Extraction and characterisation of pigment from Yanzhiguo [Prunus napaulensis (Ser.) Steud.]
- Published
- Accepted
- Received
- Academic Editor
- Naeem Khan
- Subject Areas
- Agricultural Science, Biochemistry, Plant Science
- Keywords
- Prunus napaulensis (Ser.) Steud, Natural pigment, Response surface methodology, Stability, Antioxidant activity
- Copyright
- © 2023 Shi et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Extraction and characterisation of pigment from Yanzhiguo [Prunus napaulensis (Ser.) Steud.] PeerJ 11:e15517 https://doi.org/10.7717/peerj.15517
Abstract
Yanzhiguo [Prunus napaulensis (Ser.) Steud] belongs to Rosaceae family and is consumed as wild fruit, pulp and juice. However, its potential for extracting natural pigment has not yet been explored. Herein, the components in the fresh Yanzhiguo pulp were preliminarily analyzed by liquid chromatography coupled to mass spectrometry. And, the optimal pre-treatment conditions were established for further extraction of Yanzhiguo pigment based on the a* value. Then, by combining the data from single-factor experiments and response surface methodology, the optimal extraction process was established as: 35% EtOH, a liquid-solid ratio of 200:1 mL g−1, an extraction time of 65 min, and an extraction temperature of 100 °C. Moreover, it was found that the a* value and yield had high fitness except when extracted into ethanol (EtOH) with different concentrations. Meanwhile, our result demonstrated Yanzhiguo pigment had high stability in general environments with carmine (a synthetic pigment) as control, except for extreme environments such as direct (hot) sunlight, high temperature (75 °C) and strong alkaline (pH 11). Also, Yanzhiguo pigment exhibited good antioxidant activity. Our results contribute to more information on Yanzhiguo pigment and promote its application by providing efficient extraction technology.
Introduction
Prunus napaulensis (Ser.) Steud. (commonly known as ‘Yanzhiguo’, ‘Sohiong’, or ‘Khasi cherry’) belongs to Rosaceae family (Rymbai et al., 2016) and is widely distributed in temperate region of China, India, Nepal, etc. Despite its notable nutritional and medicinal value, this species is underutilized; it remains in a transitional stage between wild and cultivated statuses (Srivastava et al., 2022). Wild Yanzhiguo fruit is sold in local markets in Yunnan (China). Also, Yanzhiguo fruit is traded in the ‘Khasi’ tribe of India trades as their principal non-food forest product and and used as a traditional medicine (Agrahar-Murugkar & Subbulakshmi, 2005). Wild Yanzhiguo is also well adapted to the Himalayan foothills, including the foothills in Nepal and Myanmar (Rymbai et al., 2016). The ripe fruit is dark purple in color, sour in flavour, and has a single stone-like seed. The fruit is often boiled or brewed to make jam or wine. The brew is bright purple-red with a sweet-and-sour taste, similar to red wine; the brew strengthens the spleen and aids digestion. Moreover, the brew contains biologically active carotenes, anthocyanins, and vitamin C, which enhance immunity, delay ageing, and exert anti-cancer effects (Boo et al., 2012; Anantharaman et al., 2014; Chaudhuri et al., 2015; Shakeri et al., 2017; Zhang et al., 2019b).
Synthetic pigments are commonly used in the food industry. However, such pigments may be of low nutritional value and cause side effects (Ardila-Leal et al., 2021). Long-term consumption of synthetic pigments may even lead to cancer and deformity (Novais et al., 2022). Human health has occasionally been affected by the misuse of industrial pigments; the ‘Sudan Red Incident’ in the United Kingdom is the most sensational example (Ou, Zhong & Li, 2017). Natural pigments derived from plants, animals or microorganisms have absorbed more attraction, which are non-toxic, have high nutritional value, and exert valuable biological activities (Li et al., 2021a). Using natural pigments to replace the synthetic pigments is the current trend. For examples, chlorophyll, curcumin, and astaxanthin have been used as sensitisers, color additives, and antioxidants in the food industry (Li et al., 2021a). Ripe Yanzhiguo fruit has abundant bright purple-red; these may promote its applications in the food, textile, and chemical industries. Ultrasound, enzyme systems, and maceration are used to extract pigments from other natural materials (Catalkaya & Kahveci, 2019; Linares & Rojas, 2022; Wojdyło, Samoticha & Chmielewska, 2021). However, there is currently a limited understanding of Yanzhiguo pigment extraction, stability, and biological activity; only a few researchers have investigated Yanzhiguo (Gao et al., 2020; Nie et al., 2021; Zhang et al., 2019b).
Here, we performed liquid chromatography coupled to mass spectrometry (LC-MS) analysis to determine the components in fresh Yanzhiguo pulp and establish the optimal pre-treatment condition to improve the a* value of Yanzhiguo pigment. Moreover, the extraction process was optimized by combining the data from single-factor experiments with response surface methodology (RSM). The optimal extraction condition was 35% EtOH, a liquid-solid ratio of 200:1 mL g−1, an extraction time of 65 min, and an extraction temperature of 100 °C. Further, we found strong fitness between the a* values and pigment yields except under different EtOH concentration. Meanwhile, the stability of Yanzhiguo pigments was similar or even stronger than that of synthetic pigment carmine except under extreme conditions (e.g., direct sunlight, high temperature, and pH 11). Also, upon extraction into 35% EtOH or water, Yanzhiguo pigment exhibited antioxidant activity. Our research would contribute to know more on Yanzhiguo pigment and promote its application by providing efficient extraction technology.
Materials and Methods
Materials, chemicals, and instruments
Materials
Fresh Yanzhiguo fruits at ripe stage were purchased from a market in Tengchong City, Yunnan Province, China and stored at –20 °C.
Chemicals
Analytical grade EtOH (95% v/v) was purchased from Sichuan Xilong Science Co., Ltd.; 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical-scavenging test kits were purchased from Suzhou Keming Biotechnology Co., Ltd.; pure water was prepared using a secondary reverse osmosis system (model JYS-500L; Shanghai Juyuan Automation Technology Co., Ltd., Shanghai, China).
Instruments
Following instruments were included in this study: an ultra-high performance liquid chromatograph (Exion; Sciex Co., Ltd., Framingham, MA, USA), a high-sensitivity mass spectrometer (QTRAP 6500+; Sciex, Framingham, MA, USA), an ultra-performance liquid chromatography (UPLC) column (Acquity UPLC HSS T3, 100 mm × 2.1 mm × 1.8 μm; Waters Co., Ltd., Milford, MA, USA), a desktop colorimeter (CM-5; Minolta Co., Ltd., Osaka, Japan), a spectrophotometer (UV-1800PC UV/Vis; Shanghai Meipuda Instrument Co., Ltd., Shanghai, China), a desktop electric fan-drying oven (HGZF-9053; Shanghai Yuejin Medical Equipment Co., Ltd., Shanghai, China), a 1/10,000 balance (AL204-IC; Mettler Toledo Instruments (Shanghai) Co., Ltd., Shanghai, China), and a refrigerated centrifuge (HR/T20MM; Hunan Hercy Instrument Equipment Co., Ltd., Changsha, China).
Mass spectrometry analysis
After lyophilisation and grinding (45 Hz, 30 s), 50 mg of sample was added to 700 μL of extraction solution, then vortexed for 30 s, homogenised at 35 Hz for 4 min, and sonicated in an ice-water bath for 5 min. This process was repeated three times; and then the supernatants were collected, held at 4 °C overnight, and centrifuged for 15 min (4 °C, 12,000 rpm). The supernatants were then passed through a membrane (pore size, 0.22 μm), diluted fivefold with extraction solution, vortexed for 30 s, and then stored at –80 °C. Mass spectrometry analysis was conducted using a QTRAP 6500+ triple quadrupole mass spectrometer with an IonDrive Turbo V electrospray ionisation ion source operating in the multiple reaction monitoring mode. The IonSpray ion source parameters were: voltage, +5,500/–4,500 V; curtain gas, 35 psi; temperature, 400 °C; ion source gas 1, 1:60 psi; ion source gas 2, 60 psi; and declustering potential, 100 V. The Waters UPLC column dimensions were 100 mm × 2.1 mm × 1.8 µm; gradient elution was conducted with mobile phase A (0.01% (v/v) formic acid) and mobile phase B (acetonitrile) (Table 1). The column temperature was 40 °C, the autosampler temperature was 4 °C, the flow rate was 0.4 mL min−1, and the injection volume was 2 µL.
| Time (min) | Mobile phase A (%) | Mobile phase B (%) |
|---|---|---|
| 0 | 98 | 2 |
| 0.5 | 98 | 2 |
| 10 | 50 | 50 |
| 11 | 5 | 95 |
| 13 | 5 | 95 |
| 13.1 | 98 | 2 |
| 15 | 98 | 2 |
Pre-treatment
After Yanzhiguo has been cleaned under running water and the stone has been removed, it was added to pure water at various liquid:solid ratios. Then, the mixtures were boiled at 800 W for 5 min; cooled at room temperature; dried successively at 40 °C, 60 °C, or 80 °C; and powdered. After passage through a 60-mesh sieve, the powder was stored at –20 °C. More details are shown in Table 2.
| Number | Sample code | Material-liquid ratio (g/mL) | Power (w) | Heating time (min) | Drying temperature (°C) |
|---|---|---|---|---|---|
| 1 | YZG0-1 | / | / | / | 40 |
| 2 | YZG0-2 | / | / | / | 60 |
| 3 | YZG0-3 | / | / | / | 80 |
| 4 | YZG1-1 | 10 | 800 | 5 | 40 |
| 5 | YZG1-2 | 10 | 800 | 5 | 60 |
| 6 | YZG1-3 | 10 | 800 | 5 | 80 |
| 7 | YZG2-1 | 5 | 800 | 5 | 40 |
| 8 | YZG2-2 | 5 | 800 | 5 | 60 |
| 9 | YZG2-3 | 5 | 800 | 5 | 80 |
Optimization of extraction process via RSM single-factor experiments
The single-factor experiments explored with four factors including the solvents, liquid-solid ratios, times, and temperatures. The solvents were pure water, 20%, 35%, 50%, and 65% EtOH; the liquid-solid ratios were 25:1, 50:1, 100:1, 200:1, and 300:1 mL/g; the extraction times were 30, 60, 90, 120, and 150 min; and the extraction temperatures were 30 °C, 45 °C, 60 °C, 80 °C, 90 °C, and 100 °C. The pigment yield was determined according to the absorbance at 277 nm.
Optimization of extraction
To optimized extraction process, a four-factors-at-three levels Box-Behnken combination was constructed using Design-Expert ver. 12 software. The ranges of variables investigated are shown in Table 3: EtOH, 20–50%; liquid-solid ratio, 100:1 to 300:1 mL g−1; extraction time, 30–90 min; and extraction temperature, 80–100 °C.
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A: EtOH volume fraction (%) | 20 | 35 | 50 |
| B: Liquid-solid ratio (mL/g) | 100 | 200 | 300 |
| C: Extraction time (min) | 30 | 60 | 90 |
| D: Extraction temperature (°C) | 80 | 90 | 100 |
Determination of pigment yield
To determine the pigment yield, absorbance from 200 to 700 nm was investigated as described by Zhang et al. (2019b), who reported that absorption peaked at 277 nm. Thus, the absorbance at 277 nm was measured to estimate the yield after appropriate dilution.
Determination of a* value
A desktop colorimeter with a D65 light source and a 10° measurement angle was used to measure a* value. The a* value was measured after the instrument was calibrated using a black-and-white board and a specular reflection light was excluded.
Stability analysis
The pigment was stored under different light, temperature, and pH conditions using carmine as the control. The light conditions were natural indoor light, ultraviolet light, normal incandescent light, strong and direct sunlight, and darkness, respectively. The temperatures were 4 °C, 25 °C, 50 °C and 75 °C, respectively. The pH values were 3, 5, 7, 9, and 11, respectively. The absorbance at 277 nm and the a* value served as indicators, which were determined one time every 1 h in 5 h. And to know pH stability, the time pointed of 24 and 48 h was added.
Antioxidant analysis
The dried powder of Yanzhiguo fruit in suitable weight was prepared to measure ABTS and DPPH free radical-scavenging abilities. Test kits (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China) were used in accordance with the manufacturer’s instructions. The scavenging rates were calculated based on the reduction of ABTS or DPPH radicals after and before adding the Yanzhiguo sample in reaction system with Trolox as standard. All the results were expressed in µmol Trolox per gram of dried Yanzhiguo powder (µmol Trolox g−1 DW).
Statistical analysis
All the measurements of in the whole manuscript were performed in triplicate with the results presented as the mean standard deviation (SD, n = 3). The factor effect in pre-treatment and the single-factor experiment was analyzed using GraphPad Prism version 8 software (GraphPad Software Inc., San Diego, CA, USA. And the three-dimensional plots of response surface and analysis of variance (ANOVA) was drawn and performed by Design Expert ver 12 software.
Results
Chromogenic components of Yanzhiguo fruit
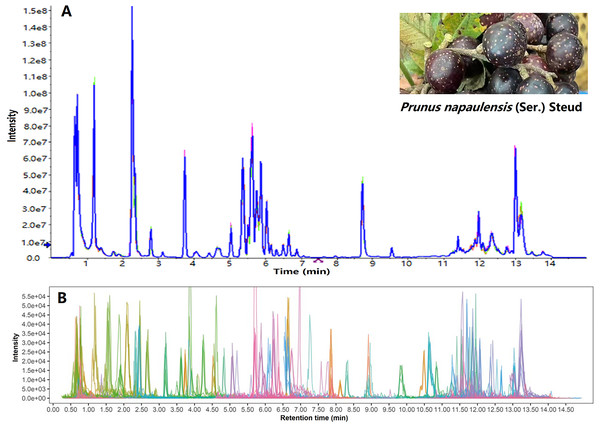
A total ion chromatogram was created by summing the intensities of all mass spectral peaks of the same scan (Fig. 1A); and then the corresponding extracted ion chromatogram is shown in Fig. 1B. More than 700 components were found in Yanzhiguo fruit, including flavonoids, terpenes, alkaloids, phenols, quinones, and organic acids. According to the structure, chromogenic components were found including tetrapyrrole derivatives (such as chlorophyll) (Shinozaki et al., 2020), isoprenes (such as carotenoids) (Li et al., 2020), polyphenols (such as anthocyanins and flavonoids) (Chen et al., 2017), quinones (such as cochineal), and ketones (such as curcumin) (Li et al., 2021b; Duval et al., 2016). Both the total ion chromatogram and extracted ion chromatogram showed that Yanzhiguo contained abundant polyphenols, isoprenes, ketones, and quinones. Among them, flavonoids were particularly diverse and abundant. The flavonoids were widely present in red dietary foods such as red cabbage (Boo et al., 2012). As typical flavonoids, anthocyanins including rutin, procyanidin B2, cyanidin, shikonin, delphinidin were plentiful in Yanzhiguo, especially rutin was prominent in peak area (Table 4).
Figure 1: TIC (A) and EIC (B) of P. napaulensis, respectively.
| No. | tR (min) | Formula | Identification | S | No. | tR (min) | Formula | Identification | S |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.56 | C15H10O3 | 7-Hydroxyflavone | 103,503.80 | 54 | 7.38 | C15H10O4 | 7,8-Dihydroxyflavone | 5,582.76 |
| 2 | 2.80 | C12H14O2 | Ligustilide | 13,298.85 | 55 | 7.38 | C15H10O4 | Alizarin 2-methyl ether | 1,904.67 |
| 3 | 3.43 | C7H6O4 | Protocatechuic acid | 350,886.05 | 56 | 7.59 | C15H10O6 | 2′-Hydroxygenistein | 6,211.75 |
| 4 | 4.60 | C10H10O4 | Methyl caffeate acid | 36,769.36 | 57 | 7.90 | C15H12O6 | Eriodictyol | 4,510.78 |
| 5 | 4.60 | C11H14O3 | Zingerone | 43,988.09 | 58 | 7.91 | C16H12O5 | Glycitein | 2,441.65 |
| 6 | 4.78 | C30H26O12 | Procyanidin B2 | 234,636.82 | 59 | 8.05 | C15H10O7 | Tricetin | 106,666.71 |
| 7 | 4.86 | C27H30O15 | Meloside A | 6,247.48 | 60 | 8.07 | C15H10O7 | Quercetin | 7,760.85 |
| 8 | 4.90 | C25H24O6 | Morusin | 30,097.82 | 61 | 8.12 | C15H10O6 | Scutellarein | 525.76 |
| 9 | 4.91 | C20H18O5 | Demethoxycurcumin | 19,203.14 | 62 | 8.16 | C28H34O14 | Poncirin | 2,384.04 |
| 10 | 4.91 | C27H30O15 | Vitexin 2″-glucoside | 6,247.48 | 63 | 8.17 | C30H26O13 | Buddlenoid A | 56,757.48 |
| 11 | 5.08 | C15H14O6 | (+)-Epicatechin | 250,710.61 | 64 | 8.37 | C15H18O3 | Curcolone | 3,035.95 |
| 12 | 5.08 | C15H14O6 | l-Epicatechin | 12,351.34 | 65 | 8.73 | C11H8O3 | Plumbagin | 5,571.71 |
| 13 | 5.10 | C15H12O2 | 2-Benzal-4-hydroxyacetophenone | 19,125.80 | 66 | 8.74 | C10H6O3 | Juglone | 17,985.76 |
| 14 | 5.14 | C16H16O5 | Shikonin | 10,319.68 | 67 | 8.78 | C15H12O5 | Chalconaringenin | 10,978.40 |
| 15 | 5.17 | C11H12O4 | Trans-3,5-dimethoxy-4-hydroxy cinnamaldehydee | 43,787.25 | 68 | 8.90 | C15H10O5 | Sulfuretin | 5,918.77 |
| 16 | 5.32 | C27H30O15 | Chrysoeriol 7-apiosylglucoside | 6,568.61 | 69 | 8.98 | C15H10O5 | 3′,4′,7-Trihydroxyisoflavone | 10,247.60 |
| 17 | 5.42 | C15H20O8 | Anisatin | 9,400.23 | 70 | 8.98 | C15H10O5 | Pelargonidin | 5,918.77 |
| 18 | 5.43 | C21H20O11 | Homoorientin | 16,362.39 | 71 | 9.06 | C15H10O5 | Apigenin | 1,923.59 |
| 19 | 5.56 | C21H20O11 | Luteolin-6-C-glucoside | 45,894.98 | 72 | 9.09 | C15H10O5 | Genistein | 3,781.94 |
| 20 | 5.56 | C21H20O11 | Orientin | 45,894.98 | 73 | 9.23 | C16H12O7 | Isorhamnetin | 8,373.62 |
| 21 | 5.57 | C22H22O10 | Glycitin | 6,775.70 | 74 | 9.36 | C16H14O6 | Hesperetin | 1,860.71 |
| 22 | 5.79 | C28H32O15 | Spinosin | 7,926.89 | 75 | 9.39 | C15H10O6 | Kaempferol | 3,375.43 |
| 23 | 5.85 | C27H30O16 | Rutin | 11,104,795.03 | 76 | 9.65 | C15H12O4 | Liquiritigenin | 5,529.71 |
| 24 | 5.85 | C21H20O10 | Isovitexin | 20,736.10 | 77 | 9.73 | C15H12O4 | Isoliquiritigenin | 7,827.86 |
| 25 | 5.90 | C15H10O6 | Cyanidin | 16,838.74 | 78 | 9.78 | C30H18O10 | Amentoflavone | 849.04 |
| 26 | 5.92 | C21H20O10 | Vitexin | 20,736.10 | 79 | 9.88 | C16H12O4 | Tectochrysin | 4,827.77 |
| 27 | 5.92 | C21H18O13 | Quercetin-3-O-glucuronide | 50,209,847.26 | 80 | 9.94 | C16H14O5 | Sakuranetin | 6,604.56 |
| 28 | 5.94 | C27H30O14 | Daidzein-4′,7-diglucoside | 13,839.33 | 81 | 10.34 | C20H20O7 | Sinensetin | 10,548.33 |
| 29 | 5.95 | C21H20O10 | Afzelin | 3,374.06 | 82 | 10.57 | C6H10OS2 | Allicin | 12,256.20 |
| 30 | 5.97 | C21H22O11 | Neoastilbin | 206,025.08 | 83 | 10.80 | C18H16O5 | Trimethylapigenin | 13,321.98 |
| 31 | 5.99 | C15H12O6 | Okanin | 44,838.49 | 84 | 11.10 | C16H12O5 | Genkwanin | 12,339.84 |
| 32 | 6.06 | C21H20O12 | Isoquercitrin | 10,644,641.98 | 85 | 11.14 | C15H10O5 | Galangin | 6,356.60 |
| 33 | 6.08 | C27H30O15 | Cyanidin 3-rutinoside;biorobin | 931,695.63 | 86 | 11.21 | C16H12O5 | Biochanin A | 31,696.78 |
| 34 | 6.10 | C21H20O11 | Cynaroside | 2,233,728.66 | 87 | 11.21 | C15H12O6 | Aromadendrin | 8,945.25 |
| 35 | 6.10 | C21H20O12 | Quercetin-7-O-β-D-glucopyranoside | 26,544.83 | 88 | 11.24 | C15H22O2 | Valerenic acid | 12,995.02 |
| 36 | 6.20 | C16H14O5 | Brazilin | 149,867.89 | 89 | 11.58 | C15H10O8 | Quercetagetin | 19,662.80 |
| 37 | 6.22 | C21H20O12 | Isoquercitrin | 161,096.90 | 90 | 11.73 | C16H14O8 | Cedeodarin | 5,944.74 |
| 38 | 6.28 | C15H12O7 | Taxifolin | 1,124,018.51 | 91 | 11.80 | C20H16O5 | Psoralidin | 33,941.29 |
| 39 | 6.29 | C27H30O15 | Kaempferol-3-O-rutinoside | 1,605,609.99 | 92 | 11.83 | C15H10O5 | Norwogonin | 6,420.40 |
| 40 | 6.38 | C28H32O16 | Narcissoside | 41,189.10 | 93 | 11.92 | C30H18O10 | 3,8’-Biapigenin | 13,583.95 |
| 41 | 6.50 | C27H30O14 | Rhoifolin | 9,368.59 | 94 | 11.93 | C30H18O10 | Hinokiflavone | 13,583.95 |
| 42 | 6.51 | C15H10O6 | Demethoxycapillarisin | 10,548.64 | 95 | 12.08 | C16H12O7 | Pedalitin | 30,829.35 |
| 43 | 6.51 | C16H16O4 | 9-Methoxy-alpha-lapachone | 10,986.33 | 96 | 12.15 | C20H26O4 | Carnosol | 1,072.05 |
| 44 | 6.53 | C16H14O5 | Isosakuranetin | 90,176.83 | 97 | 12.30 | C20H24O9 | Torachrysone 8-O-glucoside | 5,788.72 |
| 45 | 6.53 | C15H10O6 | 2′,3,5,7-Tetrahydroxyflavone | 69,190.10 | 98 | 12.44 | C21H34O4 | 10-Gingerol | 10,945.94 |
| 46 | 6.59 | C27H30O16 | Quercetin 3-O-neohesperidoside | 24,295.07 | 99 | 12.82 | C33H24O10 | Sciadopitysin | 5,648.40 |
| 47 | 6.81 | C28H34O15 | Hesperidin | 17,681.56 | 100 | 12.92 | C23H22O6 | Rotenone | 8,897.85 |
| 48 | 6.83 | C15H10O7 | Robinetin | 7,545.52 | 101 | 12.98 | C19H22O2 | Miltirone | 1,168,598.94 |
| 49 | 6.84 | C15H10O7 | 6-Hydroxykaempferol | 1,248.86 | 102 | 13.01 | C20H20O5 | Desmethylxanthohumol | 13,824.34 |
| 50 | 6.86 | C15H10O7 | Delphinidin | 7,400.09 | 103 | 13.09 | C24H26O6 | 3-Isomangostin | 7,731.58 |
| 51 | 7.03 | C15H12O6 | Fisetin | 7,090.42 | 104 | 13.16 | C20H34O4 | Kirenol | 18,970.57 |
| 52 | 7.05 | C15H10O8 | Myricetin | 3,728.17 | 105 | 13.56 | C40H52O4 | Astaxanthin | 1,681.85 |
| 53 | 7.26 | C21H18O11 | Baicalin | 37,088.10 | 106 | 13.56 | C24H28O7 | Garcinone D | 9,963.86 |
Bold values represent the important anthocyanins which might be the fundamental compounds causing Yanzhiguo fruit in dark purple, such as rutin.
Pre-treatment parameters
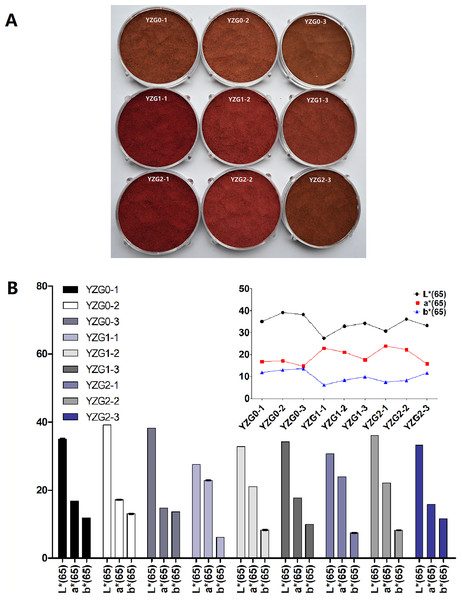
During extraction process, cell walls and cytoplasmic membranes are obstacle of the pigment transfer, which results in only partial extraction, thus appropriate pre-treatment technique was usually established to improve the content of pigments (Krakowska-Sieprawska et al., 2022). Therefore, we established pre-treatment condition to improve the quantity and quality of Yanzhiguo pigments. The appearance (Fig. 2A) and L*, a* and b* value (Fig. 2B) were observed in the pre-treatment experiment. Yanzhiguo powders under different pre-treatment parameters were mainly in the wine-red series, which was apparent to the naked eye (Fig. 2A). The a* value of Yanzhiguo pigment was approximately 20 (Fig. 2B) and more consistent with the appearance displayed in Fig. 2A, compared to L* and b* value (Fig. 2B). The a* value which represents a red-green hue (a positive value indicates redness and a negative value indicates greenness) is related to the vividness of red, such that larger a* value indicates redder color (Belasco et al., 2020). As mentioned in the Introduction, ripe Yanzhiguo fruit is dark purple in color and its juice is bright purple-red; thus, more attention was focused on Yanzhiguo pigments in red. Therefore, to express more intuitively, the a* value was set as indicator to improve the redness of Yanzhiguo pigment. According to Fig. 2B, the Yanzhiguo dried at 60 °C was reddest (a* value = 17.14) without pre-treatment process. However, it was found that the color was redder with a higher a* value after pre-treatment; Yanzhiguo dried at 40 °C before extraction had the highest a* value (22.83). Thus, the drying temperature affected redness and 40 °C was optimal. Meanwhile, it was found that boiling before drying enhanced the redness at different material-liquid ratios. Finally, we observed that the highest redness (a* value = 23.91) was achieved when Yanzhiguo was boiled at 800 W for 5 min at a material-liquid ratio of 5:1 g/mL, then dried at 40 °C. Therefore, the pre-treatment process with above parameters was conducted before further extraction.
Figure 2: The appearance, L* a* and b* values under different pre-treatment parameters.
(A) The appearance of Yanzhiguo powders pre-treat under different parameters, (B) L* a* and b* values.Effects of extraction process variables on yield
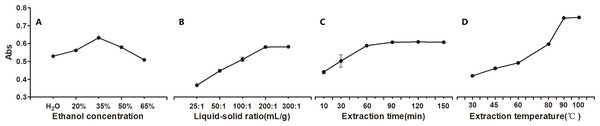
The effects of the four extraction process variables on pigment yield were investigated and are shown in Fig. 3, including the solvent, liquid-solid ratio, time, and temperature. The yield increased and then decreased with EtOH concentration increasing, peaking with the 277 nm absorbance of 0.632 when EtOH was at relatively lower level of 35% (Fig. 3). This finding suggested that EtOH at relatively lower concentration between 20% and 50% should be appropriate. Further, Fig. 3 shows that the yield increased as the liquid-solid ratio increased to 200:1 mL g−1; and then it plateaued. This finding suggested that the liquid-solid ratio should be between 100:1 mL g−1 and 300:1 mL g−1. Figure 3 shows that the yield continuously increased as the extraction time increased to 90 min, and then it remained stable. This finding suggested that the optimal extraction time was between 30 and 90 min. Figure 3 shows that the yield gradually increased as the temperature increased to 90 °C, and it then remain constant. Therefore, the optimal extraction temperature was between 80 °C and 100 °C.
Figure 3: The effect of extraction process variables on the yield of P. napaulensis pigment.
(A) EtOH concentration, (B) liquid-solid ratio, (C) extraction time, (D) extraction temperature.Optimization of extraction process
Response surface optimization test and results
RSM is widely employed to estimate the optimal level of the extraction variables (Laqui-Vilca et al., 2018; Roriz et al., 2017; Sharmila et al., 2019). In this study, a response surface experiment with four variables at three levels was designed using Design-Expert ver. 12 software according to the Box-Behnken principle. The extraction solvent (A), liquid-solid ratio (B), extraction time (C), and extraction temperature (D) as the dependent variables were employed at three levels assigned in the range of low (–1), medium (0), and high (+1); and the yield of Yanzhiguo pigment was taken as the response value. The design scheme and corresponding yields are shown in Table 5. Multiple regression analysis yielded the following equation:
| Number | A (%) | B (mL/g) | C (min) | D (°C) | Absorbance |
|---|---|---|---|---|---|
| 1 | 35 | 200 | 90 | 100 | 0.609 |
| 2 | 35 | 200 | 60 | 90 | 0.597 |
| 3 | 35 | 200 | 60 | 90 | 0.58 |
| 4 | 35 | 200 | 30 | 80 | 0.429 |
| 5 | 35 | 300 | 30 | 90 | 0.559 |
| 6 | 20 | 200 | 60 | 80 | 0.413 |
| 7 | 35 | 300 | 90 | 90 | 0.505 |
| 8 | 20 | 200 | 30 | 90 | 0.457 |
| 9 | 50 | 200 | 60 | 80 | 0.445 |
| 10 | 50 | 200 | 30 | 90 | 0.473 |
| 11 | 35 | 200 | 60 | 90 | 0.584 |
| 12 | 35 | 300 | 60 | 100 | 0.595 |
| 13 | 35 | 100 | 90 | 90 | 0.534 |
| 14 | 35 | 200 | 60 | 90 | 0.595 |
| 15 | 35 | 100 | 60 | 80 | 0.444 |
| 16 | 50 | 200 | 90 | 90 | 0.511 |
| 17 | 35 | 200 | 90 | 80 | 0.432 |
| 18 | 20 | 100 | 60 | 90 | 0.462 |
| 19 | 35 | 300 | 60 | 80 | 0.472 |
| 20 | 50 | 200 | 60 | 100 | 0.567 |
| 21 | 50 | 100 | 60 | 90 | 0.525 |
| 22 | 35 | 200 | 30 | 100 | 0.565 |
| 23 | 35 | 100 | 30 | 90 | 0.462 |
| 24 | 50 | 300 | 60 | 90 | 0.538 |
| 25 | 20 | 300 | 60 | 90 | 0.535 |
| 26 | 35 | 100 | 60 | 100 | 0.587 |
| 27 | 20 | 200 | 90 | 90 | 0.472 |
| 28 | 35 | 200 | 60 | 90 | 0.587 |
| 29 | 20 | 200 | 60 | 100 | 0.558 |
R2 explains the correlation between the actual experiment and the predicted model. Here, the higher R2 of 0.9850 indicated the sharper correlation between them. Also, the ANVOA statistics resuls are shown in Table 6. According to Table 6, the F value was 65.80 (p 0.0001), the lack of fit was 0.1805 (p 0.05) indicating that the predicted model was adequately reliable and closely matched with the actual experimental value (0.9850) to predict the yield of Yanzhiguo pigment. Meanwhile, A, B, C, D, BC, A2, B2, C2, and D2 exerted highly significant effects on yield (p 0.01) and AB a significantly effect (p 0.05). Moreover, the F value revealed that the factor ranking in terms of importance was D B A C.
| Sources model | Sum of squares | DF | Mean square | F-value | p-value | Significant |
|---|---|---|---|---|---|---|
| 0.1048 | 14 | 0.0075 | 65.80 | 0.0001 | ||
| A-A | 0.0022 | 1 | 0.0022 | 19.22 | 0.0006 | ** |
| B-B | 0.0030 | 1 | 0.0030 | 26.43 | 0.0001 | ** |
| C-C | 0.0012 | 1 | 0.0012 | 10.20 | 0.0065 | ** |
| D-D | 0.0596 | 1 | 0.0596 | 524.05 | 0.0001 | ** |
| AB | 0.0009 | 1 | 0.0009 | 7.91 | 0.0138 | * |
| AC | 0.0001 | 1 | 0.0001 | 1.16 | 0.2993 | |
| AD | 0.0001 | 1 | 0.0001 | 1.16 | 0.2993 | |
| BC | 0.0040 | 1 | 0.0040 | 34.87 | 0.0001 | ** |
| BD | 0.0001 | 1 | 0.0001 | 0.8786 | 0.3645 | |
| CD | 0.0004 | 1 | 0.0004 | 3.69 | 0.0753 | |
| A2 | 0.0203 | 1 | 0.0203 | 178.78 | 0.0001 | ** |
| B2 | 0.0035 | 1 | 0.0035 | 30.83 | 0.0001 | ** |
| C2 | 0.0159 | 1 | 0.0159 | 139.69 | 0.0001 | ** |
| D2 | 0.0084 | 1 | 0.0084 | 73.90 | 0.0001 | ** |
| Residual | 0.0016 | 14 | 0.0001 | |||
| Lack of fit | 0.0014 | 10 | 0.0001 | 2.65 | 0.1805 | |
| Pure error | 0.0002 | 4 | 0.0001 | |||
| R2 | 0.1064 | 28 |
*Significant difference.
**highly significant difference.
Interactions between the extraction process variables
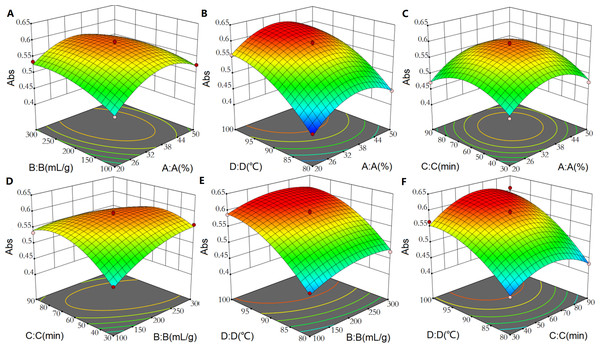
Based on the regression equation, 3D response surface plots were established to depict the interactions between the extraction process variables (Fig. 4). In these response surface plots, a steeper surface indicated a greater factor influence on yield and a more significant interaction between two factors; a smoother surface indicated a smaller influence on yield and a less significant interaction between two factors. The significance of the interaction between two variables could be revealed by the contour line; the line is higher in an ellipse (more significant interaction) but lower in a circle (less significant interaction). As shown in Fig. 4, the surfaces of AB and BC were steep while the surfaces of AC, AD, BD, and CD were gentle; this finding indicated that the A, B and B, C had greater interactions affecting pigment yield.
Figure 4: 3D surface plot showing interaction effect of extraction process variables on the yield of P. napaulensis pigment.
(A) EtOH concentration and liquid-solid ratio, (B) ethanol concentration and extraction temperature, (C) ethanol concentration and extraction time, (D) liquid-solid ratio and extraction time, (E) liquid-solid ratio and extraction temperature, (F) extraction time and temperature.To further optimize the extraction process, the effects of individual variable on yield was analyzed using Desk-Expert version 12. Based on the analysis, the optimal predicted condition was an EtOH volume fraction of 33.92%, a liquid-solid ratio of 224.41:1 mL g−1, an extraction time of 65.04 min, and an extraction temperature of 99.96 °C. Absorbance at 277 nm of the pigment extracted with above conditions was 0.624. In practice, we adjusted the EtOH volume fraction to 35%, the liquid-solid ratio to 200:1 g mL−1, the extraction time to 65 min, and the extraction temperature to 100 °C; then yielded the sample with the absorbance value of 0.625, which just differed from the predicted value by only 0.001, confirming the accuracy of the model.
Fitness between the a* value and the yield
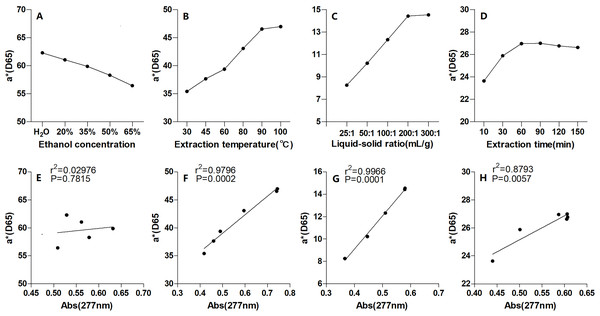
As mentioned above, the a* value is related to the vividness of red, thus a higher a* value is desirable (Belasco et al., 2020). To balance the redness and the yield, the a* values of pigment extracted under the single-factor conditions were investigated and are shown in Figs. 5A–5D; the fitness between the a* values and the yields are shown in Figs. 5E–5H. Figures 5B–5D show that the a* values closely matched with the yields under different extraction temperatures, liquid-solid ratios, and times (R2 values of 0.9796, 0.9966, and 0.8793, respectively; all p 0.01). However, the fitness between a* values and yields at different EtOH volume fractions was low (R2 values of 0.02976; p 0.01) (Fig. 5A). And, the pigment extracted into pure water had the largest a* value, while the pigment extracted into 35% EtOH exhibited the highest absorbance at 277 nm. Thus, it was suggested that the red pigment of Yanzhiguo may be soluble in water. Flavonoids which acted as the main chromogenic substracts might increase the water-solubility of Yanzhiguo pigments, this suppose well supports the comment of Jurić et al. (2020). Jurić et al. (2020) reviewed that natural pigments might be water-soluble (hydrophilic molecules) or lipid-soluble (hydrophobic molecules), and the generally water-soluble natural pigments such as red-blue-purple anthocyanins and red betalains belong to the group of flavonoids.
Figure 5: The a* value of P. napaulensis pigment and its correlation with absorbance under different extraction process variables.
(A) Ethanol concentration, (B) liquid-solid ratio, (C) extraction time, (D) extraction temperature and (E–H) its correlation with absorbance, respectively.Pigment stability
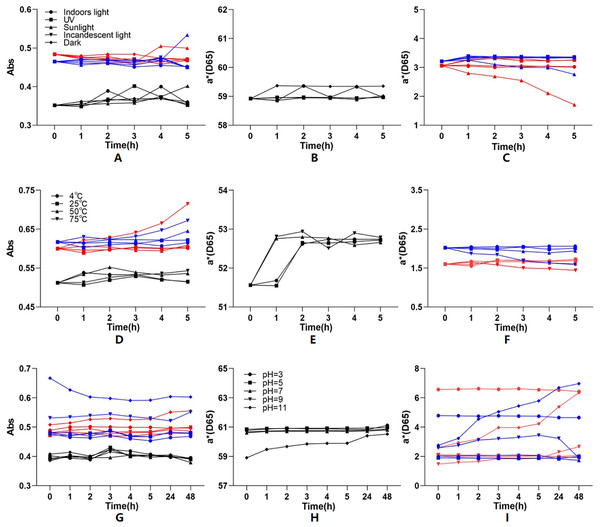
To know the stability, Yanzhiguo pigment absorbances at 277 nm and a* values were investigated under different light, temperature, and pH conditions, then compared with that of carmine (a typical synthetic pigment) (Fig. 6). To ensure the comparability, the absorbance of both Yanzhiguo pigments (respectively extracted into 35% EtOH or water) were firstly adjusted to between 0.3 and 0.7. Figure 6A-1 shows that the absorbance of carmine and Yanzhiguo pigment extracted into water increased to 114.22% and 114.84% under direct (hot) sunlight for 5 h, respectively. While the absorbance of pigment extracted into 35% EtOH remained stable at 103.41%; the smaller fluctuation indicated more stable. Meanwhile, Fig. 6B-1 shows that the absorbance of pigments extracted into 35% EtOH and water were 101.37% and 104.53% after 5 h at 50 °C, respectively, while the absorbance of carmine increased to 104.63%. Thus, pigment extracted into 35% EtOH was stabler than carmine and water-extracted pigment at 50 °C; the stability of water-extracted pigment was close to that of carmine. However, the absorbance of the two Yanzhiguo pigments respectively increased to 119.01% and 108.84% after 5 h at 75 °C, which was greater than the increase in the absorbance of carmine; therefore, the two Yanzhiguo pigments were less stable than carmine at 75 °C. Figure 6G shows that after 48 h in strongly alkaline condition (pH = 11), the absorbance of the two Yanzhiguo pigments changed more than that of carmine. This indicated the two Yanzhiguo pigments were less stable than carmine at strongly alkaline condition (pH = 11).
Figure 6: The effect of different light, temperature and pH conditions on the stability of P. napaulensis pigment.
(A–C) Different light, (D–F) different temperature, (G–I) different pH.According to Figs. 6B and 6C, the a* value fluctuation of pigment extracted into 35% EtOH was lower than that of the water extract and carmine, further confirming that Yanzhiguo pigment extracted into 35% EtOH were more stable under direct (hot) sunlight. The a* values of the two Yanzhiguo pigments exhibited less fluctuation than did carmine under indoor light, ultraviolet light, normal incandescent light, or darkness. Moreover, Figs. 6E and 6F show that although the two Yanzhiguo pigments were less stable than carmine at 75 °C, their a* values were more stable than the a* values of carmine at 4 °C, 25 °C, and 50 °C. Figures 6H and 6I shows that the two Yanzhiguo pigments were less stable than carmine in strongly alkaline conditions, but their stability was similar to that of carmine under neutral or acidic conditions. In total, the a* value fluctuation was consistent with the yield fluctuation.
Usually, natural pigments are more sensitivity and instability than synthetic pigments (Coultate & Blackburn, 2018; Khoo et al., 2017; Abdollahi, Jahadi & Ghavami, 2021). Pigments such as red pigment of Monascus were affected at lower pH values, high salt concentration at lower temperatures (Abdollahi, Jahadi & Ghavami, 2021). In our study, as compared to carmine, Yanzhiguo pigments were slightly unstabler with slightly larger fluctuation under direct (hot) sunlight, at high temperatures ( 75 °C), and in strongly alkaline conditions (pH 11); however, they were more stable with less fluctuation in absorbance and a* value under indoor light, ultraviolet light, normal incandescent light, and darkness; at 4 °C, 25 °C, 50 °C; and at pH = 3, 5, 7, and 9.
Antioxidant activity of Yanzhiguo pigment
The food industry requires natural radical-scavenging pigments. Hence, the ABTS and DPPH radical scavenging capacities of Yanzhiguo pigments extracted into 35% EtOH or water were analyzed and are shown in Table 7. The ABTS and DPPH radical scavenging capacity of the pigment extracted into water were 6.125 µg TE/g and 1.600 µg TE g−1, respectively, which were higher than that of the pigment extracted into 35% EtOH. Flavonoids acted as substrates in color and antioxidant activity in thirteen kinds dietary foods including black rice, purple sweet potato, mature bitter melon (Boo et al., 2012). And, red and black rice varieties which contented higher quercetin and catechin displayed significantly higher antioxidant capacity than white rice (Chen et al., 2022). Here, Yanzhiguo fruit was also confirmed to be rich in flavonoids by LC-MS, which might be the main substrates in color and radical-scavenging reactions of Yanzhiguo pigment. However, more information such as the relationship between antioxidant capacities and Yanzhiguo pigments concentration requires further investigation.
| ABTS+ (μmol Trolox g−1 DW) | DPPH (μmol Trolox g−1 DW) | |
|---|---|---|
| Yanzhiguo pigment extracted with water | 6.125 0.0010 | 1.600 0.0008 |
| Yanzhiguo pigment extracted with 35% EtOH | 4.175 0.0025 | 1.275 0.0024 |
Discussion
Color is important to evaluate the quality of food, as it is related to food safety, nutritional value, flavor, and so on (Delgado-Vargas et al., 2000; Spence, 2015). Hence, uniform, attractive, and pleasantly color is required in food industry including processing and storage (Jurić et al., 2020). Therefore, pigments of various kinds and forms have been applied in food industry. Although synthetic pigments have the advantages of good stability, low cost, bright color, good dyeing force and the wide range of hues, etc., such pigments may be of low nutritional value and cause side effects (Jurić et al., 2020; Ardila-Leal et al., 2021). Recently, natural pigments has been paid more attention because their advantages in safety, natural color and biological functions.
Based on solubility, natural pigments are divided into water-soluble (hydrophilic molecules) and lipid-soluble (hydrophobic molecules) (Sigurdson, Tang & Giusti, 2017). Chlorophylls and carotenoids which have been used as sensitisers, color additives, and antioxidants in the food industry are lipid-soluble (Li et al., 2021a). Anthocyanins and betalains which can be supplied by vegetables and fruits are water-soluble (Rodriguez-Amaya, 2016). However, there is little information on the chromogenic components in Yanzhiguo fruit, which restricts its applications. LC-MS with high sensitivity, high specificity and large dynamic range has been widely applied in detection of phytochemical compounds (Zhang et al., 2019a; Munyai, Raletsena & Modise, 2022; Cupido et al., 2022). In our study, LC-MS analysis was performed to investigate the chromogenic components in Yanzhiguo fruit. Baese on LC-MS analysis, polyphenols, isoprenes, ketones, and quinones were found in Yanzhiguo fruit. Among them, flavonoids were particularly diverse and abundant. Anthocyanins including rutin, procyanidin B2, cyanidin, shikonin, delphinidin were plentiful, especially rutin was prominent in peak area. As we know, anthocyanins are typical flavonoid compounds, and foods rich in anthocyanins predominated in deep color although it alters from dark blue (blueberry, Vaccinium sp. L) to purple (eggplant, Solanum melongena L.) and red (strawberry, Fragaria × ananassa) (Rodriguez-Amaya, 2016). Furthermore, studies have shown that the anthocyanins content of red plum is higher than yellow plum (Liu et al., 2022).
Thus, we assumed that anthocyanins mainly composed by rutin might the fundamental compounds causing Yanzhiguo fruit in dark purple. However, more refined information should be provided to verify this assume. Meanwhile, Yanzhiguo pigment gradually varies from red to yellow after diluting, this phenomenon might be bound up with the contribution of anthocyanins and non-anthocyanin flavonoid to color. A study on 207 cultivated lotus (Nelumbo nucifera) validated that anthocyanins contribute to red, while non-anthocyanin flavonoids contribute to yellow, controlling colors from deep yellow to light yellow and even colorlessness (Liu et al., 2022).
Also, the application of natural pigments is limited by lacking of extraction and purification technology. Ultrasound, enzyme systems, and maceration are used to extract pigments from other natural materials (Catalkaya & Kahveci, 2019; Sharmila et al., 2019; Wojdyło, Samoticha & Chmielewska, 2021). Techology combining enzymatic and solvent extraction was established to extract lycopene from industrial tomato waste (Catalkaya & Kahveci, 2019). First, lycopene-rich oleoresins was obtained after treatment of tomato waste by combination of cellulolytic and pectinolytic enzymes. Then, the optimized ethyl acetate extraction conditions were established: enzymatic reaction temperature = 40 °C, enzymatic reaction time = 5 h, enzyme:substrate ratio = 0.2 ml/g, solvent:substrate ratio = 5 ml/g, extraction time = 1 h, enzyme:enzyme ratio = 1. And, RSM was employed to optimize ultrasonic extract parameters to obtain yellow pigment from Tecoma castanifolia floral petals (Sharmila et al., 2019). Flower mass, methanol concentration, extraction time, and sonication power as 2 g, 100 %, 15 min, and 30 W was the optimized. However, there is limited understand on Yanzhiguo pigment extraction. In our study, the appropriate pre-treatment process and extraction condition were established for Yanzhiguo pigment. Pre-treatment which involved boiling at 800 W for 5 min at a material-liquid ratio of 5:1 g mL−1, followed by drying at 40 °C was appropriate, and the extraction with 35% EtOH as solvent, a liquid-solid ratio of 200:1 mL g−1, an extraction time of 65 min, and an extraction temperature of 100 °C was derived by combining the data from single-factor experiments and RSM.
The use of natural pigments into food industry is challenging due to their instability. The stability of natural pigments is easily influenced by a number of factors such as pH, light, temperature, co-pigmentation, sulfites, ascorbic acid, oxygen and enzymes (Enaru et al., 2021). Among them, pH and temperature are the major destroy factors (Xing & Wang, 2021). However, it was found that Yanzhiguo pigment was very stable under normal environment, including different light, temperature, and pH conditions. Also, high stability, strong oxidation activities were also detected in Yanzhiguo pigment. Similarly, thirteen natural plant pigments were reported with DPPH radical scavenging activity ranged from 88.9% (red cabbage) to 18.0% (blue gardenia), and the red cabbage had not only highest DPPH radical scavenging activity but also the most abundant total flavonoid (Boo et al., 2012). Compared to white rice, red and black rice varieties which contented higher quercetin and catechin displayed significantly higher antioxidant capacity (Chen et al., 2022). It is universally known that flavonoids usually have antioxidant activities (Liu et al., 2022). Diverse and abundant flavonoids confirmed by LC-MS might the material base for the antioxidant activities in Yanzhiguo pigment. However, more investigation such as the relationship between antioxidant capacities and Yanzhiguo pigments concentration will be required.
Conclusion
To promote the development and application of natural and stable pigment, the extraction and characterization of pigment of Yanzhiguo were investigated in this study. Firstly, more than 700 components in various categories such as flavonoids, terpenes, alkaloids, phenols, and quinones were identified. Anthocyanins, as typical flavonoids including rutin, procyanidin B2, cyanidin, shikonin, delphinidin, were assumed to be the fundamental compounds causing Yanzhiguo fruit in dark purple. Then, the optimal pre-treatment conditions were established before further extraction of Yanzhiguo pigment, which involved boiling at 800 W for 5 min at a material-liquid ratio of 5:1 g mL−1, followed by drying at 40 °C. After that, the extraction process was optimized by combining the data from single-factor experiments and RSM, with 35% EtOH as extration solvent, a liquid-solid ratio of 200:1 mL g−1, an extraction time of 65 min, and an extraction temperature of 100 °C. We found strong fitness between the a* values and pigment yields except under different EtOH concentration. Meanwhile, Yanzhiguo pigment was confirmed with high stability with only small fluctuations in absorbance and a* value, except under the extreme environments such as direct (hot) sunlight, high temperature (75 °C) and strong alkaline (pH 11). Moreover, Yanzhiguo pigment exhibited good antioxidant activity. Our results contribute to know more on Yanzhiguo pigment and promote its application by providing efficient Yanzhiguo pigment extraction technology.