 ± 0.4 | 3.5–4.4 | 4.1 ± 0.4 | 5.6–6.9 | 6.3 ± 0.6 | 0.341 | 0.001 |
| IOD | 4.0–4.7 | 4.4 ± 0.2 | 3.4–3.7 | 3.6 ± 0.1 | 3.8–5.2 | 4.5 ± 0.7 | 0.000 | 0.214 |
| UEW | 3.7–4.5 | 4.2 ± 0.3 | 3.1–3.1 | 3.1 ± 0.0 | 3.2–4.4 | 3.8 ± 0.5 | 0.001 | 0.638 |
| ED | 6.0–7.0 | 6.4 ± 0.4 | 4.4–5.0 | 4.6 ± 0.3 | 5.2–6.2 | 5.8 ± 0.4 | 0.003 | 0.181 |
| TYD | 4.7–5.6 | 5.0 ± 0.4 | 3.6–4.3 | 3.9 ± 0.3 | 4.0–5.6 | 4.7 ± 0.7 | 0.002 | 0.975 |
| LAL | 20.6–25.3 | 23.4 ± 1.9 | 14.8–15.1 | 15.0 ± 0.2 | 19.8–23.7 | 22. 0 ± 1.6 | 0.132 | 0.279 |
| LW | 4.4–5.9 | 4.9 ± 0.6 | 2.0–2.9 | 2.5 ± 0.4 | 4.0–4.4 | 4.2 ± 0.2 | 0.021 | 0.639 |
| HAL | 12.5–15.2 | 13.8 ± 1.3 | 9.0–9.7 | 9.2 ± 0.3 | 12.0–14.9 | 13.7 ± 1.2 | 0.028 | 0.162 |
| HLL | 82.2–97.4 | 89.3 ± 6.0 | 58.0–61.0 | 59.0 ± 1.4 | 79.0–92.7 | 86.2 ± 5.7 | 0.003 | 0.462 |
| TL | 26.1–31.5 | 29.2 ± 2.1 | 17.6–19.4 | 18.6 ± 0.8 | 25.2–31.2 | 27.9 ± 2.5 | 0.260 | 0.719 |
| TW | 7.3–8.6 | 8.0 ± 0.7 | 4.5–5.4 | 4.9 ± 0.4 | 6.3–8.3 | 7.2 ± 0.8 | 0.397 | 0.299 |
| TEL | 37.0–46.5 | 42.1 ± 3.7 | 26.1–27.7 | 26.8 ± 0.7 | 37.3–42.4 | 39.9 ± 2.2 | 0.341 | 0.457 |
| FL | 27.3–34.6 | 30.7 ± 2.7 | 18.5–18.8 | 18.7 ± 0.1 | 26.3–32.4 | 29.4 ± 2.5 | 0.327 | 0.444 |
Notes:
Unit: mm. ANOVA, one-way analyses of variance. Significant level: p <0.05. See abbreviations for the measurements in ‘Methods’.
Figure 4: Plots of principal component analyses of Nidirana leishanensis sp. nov. and N. hainanensis.
PC1, the first principal component; PC2, the second principal component. Different species were denoted as different colors and shapes.Bioacoustics comparisons
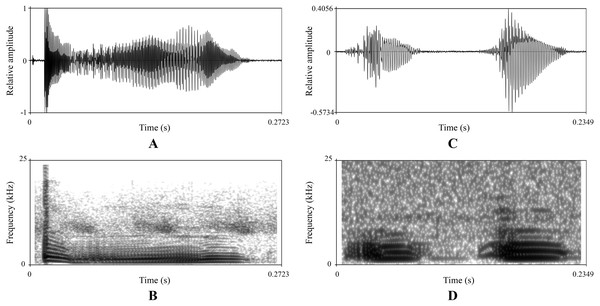
There were obvious differences on sonograms and waveforms of calls between the new taxon (Figs. 5A and 5B) and N. hainanensis (Figs. 5C and 5D). Firstly, the call of the new taxon had only one strophe with one syllable, but the call of N. hainanensis had one strophe containing two syllables. Secondly, the new taxon had a longer time of syllable duration (0.33–0.43 s) than N. hainanensis (0.20–0.25 s). Thirdly, the new taxon had a longer time of interval between syllables duration (8.85–15.77 s) than N. hainanensis (0.13–0.15 s). Finally, the new taxon had a broader frequency range (480–20,640 Hz) than N. hainanensis (440–7,170 Hz).
Figure 5: Advertisement calls of a male Nidirana leishanensis sp. nov. (the holotype CIBLS20170727002) and a male N. hainanensis (specimen CIB20110629003).
(A) and (B) waveform and sonogram showing a strophe of Nidirana leishanensis sp. nov. respectively. (C) and (D) waveform and sonogram showing a strophe of N. hainanensis respectively.In all, molecular phylogenetic analyses, morphological comparisons and bioacoustics comparisons consistently indicated that our specimens from the Leigong Mountain of Guizhou Province, China represented a new taxon of the genus Nidirana. It is described as a new species in the following sections:
Nidirana leishanensis sp. nov.
urn:lsid:zoobank.org:act:72C795CB-8FCF-40B7-9C0F-6F9D04B47C5E
Holotype
CIBLS20170727002, adult male (Figs. 6A–6C, 7A, 7B and 7F), collected by S. Z. Li in the Leigong Mountain National Nature Reserve (26.37533°N, 108.16107°E; elevation 1,298 m a.s.l.), Leishan County, Guizhou Province, China on 27 July 2017.
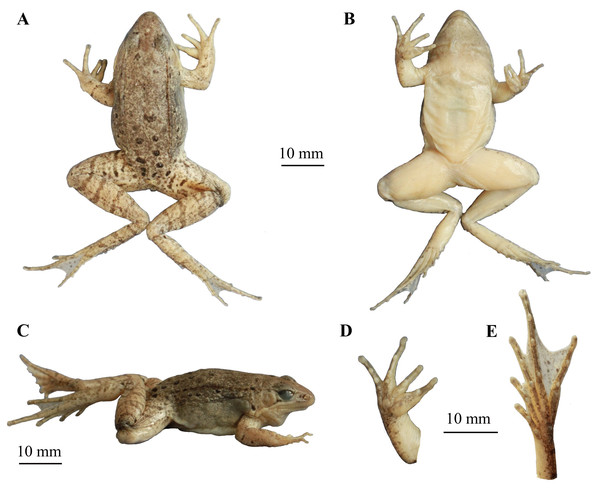
Figure 6: The holotype specimen (CIBLS20170727002) of Nidirana leishanensis sp. nov.
(A) Dorsal view. (B) Ventral view. (C) Lateral view. (D) Ventral view of hand. (E) Ventral view of foot. Photographed by Shize Li.Figure 7: Photos of the holotype CIBLS20170727002 of Nidirana leishanensis sp. nov. in life.
(A) Dorsal view. (B) Ventral view. (C) Ventral view of hand. (D) Ventral view of foot. (E) Nuptial pads on the first and second fingers. (F) Lateral view showing subgular external vocal sac. 1, nuptial pad on inner side of base of fingers I and II; 2, a pair of subgular internal vocal sac at corners of throat; 3, suprabrachial gland. Photographed by Shize Li.Paratypes
A total of nine adult specimens (five males and four females) collected by S. Z. Li from Leigong Mountain National Nature Reserve in Leishan County, Guizhou Province, China. Five male specimens: CIBLS20150628001, CIBLS20150628002, CIBLS20150628005, CIBLS20150628006 and CIBLS20150628007 collected on 28 June 2015; four female specimens: CIBLS20150627001, CIBLS20150627002 and CIBLS20150627003 collected on June 27, 2015 and CIBLS20170727001 on 27 July 2017. Seven tadpoles (CIBLS20180826001–CIBLS20180826007) collected by S. Z. Li on 6 August 2018 in the same site.
Diagnosis
Nidirana leishanensis sp. nov. is assigned to the genus Nidirana based on molecular data and the following morphological characters: disks of digits dilated, pointed; lateroventral grooves present on digits; feet full webbed or half webbed; dorsolateral folds distinct; the presence of large suprabrachial gland in males; nuptial pad present at the base of first finger in males; vocal sacs present in males.
Nidirana leishanensis sp. nov. could be distinguished from its congeners by a combination of the following characters: (1) a large body size in males (SVL > 49 mm); (2) the presence of lateroventral grooves both on fingers and toes; (3) relative finger lengths: II < IV < I < III; (4) tibiotarsal articulation reaching to the level between eye and nostril when leg stretched forward; (5) a pair of subgular internal vocal sacs at corners of throat present in males; (6) the presense of nuptial pad on the inner side of base of fingers I and II in males; (7) webbing formula: I 2 –2 II 2 –2 III 3 –3 IV 3 –3V.
Description of holotype
Body size large, SVL 55.8 mm; head longer than wide (HDL/HDW = 1.08), flat above; snout round, slightly projecting beyond lower jaw in ventral view; snout and tympanum to axial region, forming a maxillary gland in posterior corner of mouth, behind the gland a soya bean size gland present; supratympanic fold absent; interorbital space narrower than internarial distance (IND/IOD = 1.4); eye large and convex, ED 0.78 times of SL; tympanum round, distinct, and close to eye; vomerine teeth on well-developed ridges; tongue deeply notched posteriorly; a pair of subgular internal vocal sacs at corners of throat.
Forelimbs moderately robust (LW/SVL = 0.11); lower arm and hand less than a half of body length (LAL/SVL = 0.44); relative finger lengths: II < IV < I < III; tip of fingers weakly dilated, forming elongated and pointed disks; lateroventral grooves on the disks of finger; fingers free of webbing, with lateral fringes; subarticular tubercles and supernumerary tubercles below the base of finger present, and subarticular tubercles prominent; palmar tubercles three, elliptic, distinct.
Hindlimbs relatively robust, tibia 54% of SVL; heels overlapping when hindlimbs flexed at right angles to axis of body; tibiotarsal articulation reaching the level between eye to nostril and closer to eye when leg stretched forward; toes long and thin, relative toe lengths: I < II < V < III < IV; tip of toes dilated, forming significantly elongated disks; distinct lateroventral grooves on toes; webbing moderate, webbing formula: I 2 –2 II 2 –2 III 3 –3 IV 3 –3V; toes with lateral fringes; subarticular tubercles oval, prominent; inner metatarsal tubercle elliptic, prominent; outer metatarsal tubercle absent.
Dorsal skin of head and anterior part of body smooth, posterior part and flanks with several tubercles, on some tubercles with black spot; a large suprabrachial gland behind base of forelimb; dorsolateral fold extending from posterior margin of upper eyelid to above groin; several granules on the dorsal surfaces of thigh, tibia and tarsus; ventral surface of head, body and limbs smooth, several flattened tubercles densely arranged on the rear of thigh and around vent.
Coloration in life
Dorsal surface and suprabrachial gland olive; flank light yellow; several black spots on flank and dorsum; a light yellow streak from posterior head to cloacae discontinuously; dorsal forelimbs reddish brown and one brown stripe in front of the base of forelimb; dorsal hindlimb reddish brown, two brown bands on the thigh, four on the tibia and three on the tarsus; tympanum and temporal region light yellow; maxillary gland white; ventral surface smooth, throat and ventral of thigh and forelimbs incarnadine, belly and chest light yellow; lips incarnadine and lower jaw brownish white (Fig. 7).
Color in preservative
Dorsal surface faded to brown; black spots on dorsum and flank more distinct; limbs faded light brown and the crossbars becoming clearer; ventral surface faded to pale cream (Fig. 6).
Variation
Measurements and basic statistics of adult specimens were presented in Table S1 and Table S4, respectively. All specimens were similar in morphology but some individuals different from the holotype in color pattern. In some adult males, the dorsal coloration was pale brown (Fig. 8A); in some adult males, the dorsal coloration was olive-grey without black spots on the posterior of dorsum, and the gland was smaller and obvious (Fig. 8B); in some adult males, the dorsolateral folds not continuous and split from the middle dorsum (Fig. 8C); in some adult females, dorsal coloration was brown with brownish red (Fig. 8D); in some adult females, dorsal coloration was olive-grey and the black spots on dorsum was more intensive (Fig. 8E); in some adult females, the ventral surface was creamy white (Fig. 8F).
Figure 8: Color variations in Nidirana leishanensis sp. nov. in life.
(A), (B) and (C) dorsolateral view of the male specimens CIBLS20150628001, CIBLS20150628005 and CIBLS20150628007, respectively. (D) and (E) dorsolateral view of the female specimens CIBLS20150627002 and CIBLS20150627003, respectively. (F) ventral view of the female specimen CIBLS20150627002. Photographed by Shize Li.Skull
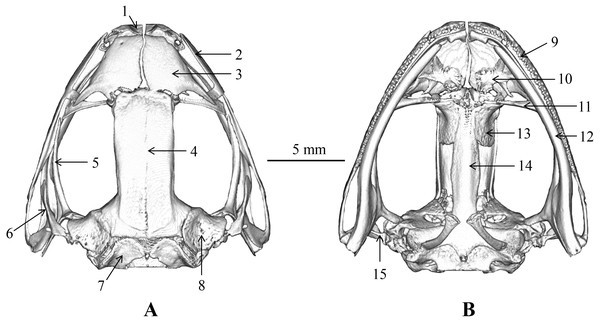
The skull morphology of the five scanned specimens were almost identical, and thus, only one representative (voucher number: CIBLS20170727002) was presented (Fig. 9) and described as following: skull flat, maxillary teeth well developed, vomerine teeth present; mandible without teeth; a pair of premaxillas, separated from each other and connects with nasal; nasals large, separated from each other, and connects with the sphenethmoid; sphenethmoid tubular, forms the anterolateral walls of the braincase; frontoparietal roof the braincase and wider anteriorly than posteriorly; prootic large and separated from the exoccipital; a pair of exoccipitals situated the end of the brain; palatines arcuate and long, behind the prevomers; a pair of prevomers obliquely lie anterior to the palatines, vomerine teeth on the prevomers distinct; parasphenoid in sword shape, supports the braincase ventrally, connects with palatines; a pair of squamosals on the dorsolateral side of the prootic, each squamosal consists of three rami: the zygomatic ramus, the otic ramus, and the ventral ramus; zygomatic ramus pointing to the orbit, otic ramus shorted, ventral ramus outboard and covered the posterior of pterygoid; a pair of pterygoids, outside of the ventral surface of the squamosal, anterior ramus is the longest, center is inward and leading edge extends to the orbit, medial ramus shorted and attached to the anterior lateral part of the prootic, posterior ramus edge-on and extend to maxillary; a pair of columellaes situated ventral to the crista parotica (Fig. 9).
Figure 9: Skull of Nidirana leishanensis. sp. nov., the holotype CIBLS20170727002.
(A) Dorsal view. (B) Ventral view. 1, premaxilla; 2, maxillary; 3, nasal; 4, frontoparietal; 5, pterygoid; 6, squamosal; 7, exoccipital; 8, prootic; 9, maxillary teeth; 10, prevomer; 11, palatine;12, mandible; 13, sphenethmoid; 14, parasphenoid; 15, columella. Photographed by Shize Li.Tadpole description
Measurements of the seven tadpole specimens were presented in Table S2. All specimens of tadpole were similar in morphology and color pattern, the following tadpole description is based on a single specimen (CIBLS20180826007) at Stage 31 (Fig. 10), which was confirmed as Nidirana leishanensis sp. nov. by the molecular analyses (Figs. 2 and 3).
Figure 10: Tadpole of Nidirana leishanensis. sp. nov., the specimen CIBLS20180826007, in life.
(A) Dorsal view. (B) Lateral view. (C) Ventral view. (D) Mouth structure. 1, Spiracle; 2, lower keratodonts; 3, upper keratodonts; 4, labial papillae on lower lips; 5, labial papillae on upper lips; 6, additional tubercles at the angles of mouth. Photographed by Shize Li.Body oval, body and tail yellowish-brown, flattened above; several brown spots on dorsum; eyes lateral, nostril near snout; spiracle on left side of body, directed dorsoposteriorly; keratodont formula I: 1–2/II: 1–1; ventral of body oval, creamy white; both upper and lower lips with labial papillae; some additional tubercles at the angles of the mouth, usually with small keratodonts; TOL 38.3 mm; tail fusiform, approximately 2.0 times as long as snout-vent length, tail height 21.3% of tail length; dorsal fin arising behind the origin of the tail; maximum depth near mid-length, and larger than body depth; body width longer than body height (BW/ BH = 1.5); several brown spots on the dorsal of body (Fig. 10).
Vocalization
The sonograms and waveforms are shown in Figs. 5A and 5B respectively. The call has only one strophe with one syllable. The syllable has a duration of 0.33–0.43 s (mean ± SD: 0.37 ± 0.053 s, N = 10). The interval between syllables has a duration of 8.85–15.77 s (mean ± SD: 12.31 ± 4.89 s, N = 9). Amplitude modulation within strophe is apparent, beginning with highest energy pulses, decreasing slightly to a minimum then increase to mid strophe approximately, subsequently increasing to a peak then decreasing rapidly and then increasing to another peak and subsequently decreasing towards the end of each strophe. Calls have a broad frequency range of 480–20,640 Hz.
Secondary sexual characteristics
A pair of subgular internal vocal sacs, a pair of slit-like openings at posterior of jaw (Fig. 7F); linea musculina present on dorsum; incarnadine nuptial pad on the inner side of base of fingers I and II (Fig. 7E); nuptial spinules invisible; suprabrachial gland present.
Comparisons
Nidirana leishanensis sp. nov. differs from N. chapaensis, N. nankunensis and N. okinavana by having larger body size in males (SVL > 49 mm in the new species vs. SVL < 43 mm in the latter).
Nidirana leishanensis sp. nov. differs from N. pleuraden by the presence of lateroventral grooves both on fingers and toes (vs. absent in the latter).
Nidirana leishanensis sp. nov. differs from N. chapaensis, N. daunchina, N. lini, N. nankunensis, N. okinavana and N. pleuraden by the relative finger lengths II < IV < I < III (vs. II < I < IV < III or II < I = IV < III in the latter).
Nidirana leishanensis sp. nov. differs from N. chapaensis, N. daunchina, N. lini and N. nankunensis by tibiotarsal articulation reaching the level between eye to nostril when leg stretched forward (vs. reaching nostril or beyond snout in the latter).
Nidirana leishanensis sp. nov. differs from N. okinavana by having a pair of subgular internal vocal sacs in males (vs. gular vocal sacs absent in the latter).
Nidirana leishanensis sp. nov. differs from N. daunchina, N. lini, N. nankunensis, N. okinavana and N. pleuraden by having nuptial pad on the inner side of base of fingers I and II in males in breeding season (vs. nuptial pad only on the finger I in males in the latter).
Nidirana leishanensis sp. nov. differs from N. daunchina, N. lini and N. nankunensis by the webbing formula I 2 –2 II 2 –2 III 3 –3 IV 3 –3V (vs. I 2 –2 II 2 –III 2 –3 IV 3 –2V in N. daunchina, I 2 –II 2 –2 III 3 –3 IV 3 –3 V in N. lini and I 2 –2 II 1 –3 III 2 –3 IV 3 –2 V in N. nankunensis).
Nidirana leishanensis sp. nov. is superficially resembling N. adenopleura on morphology but can be distinguished from the latter by a series of morphological characters as follows: the relative finger lengths II < IV < I < III (vs. II < I < IV < III); having nuptial pad on the inner side of base of fingers I and II in males in breeding season (vs. nuptial pad only on the finger I in males); tibiotarsal articulation reaching the level between eye to nostril when leg stretched forward (vs. reaching the eye); webbing formula: I 2 –2 II 2 –2 III 3 –3 IV 3 –3V (vs. I 2 –2 II 2 –3 III 3 –4 IV 4 –3V).
Nidirana leishanensis sp. nov. is genetically most closer to N. hainanensis. It differs from N. hainanensis by the following characters: having larger body size in males (SVL 49.5–56.4 mm in the new species vs. SVL 32.8–33.5 mm in N. hainanensis); having nuptial pad on the inner side of base of fingers I and II in males in breeding season (vs. nuptial pad absent in breeding season); tibiotarsal articulation reaching the level between eye to nostril when leg stretched forward (vs. reaching nostril); the webbing formula: I 2 –2 II 2 –2 III 3 –3 IV 3 –3V (vs. I 2 –II 2 –2 III 3 –3 IV 3 –3V); having significantly lower ratios of SL, IOD, UEW, ED, TYD, HAL and HLL to SVL in males and having significantly higher ratios of LW to SVL in males (all p-values < 0.05; Table 3).
Ecology
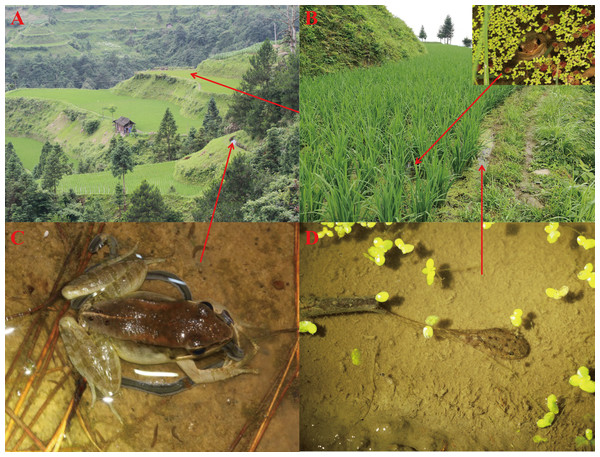
Nidirana leishanensis sp. nov. is currently known only from Leigong Mountain (26.25°N–26.53°N, 108.08°E–108.41°E) in Leishan County, Guizhou Province, China (Fig. 1). Nidirana leishanensis sp. nov. inhabited the paddy field or nearby the artificial trench where the water flows very slowly (Fig. 11A), at elevations from 650 to 1,300 m a. s. l., and the individuals could be found on the ridge of paddy field or in the paddy field (Figs. 11A–11C). The tadpoles of the species could be found in the water of ponding in the paddy (Fig. 11D). The species could eat some small mollusks for food (Fig. 11C). Two sympatric amphibian species, Microhyla fissipes (Boulenger, 1884) and Polypedates megacephalus (Hallowell, 1861 “1860”) were found in the type locality.
Figure 11: Habitats of Nidirana leishanensis. sp. nov. in the type locality, Leigong Mountain, Leishan County, Guizhou Province, China.
(A) Landscape. (B) A paddy field with a Nidirana leishanensis sp. nov. in the water (insert). (C) A frog eating a small mollusk for food. (D) A tadpole swimming in the paddy field. Photographed by Shize Li.Etymology
This specific epithet “leishanensis” is a Latinize toponymic adjective that refers to Leigong Mountains, Leishan County, Guizhou Province, China, where the new species was collected. For the common name, we suggest Leishan Music Frog (English) and Lei Shan Qin Wa (Chinese).
Discussion
There has been a lot of controversy on systematic profiles of the genus Nidirana. At first, some populations in this genus had been recognized as separated species, such as “psaltes” and “caldwelli” which were suggested to be the subjective junior synonym of “okinavana” (Matsui, 2007) and “adenopleura” (Lyu et al., 2017), respectively. Next, species assignments of some populations were suggested to be misguided. For example, several populations in the southwestern corner of Sichuan Province and Chongqing City and northwestern part of Guizhou Province of China, previously ever recognized as N. adenopleura, were later identified as N. daunchina (Fei, Ye & Huang, 1990; Fei et al., 2009; Fei & Ye, 2005); and the recently described species (N. nankunensis) had also been assigned to N. adenopleura, although molecular phylogenetic analyses indicated that the two species were paraphyly. Similarly, the new species, Nidirana leishanensis sp. nov., were misidentified as N. adenopleura though the population had been found for many decades (Fei et al., 2009; Wu, Dong & Xu, 1986). It is assumed that morphological similarity of the related species in the genus much probably led to the misclassifications. Thus, integrative taxonomy with multiple evidences especially molecular phylogenetic reconstructions that have become the main trend and has been proved to be quite effective (e.g., Lyu et al., 2017; Li et al., 2018) is expected to be applied to resolve the taxonomic confusions of the genus Nidirana. In this study, molecular phylogenetics based on mitochondrial DNA and nuclear DNA indicated that Nidirana leishanensis sp. nov. was an independent clade from its congeners, and the genetic distance between the new species and its sister species (N. hainanensis; mean 5.7% on COI gene) was higher than those between several pairs of sister species of Nidirana and also match the level about interspecific divergences in amphibians (3.1–28.2% on COI gene; Che et al., 2012). Moreover, the distinct genetic separations between the new species and its related species on two nuclear locus implicated very restricted gene flow among these species. Finally, morphological comparisons and bioacoustics comparisons also supported the validity of the new species. As noted, we found that the species are seriously threatened by local villagers and pesticides use in the paddy fields. Hence, it is urgent to make further investigations to evaluate its distributional range and population status of the new species.
Based on currently available information, it could presume that species diversity of the genus Nidirana is still underestimated, requiring future more studies. On one hand, the populations distinctly distributed far from the type locality of its temperately-ranked species should be carefully investigated especially using sophisticated molecular phylogenetic approaches. For examples, the populations previously recognized as N. adenopleura in Fanjing Mountain of Guizhou Province and Dayao Mountain of Guangxi Province in China are expected to be different species because their morphology was supposed to be different from N. adenopleura (Wu, Dong & Xu, 1986; Fei et al., 2009) while the localities were far from the type locality of N. adenopleura (Taiwan Island); and the populations previously assigned to N. lini in northern Thailand and that identified as N. chapaensis in the southern Laos (Chuaynkern et al., 2010) may be undescribed species because of their far and probably isolated distributional areas. On the other hand, further field surveys should be carried out in the poorly studied regions, such as west part of Guizhou-west part of Guangxi provinces of China, Yunnan Province of China and tropical regions along the northeastern Indo-China Peninsula, which are the biodiversity hotspots and are expected to bear much underestimated species diversity (Myers et al., 2000).
Conclusion
We described a new species of the Asian music frog genus Nidirana (Amphibia, Anura, Ranidae) from Southwestern China, and confirmed its phylogenetic placements. Nidirana leishanensis sp. nov. is currently only known from Leigong Mountain, Guizhou Province of China, and inhabits still waters in the mountains at mid and low elevations similar to most music frogs. Although there were still a mass of unresolved systematic assignments and assumed underestimated diversity in the genus Nidirana, the findings here give a better knowledge on species diversity and phylogenetic assignments of the group. Future works are expected mainly on re-examination of the doubtful classifications of proposed-misidentified populations using credible molecular phylogenetic approaches and painstaking field surveys especially in the poorly investigated regions that harboring high species richness.
Supplemental Information
Measurements of the adult specimens of Nidirana leishanensis sp. nov. and N. hainanensis
Unit: mm. See abbreviations for the morphometric measurements in Methods section.
Measurements of the tadpoles of Nidirana leishanensis sp. nov
Unit: mm. See abbreviations for the morphometric measurements in Methods section.
The best partition schemes and the best-fit substitution model for each partition selected by the program PARTITIONFINDER for the concatenated data of three mitochondrial genes and the concatenated data of three mitochondrial genes and two nuclear genes
Maximum likelihood (ML) tree of Nidirana leishanensis sp. nov. and the related species reconstructed based on the concatenated sequences of the mitochondrial 12S, 16S and COI genes
Bootstrap supports from ML analyses/Bayesian posterior probabilities from BI analyses were labeled beside nodes. See information of samples 1-31 in Table 1.