Genome-wide identification and expression analyses of C2H2 zinc finger transcription factors in Pleurotus ostreatus
- Published
- Accepted
- Received
- Academic Editor
- Joseph Heitman
- Subject Areas
- Agricultural Science, Bioinformatics, Microbiology, Molecular Biology, Mycology
- Keywords
- Pleurotus ostreatus, C2H2-ZFPs, Expression patterns, Hormone response, Abiotic stress
- Copyright
- © 2022 Ding et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Genome-wide identification and expression analyses of C2H2 zinc finger transcription factors in Pleurotus ostreatus. PeerJ 10:e12654 https://doi.org/10.7717/peerj.12654
Abstract
The C2H2-type zinc finger proteins (C2H2-ZFPs) regulate various developmental processes and abiotic stress responses in eukaryotes. Yet, a comprehensive analysis of these transcription factors which could be used to find candidate genes related to the control the development and abiotic stress tolerance has not been performed in Pleurotus ostreatus. To fill this knowledge gap, 18 C2H2-ZFs were identified in the P. ostreatus genome. Phylogenetic analysis indicated that these proteins have dissimilar amino acid sequences. In addition, these proteins had variable protein characteristics, gene intron-exon structures, and motif compositions. The expression patterns of PoC2H2-ZFs in mycelia, primordia, and young and mature fruiting bodies were investigated using qRT-PCR. The expression of some PoC2H2-ZFs is regulated by auxin and cytokinin. Moreover, members of PoC2H2-ZFs expression levels are changed dramatically under heat and cold stress, suggesting that these genes may participate in abiotic stress responses. These findings could be used to study the role of P. ostreatus-derived C2H2-ZFs in development and stress tolerance.
Introduction
Pleurotus ostreatus is a mushroom that is widely cultivated for its nutritional value and relatively simple cultivation techniques (Chang & Miles, 2004; Khan & Tania, 2012). The production of P. ostreatus relies on the precise control of fruiting body development. The formation of fruiting bodies starts when two hyphae with different mating types combine to form dikaryotic hyphae during a process called plasmogamy. If these dikaryotic hyphae aggregate, they will develop into primordia, which will then differentiate into fruiting bodies. Genome sequencing of model mushroom Schizophyllum commune indicated that many predicted transcription factors like zinc finger proteins (ZFPs), MYB, fungal specific transcription factor (fst), and so on are differentially expressed during sexual development (Ohm et al., 2010). The Pofst3 gene in P. ostreatus is a homolog of the fst3 gene in S. commune and was determined to play a role in primordia formation (Qi et al., 2019). These results indicated that certain transcription factors may mediate the development of P. ostreatus. However, only a few transcription factors have been identified in this commercial mushroom. The identification and characterization of more transcription factors in P. ostreatus could help researchers identify interesting proteins involved in various development processes or help breeders selectively breed for controlled mushroom development.
ZFPs are one of the largest transcription factor families in eukaryotic genomes (Laity, Lee & Wright, 2001). The term “zinc finger” refers to proteins harbor a conserved domain consisting of cysteine (C) and/or histidine (H) residues. This domain binds with a zinc ion and, structurally, consists of a two-stranded antiparallel beta-sheet and a helix (Takatsuji, 1998). ZFPs can be divided into the following categories based on the number and location of C and H residues in this conserved domain: C2H2, C2HC, C2HC5, C2C2, C3H, C3HC4, C4, C4HC3, C6, and C8 (Berg & Shi, 1996). Among these, C2H2-type zinc finger proteins (C2H2-ZFPs) are the most widely studied. The zinc finger domain in these proteins contains two C and two H residues, which are described as CX(2-4)CX12HX(3-5)H (where X represents any amino acid) (Pabo, Peisach & Grant, 2001).
Functional analysis has shown that C2H2-ZFs participate in vegetative growth and reproductive development in plants (An et al., 2012; Lu et al., 2012; Sun et al., 2015). They also mediate growth and development (Tian et al., 2017), sexual development (Kim et al., 2009), oospores production (Wang et al., 2009), and so on in fungi and fungal hyphae. Moreover, while c2h2-overexpression strains did not affect normal development in Agaricus bisporus, the yield per day of the transgenic strains peaked 1 day earlier than the control strains did (Pelkmans et al., 2016). The effect of C2H2-ZFs on mushroom formation makes them a target for breeding of this commercial mushroom. However, no further studies have been conducted on the roles of C2H2-ZFs in P. ostreatus development thus far.
Apart from regulating various development processes, C2H2-ZFs have been found to play crucial roles in abiotic stress defense. In plants, they have been shown to respond to heat (Mittler et al., 2006), and functional analysis has shown they help temper the effects of drought (Yin et al., 2017), cold (Liu et al., 2017), and salt stress (Ciftci-Yilmaz et al., 2007). In China, traditional greenhouses are mainly used for cultivating P. ostreatus, but they often lack proper environmental control. Environmental stress, especially heat, consistently threatens the supply of greenhouse-grown mushrooms. Extremely and continuously high temperatures (>36 °C) disrupt the cell wall integrity of P. ostreatus and enhance the ability of Trichoderma asperellum to infect mycelia (Qiu et al., 2018). In addition, mycelia exposed to 40 °C for 3 h leads to the accumulation of lactate, which inhibits mycelial growth (Yan et al., 2020). On the other hand, treatment at 5 °C significantly decreases the activity of enzymes like laccase and Mn-peroxidase in mycelia (Snajdr & Baldrian, 2007). Therefore, identification of C2H2-ZFs in P. ostreatus could also be used to in breeding programs to improve environmental stress tolerance in mushrooms.
In this study, C2H2-ZFs in the P. ostreatus genome were identified and characterized using bioinformatic analysis. Then, the expression profiles of each transcription factor were measured in different tissues to better understand their roles in regulating P. ostreatus development and stress response. The results of this work provide useful information about the characterization of C2H2-ZFs in mushrooms and candidate genes for the control the development and abiotic stress tolerance in P. ostreatus.
Materials & Methods
Identification and characterization of C2H2-ZFPs in P. ostreatus
A Hidden Markov Model (HMM) profile of the C2H2 domain sequences (PF00096) was downloaded from the Pfam database and used as a query in the HMMER3.0 program against the publicly available genome of P. ostreatus from JGI (http://genome.jgi.doe.gov/PleosPC15_2/) to search for C2H2-ZFPs with an E-value less than 1e−4. The candidate C2H2-ZFPs were submitted to SMART (http://smart.embl-heidelberg.de) to confirm the presence of a C2H2 domain. C2H2 domains that did not contain the “CX(2-4)CX12HX(3-5)H” motif were deleted manually, and the rest were regarded as PoC2H2-ZFPs. The subcellular localizations of PoC2H2-ZFPs were predicted using WoLF PSORT (http://wolfpsort.org/). The ExPasy site (http://web.expasy.org/protparam/) was used to calculate the molecular weight (MW) and isoelectric point (pI) of the proteins.
Phylogenetic analysis and multiple sequence alignment
A phylogenetic tree was constructed for C2H2-ZFPs in P. ostreatus using the MEGA-X program (Sudhir et al., 2018). A neighbor-joining (NJ) method based on the JTT model with bootstrapping was performed 1000 times to calculate phylogenetic distances.
Multiple sequence alignment was performed on full C2H2-ZFP and C2H2 domains using MEGA-X. The results were loaded into JaLview for visualization (Waterhouse et al., 2009).
Gene structure and motif analysis
The exon-intron organization of the C2H2-ZF genes was obtained from genomic information and drawn using Tbtools (Chen et al., 2020). Then, the proteins were submitted to MEME (http://meme-suite.org/tools/meme) to identify conserved motifs with five motif numbers. The optimum motif length was fixed using the default parameters (6–50 residues).
Strains, culture conditions, and sample collection
The P. ostreatus 3125 strain was provided by the Institute of Scientific Edible Fungi, Gaoyou, China. The fungi were grown in potato dextrose agar (PDA) medium, then transferred to sterile wheat grain medium and cultured at 25 °C in the dark in a temperature-controlled incubator. Five days later, a bit of wheat grain and mycelium were placed into sterile growth bags composed of 60% cottonseed hulls, 35% corncob, 10% bran, 3% gypsum, and 2% kalk. They were cultured at 55% humidity, in the temperature-controlled incubator. The mycelium were collected once they were fully grown. To obtain primordia, the growth bags were transferred to the culture room (10–13 °C, 80% relative humidity). Young and mature fruiting bodies were collected on day 6 and 12, respectively, as primordia differentiated into fruiting bodies. All samples were frozen in liquid nitrogen and immediately stored at −80 °C.
Hormonal and abiotic stress treatments
Selected primordia were exposed to hormones and environmental stresses and the response of PoC2H2-ZFs was analyzed. For the hormonal treatment, the primordia were covered with absorbent cotton soaked in 200 ul 0.01 mM IAA, 0.01 mM zeatin, and H2O then collected after 1 and 3 h (h). For the heat and cold stress treatments, the primordia were cultured at 38 °C and 4 °C in a temperature-controlled incubator for 1 and for 3 h, respectively. For the control, primordia were grown in the culture room at 10–13 °C.
Isolation of RNA, cDNA synthesis, and qRT-PCR analysis
Total RNA was isolated from the sampled primordia using the Plant Total RNA Isolation Kit (Sangon Biotech Co., Ltd, ShangHai). cDNA was generated using the MightyScript First Strand cDNA Synthesis Master Mix (Sangon Biotech Co., Ltd, ShangHai) according to the manufacturer’s protocol. The 2X SG Fast qPCR Master Mix (Sangon Biotech Co., Ltd, ShangHai) was used to perform qRT-PCR. The sar gene was used as the reference (Castanera et al., 2015) and the relative expression level of genes was analyzed using the 2−ΔCT or 2−ΔΔCT method. The primer sequences used for qRT-PCR are listed in Table S3. A heatmap showing relative expression levels of PoC2H2-ZF genes was generated using Tbtools (Chen et al., 2020).
Results
Identification, characterization, and phylogenetic analysis of C2H2-ZFPs in P. ostreatus
Using an HMM and manual correction, 18 C2H2-ZFPs were identified in the P. ostreatus genome. All these proteins contained one to four conserved C2H2 domains either in the N-terminus or the C-terminus (Fig. S1). Detailed information about the characteristics of these proteins like amino acid size, MW, isoelectric points, and so on were also analyzed (Table S1). The results showed that the PoC2H2-ZFPs had between 149 and 688 amino acids, molecular weights ranging from 16.4 (PleosPC15_2—1089905) to 74.8 kDa (PleosPC15_2—1054163), and isoelectric points ranging from 4.66 (PleosPC15_2—1079678) to 10.8 (PleosPC15_2—1089905). All the PoC2H2-ZFPs are predicted to be nuclear proteins based on subcellular localization analysis (Table S1).
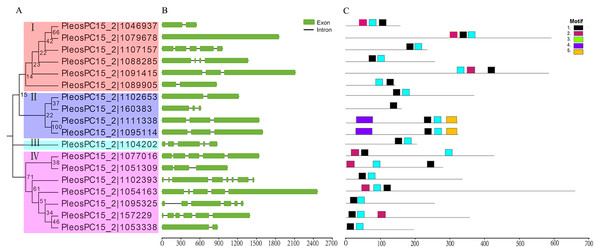
To analyze the phylogenetic relationships of these C2H2-ZFPs in P. ostreatus, a phylogenetic tree was constructed with their full protein sequences. As a result, the 18 PoC2H2-ZFPs were clustered into four separate clades (Fig. 1A). PleosPC15_2—1104202 is the only gene in clade III, indicating that it originated independently of the other genes. Based on bootstrapping values, the other genes are distantly related to each other. Most PoC2H2-ZFPs in the same clade had low bootstrap values (<60%). Only PleosPC15_2—1111338 and PleosPC15_2—1095114, which are regarded as duplicate genes, had strong bootstrap values (100%). The results of sequence alignment indicates that the PoC2H2-ZFPs share low sequence homology (Fig. S1).
Figure 1: Phylogenetic relationships, gene structures, and conserved motifs analysis of C2H2-ZFs in P. ostreatus.
(A) The Phylogenetic tree of PoC2H2-ZFPs. The four major subfamilies are marked with different colored backgrounds and indicated by Roman numerals on the left. (B) Gene structures of PoC2H2-ZF. Exons are represented by green boxes, and introns by black lines. (C) Conserved motifs in PoC2H2-ZFPs. Five colored boxes represent the various putative motifs. The sequences of each putative motif encoded are shown in Table S2.Gene structure and conserved motif analysis of PoC2H2-ZFs
To gain insights into the genetic structure of PoC2H2-ZFs, their exon-intron organization was analyzed. The results show that PoC2H2-ZFs have diverse gene structures and also appeared to have high inter-clade variation (Fig. 1B). The number of introns varied from 0 to 4 in clade I, 1 to 2 in clade II, PleosPC15_2—1104202 showed 5 exons and 4 introns, and clade IV had 1 to 7 introns (Fig. 1B).
To further investigate the structural diversity of the PoC2H2-ZFPs, the motif composition was analyzed using MEME (Fig. 1C). The results identified five putative conserved motifs (while the number was set beyond five, the E-value of the motif (X >5) was greater than one; data not shown). It was predicted that motif 1 encodes the conserved region (CX2CX12HX3H) that corresponds to the characteristic motif of the C2H2 domain (Table S2). This motif was detected in either at the N-terminus or the C-terminus of all the PoC2H2-ZFPs (Fig. 1C). Motif 3 represented one type of C2H2 domain (CX4CX12HX3H) (Table S2). PleosPC15_2—1046937, PleosPC15_2—1079678, and PleosPC15_2—1091415 in group I and PleosPC15_2—1077016, PleosPC15_2—1051309, PleosPC15_2—1054163, and PleosPC15_2—157229 in group IV possessed this sequence (Fig. 1C). It was predicted that Motif 2 encodes a false motif of the C2H2 domain lacking an H residue in the C-terminus (Table S2). It was also found in all 17 PoC2H2-ZFPs (Fig. 1C). The PoC2H2-ZFPs had diverse motif compositions. In group I, PleosPC15_2—1107157, PleosPC15_2—1088285, and PleosPC15_2—1089005 contained motifs 1 and 3, while the other genes had motifs 1, 2, and 3 (Fig. 1C). In group II, PleosPC15_2—160383 only had a single copy of motif 1, while PleosPC15_2—1111338 and PleosPC15_2—1095114 possessed motif 1 and motifs 4 and 5 in the N-terminus and the C-terminus, respectively (Fig. 1C). In group IV, PleosPC15_2—1102393, PleosPC15_2—1095325, and PleosPC15_2—1053338 shared the same motif composition, in that they all had motifs 1 and 2 in the N-terminal region. The other genes in this group had this motif composition and a single copy of motif 3 in the C-terminal region (Fig. 1C).
Conserved domain analysis of the PoC2H2-ZFPs
To better understand the characteristics of C2H2 domains in P. ostreatus, multiple sequence alignment was performed to identify conserved amino acids. The results revealed that the 29 predicted C2H2 domains consisted of 23-26 amino acids (Fig. 2A). The variation in sequence length was caused by amino acid changes in two regions: CX(2-4)C and HX(3-5)H. More specifically, 15 of the C2H2 domains contained two amino acids in the CX(2-4)C region while the others contained four amino acids (Fig. 2A). In the HX(3-5)H region, 24 of the C2H2 domains had three amino acids, three had four amino acids, and two had five amino acids (Fig. 2A). Two C and two H residues were conserved in every one of these domains. An F and an L residue were also highly conserved in these domains (Fig. 2A). A sequence similar to motif 1 (CX(2-4)CX3FX5LX2HX(3-5)H) was also found (Fig. 2B), suggesting that motif is conserved in PoC2H2-ZFPs.
Figure 2: Multiple alignment and conserved amino acids analysis of the C2H2 domains in PoC2H2-ZFPs.
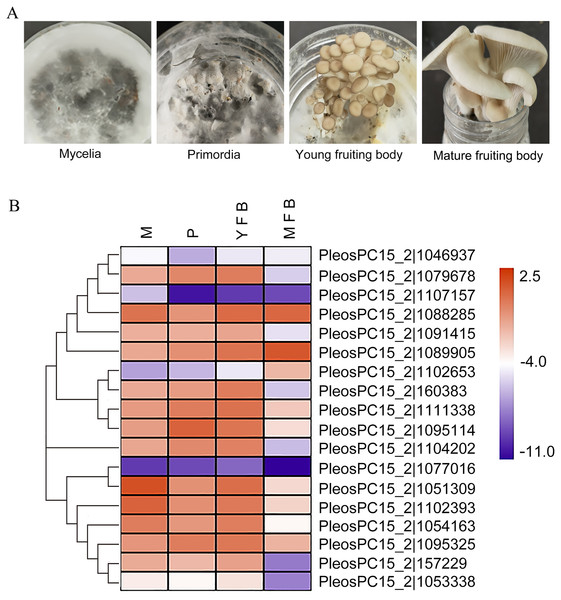
(A) Multiple sequence alignments of the C2H2 domains in PoC2H2-ZFPs. The C2H2 domains in PoC2H2-ZFPs were predicted on SMART (http://smart.embl-heidelberg.de) (with E-value < 1e−2). The conserved amino acid sequence with 100% identity was marked with an asterisk. (B) Conserved amino acid analysis of motif 1. The height of amino acids indicates the conservation ratio.Figure 3: Expression analysis of PoC2H2-ZFs in different tissues.
(A) Mycelia (M), primordia (P), young fruiting bodies (YFB), and mature fruiting bodies (MFB) were sampled to analyze the expression profiles of PoC2H2-ZFs in these tissues. (B) The results from RT-qPCR were log-transformed for ease of visualization in the heatmap.Expression analysis of PoC2H2-ZFs during different developmental stages
The expression profiles of the PoC2H2-ZFs were measured in four different tissues (mycelia, primordia, young fruiting body, and mature fruiting body) using qRT-PCR (Fig. 3A). The results showed that PoC2H2-ZFs have distinctive spatial and temporal expression patterns. All the PoC2H2-ZFs were continuously expressed in all four tissues (Fig. 3B). PeosPC15_2—1046937, PeosPC15_2—1107157, PeosPC15_2—1102653, PosPC15_2—1077016, and PeosPC15_2—1053338 had the lowest expression levels in all four tissues (Fig. 3B). In general, though, PoC2H2-ZF expression was relatively high in mycelia, primordia, and young fruiting bodies and was low in mature fruiting bodies (Fig. 3B). PeosPC15_2—1077016, PeosPC15_2—112393, and PeosPC15_2—1088285 were highly expressed in mycelia. Five genes (PeosPC15_2—1079678, PeosPC15_2—1089905, PeosPC15_2—1111338, PeosPC15_2—1095114, and PeosPC15_2—1095325) were expressed more in mycelia than in primordia (Fig. 3B). The expression levels of all PoC2H2-ZFs increased when the primordia differentiated into fruiting bodies (Fig. 3B). As the fruiting bodies started to ripen, the expression levels of the PoC2H2-ZFs generally decreased. However, the expression levels of three genes (PeosPC15_2—1088285, PeosPC15_2—1089905, and PeosPC15_2—1102653) continued to increase (Fig. 3B).
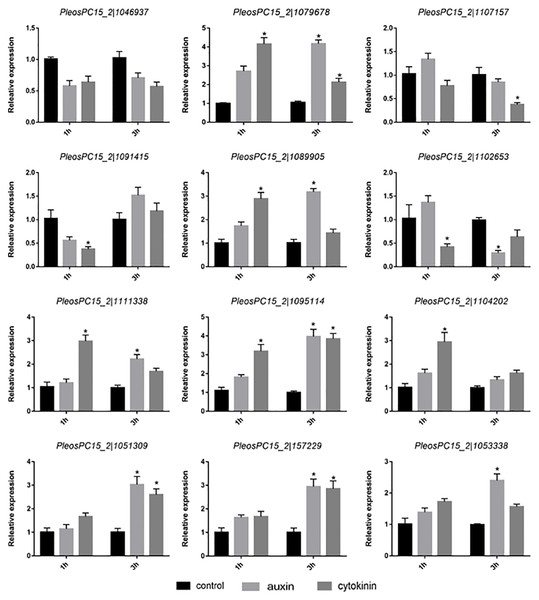
Expression analysis of PoC2H2-ZFs under auxin and cytokinin
To study the response of PoC2H2-ZFs to hormones, the primordia were treated with IAA and zeatin for 1 h and 3 h, respectively. Of the twelve genes studied, only the expression of PeosPC15_2—1046937 was not affected treatment with auxin or cytokinin (Fig. 4). The expression of PeosPC15_2—1091415 and PeosPC15_2—1102653 was down-regulated following 1 h of treatment with zeatin, whereas the expression of PeosPC15_2—1079678 and PeosPC15_2—1089905 increased (Fig. 4). In addition, four genes (PeosPC15_2—1079678, PeosPC15_2—1095114, PeosPC15_2—1051309, and PeosPC15_2—157229) were up-regulated following 3 h of treatment with zeatin (Fig. 4). On the other hand, the expression of PeosPC15_2—1079678 increased after 1 h of auxin treatment. The expression levels PeosPC15_2—1079678, PeosPC15_2—1089905, and PeosPC15_2—1095114 increased after 3 h of treatment with auxin, whereas PeosPC15_2—1102653 was down-regulated after 3 hours (Fig. 4). This shows that the PoC2H2-ZFs identified in this study are differentially regulated by auxin and cytokinin.
Figure 4: Expression patterns of PoC2H2-ZFs under auxin and cytokinin treatment.
The level of each gene was defined as 1 in the control, and levels in IAA and zeatin treatment are presented as relative ratios. The data were analyzed using the student’s t-test, and the asterisk indicates a significant difference at P < 0.05 (n = 3).Expression analysis of PoC2H2-ZFs under different abiotic stresses
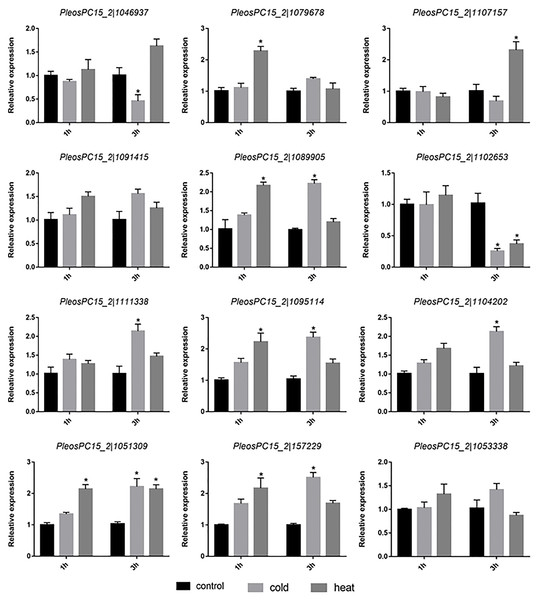
To investigate the potential roles of PoC2H2-ZFs in abiotic stress, their expression profiles were analyzed under heat and cold stress. The results showed that five genes (PeosPC15_2—1079678, PeosPC15_2—1089905, PeosPC15_2—1095114, PeosPC15_2—1051309, and PeosPC15_2—157229) were up-regulated after 1 h of heat stress (Fig. 5). After 3 h of heat treatment, PeosPC15_2—1102653 was down-regulated and PeosPC15_2—1102653 and PeosPC15_2—1051309 were up-regulated (Fig. 5). The expression of PeosPC15_2—1046937 and PeosPC15_2—1102653 was significantly suppressed by 3 h of cold stress, whereas PeosPC15_2—1111338, PeosPC15_2—1051309, PeosPC15_2—1089905, PeosPC15_2—157229, and PeosPC15_2—1104202 were up-regulated (Fig. 5). The expression levels of two PoC2H2-ZFs (PeosPC15_2—1091415 and PeosPC15_2—1053338) were not affected by cold and heat stress (Fig. 5). These data suggest that PoC2H2-ZFs are differentially regulated by abiotic stresses.
Figure 5: Expression patterns of PoC2H2-ZFs response to cold and heat stress.
The level of each gene was defined as 1 in the control, and levels in cold and heat treatment are presented as relative ratios. The data were compared using the student’s t-test, and the asterisk indicates a significant difference at P < 0.05 (n = 3).Discussion
C2H2-ZF proteins are one of the largest and most conserved transcription factor families in the eukaryotic kingdom. They have been reported to play important roles in mediating plant growth and responses to stress (An et al., 2012; Ciftci-Yilmaz et al., 2007; Liu et al., 2017; Lu et al., 2012; Sun et al., 2015; Yin et al., 2017). Moreover, it has been demonstrated that C2H2-ZFs participate in growth and development (Tian et al., 2017), microsclerotia formation (Tian et al., 2017), sexual development (Kim et al., 2009), and so on in fungi. For instance, it affected the yield of Agaricus bisporus (Pelkmans et al., 2016), one of the most widely cultivated commercial mushrooms. C2H2-ZFs could be candidate genes for edible mushrooms breeding. P. ostreatus is one of the widely cultivated mushrooms in China. Hence, it is useful to study C2H2-ZFs transcription factors in this species. The genome of P. ostreatus PC15 has been widely used since its release, but a systematic analysis of C2H2-ZFs has not yet been performed. The strain 3125 is the main cultivar for our laboratory work. However, the genome sequence of it was not obtaining. Thus, this study used the PC15 genome to identify phylogenetic relationships, gene structures, and conserved motifs among PoC2H2-ZFs. In addition, the effects of growth and development, various hormones, and abiotic stress treatments on the expression of PoC2H2-ZFs were analyzed.
Eighteen C2H2-ZFs were identified in the P. ostreatus genome using genome-wide analysis. The proteins were further divided into four subfamilies using phylogenetic relationship analysis. However, the C2H2-ZFPs in each subfamily had low bootstrap values (Fig. 1A), which may have been caused by the low sequence similarity of the non-C2H2 domain sequences (Fig. S1). The results show that the the molecular weight, pI values, protein length, exon and intron number, and motif composition of these PoC2H2-ZFs (Table S1, Figs. 1B and 1C) vary widely, suggesting that PoC2H2-ZFs have diverse structural and physicochemical properties, as well as distinct origins and functions.
The conserved “QALGGH” sequences located in the C2H2 domain was considered the plant-specific motif that animals and yeasts lacked (Takatsuji, 1999)). Like the rice and tomato, most of the C2H2-ZFPs that were detected had this sequence in their genomes (Cao et al., 2016; Xin et al. 2019). Such sequence was not detected in the C2H2-ZFPs of P. ostreatus (Fig. 2A). The sequence alignment indicated that the C2H2 domains in P. ostreatus have a CX(2-4)CX3FX5LX2HX(3-5)H motif, and that the F and L residues are highly conserved (Fig. 2A). This signature sequence can also be written as CX2CX3FX5LX2HX3H (Table S2). This motif, called, motif 1, was detected in all PoC2H2-ZFPs (Table S2, Fig. 1C). These results suggest that the CX2CX3FX5LX2HX3H sequence was conserved in PoC2H2-ZFPs.
C2H2-ZFs have been shown to participate in multiple processes related to growth and development in fungi. An investigatioin of the expression profiles of PoC2H2-ZFs could provide information about their role in regulating the growth and development of P. ostreatus. In a strain of Verticillium dahlia with a C2H2 transcription factor loss-of-function mutation (VdMsn2), a significant reduction in hyphal growth was seen (Tian et al., 2017). It was hypothesized that C2H2-ZFPs could also influence hyphal growth in P. ostreatus. PeosPC15_2—1077016, PeosPC15_2—112393, and PeosPC15_2—1088285 were highly expressed in mycelia (Fig. 3B), suggesting they play an important role in mycelial growth. Previous studies have also shown that C2H2-ZFs regulate the formation of primordia. Inactivation of C2H2 in S. commune resulted in the formation of aggregates but not subsequent differentiation into primordia, for instance (Ohm et al., 2011). In this study, five genes (PeosPC15_2—1079678, PeosPC15_2—1089905, PeosPC15_2—1111338, PeosPC15_2—1095114, and PeosPC15_2—1095325) were expressed more in primordia than in mycelia (Fig. 3B), indicating they are involved in the formation of primordia in P. ostreatus. Moreover, it was shown that C2H2-ZFs are involved in the development of fruiting bodies. In Aspergillus nidulans, the deletion of nsdC (a gene that encoded one C2H2 transcription factor) resulted in the loss of fruiting body formation (Kim et al. 2009). Here, all the PoC2H2-ZFs was expressed more in the young fruiting bodies than in the primordia (Fig. 3B), suggesting that C2H2-ZFs play an important role in fruiting body development in P. ostreatus. Three genes (PeosPC15_2—1088285, PeosPC15_2—1089905, and PeosPC15_2—1102653) showed increased expression in the mature fruiting body (Fig. 3B), as well, implying that they are involved in the ripening process of P. ostreatus. The PsCZF1 gene (encoding a C2H2-ZFs in Phytophthora sojae) has been implicated in the production of oospores and swimming zoospores (Wang et al., 2009). Indeed, PeosPC15_2—1088285, PeosPC15_2—1089905, and PeosPC15_2—1102653 seem to participate in spore development in P. ostreatus (Fig. 3B).
In Arabidopsis thaliana, ZINC FINGER PROTEIN 5 (ZFP5) mediates the effects of cytokinin and ethylene on the formation and growth of root hairs (An et al., 2012). ZFP6 has been identified as essential regulator of trichome initiation by and is responsive to gibberellin and cytokinin (Zhou et al. 2013). In addition, GhWIP2 (encoded a C2H2-ZFP) mediates cell expansion during organ growth by modulating crosstalk between auxin, gibberellins, and abscisic acid in Gerbera hybrida (Ren et al., 2018). Thus, it was hypothesized that various hormones can regulate C2H2-ZFs which can, in turn, affect developmental processes. P. ostreatus produces auxin (Bose, Shah & Keharia, 2013), and exogenous auxin and cytokinin have been reported to affect mycelial growth (Ramachela & Sihlangu, 2016). In this study, expression levels of PoC2H2-ZFs in primordia changed significantly in the presence of auxin and cytokinin (Fig. 4). Therefore, auxin and cytokinin have the potential to affect PoC2H2-ZF-mediated growth and developmental processes.
Previous studies have revealed that C2H2-ZFs confer resistance to abiotic stress in plants. In Arabidopsis, root growth in transgenic plants constitutively expressing Zat10 were more tolerant to heat stress (Mittler et al., 2006)). Overexpression of ZAT18 in transgenic Arabidopsis plants also increased drought tolerance, whereas the mutation of this gene resulted in decreased drought tolerance (Yin et al., 2017). In soybean, the expression of the C2H2-ZF gene GmSCOF-1 was induced by low temperature, and the overexpression lines not only had increased cold tolerance, but also had increased expression levels of cold-responsive genes (Kim et al. 2001). During P. ostreatus cultivation, bad environmental conditions negatively impact the growth and development of the mushrooms by inhibiting mycelial growth and disrupting the integrity of the cell wall, thereby increasing the risk of fungal contamination and reducing yield (Qiu et al., 2018). In this study, PoC2H2-ZFs were induced by heat and cold stress (Fig. 5), meaning that this transcription factor may have a conserved function related to heat and cold tolerance in fungi. Notably, PeosPC15_2—1089905 and PeosPC15_2—1102653 were induced by heat and cold stress (Fig. 5), suggesting that these genes play a variety of roles in response to various stresses.
Conclusions
In this study, we identified 18 C2H2-ZFs in the P. ostreatus genome. Their phylogenetic relationship, gene structure, motif composition, and other structural factors were highly variable. The expression profiles of these PoC2H2-ZFs suggest they play diverse roles in tissue growth and development. In addition, hormones and abiotic stress treatments induced the expression of these PoC2H2-ZFs, meaning that they could also participate in hormone signaling and abiotic stress response pathways.
Supplemental Information
Sequence alignment of PoC2H2-ZFPs
The C2H2 domains predicted on SMART (http://smart.embl-heidelberg.de) (with E-value < 1e−2) was enclosed by red box.