Brain Development: The dangers of rubella virus
Some pathogens can be extremely harmfull during pregnancy as they can cross the placenta, infect the fetus, and go on to cause congenital birth defects, miscarriages and stillbirths (Pereira, 2018). The rubella virus, for example, can cause a range of congenital brain defects, and it is also associated with a higher risk of babies developing congenital rubella syndrome, a complex condition associated with developmental delays, cardiac anomalies, hearing impairment and eye abnormalities (Bardeletti et al., 1975; Lazar et al., 2016; Toizumi et al., 2017). These viruses and other pathogens are collectively referred to as TORCH pathogens, which is short for toxoplasmosis (which is caused by a parasite), other pathogens (such as syphilis, varicella, mumps, parvovirus B19 and HIV), rubella, cytomegalovirus, and herpes simplex virus.
Despite the threat they pose to public health, the mechanisms by which TORCH pathogens affect brain development remain poorly understood. Now, in eLife, Tomasz Nowakowski, Joseph DeRisi and colleagues at the University of California San Francisco – including Galina Popova and Hanna Retallack as joint first authors – report new insights into the infection of brain cells by the rubella virus (Popova et al., 2023).
The researchers combined analyses of live human fetal brain slices that were maintained in the laboratory and two-dimensional cell cultures to study how the rubella virus affects brain cells. This revealed that the virus predominantly infects immune cells called microglia, which patrol and scavenge the central nervous system for damaged cells and pathogens. Microglia also have an important role in protecting the brain during development.
The experiments revealed that the rubella virus can only infect microglia when a variety of other brain cells are present. This is likely due to some yet-to-be-identified diffusible factors released by the other brain cells, which could render microglia susceptible to infection. However, the microglia do not need to make direct contact with these other cells in order to get infected.
Microglia play a crucial role in the antiviral immune response by releasing inflammatory cytokines, such as interferons (Sala and Kuka, 2020). Popova et al. found that infection with the rubella virus leads to an excessive interferon response by neighbouring neuronal cells, and this could have a deleterious effect on brain development. This is consistent with previous research, which showed that prenatal infection with rubella and HIV can trigger the overproduction of interferons, leading to prolonged inflammation that may contribute to the atypical development of the fetus (Crow et al., 2003). Certain inflammatory disorders, such as systemic lupus erythematosus and Aicardi-Goutières syndrome, are also characterized by an increased interferon response, and it is possible that some TORCH infections (in particular HIV and Rubella) share certain phenotypic similarities with these conditions.
As with many other TORCH pathogens, the fetus is most vulnerable to the rubella virus during the first trimester of pregnancy, due to the lack of immune defense in the developing fetus. Microglia populate the brain about a month into pregnancy, and the blood-brain barrier in the fetal brain only starts to be functional about two months into pregnancy (Menassa and Gomez-Nicola, 2018; Goasdoué et al., 2017). The brain is therefore extremely vulnerable to viruses during the first trimester of pregnancy, which coincides with a higher risk of developing severe developmental disorders.
The study of Popova et al. highlights the importance of using human cell-based models to better understand the pathophysiological mechanisms of congenital rubella syndrome. It remains to be seen why and how the rubella virus specifically attacks microglia, and what its molecular targets are. Identifying the molecular cues released by other brain cells, which potentially increase infection, will be necessary to eventually develop therapies against congenital rubella syndrome.
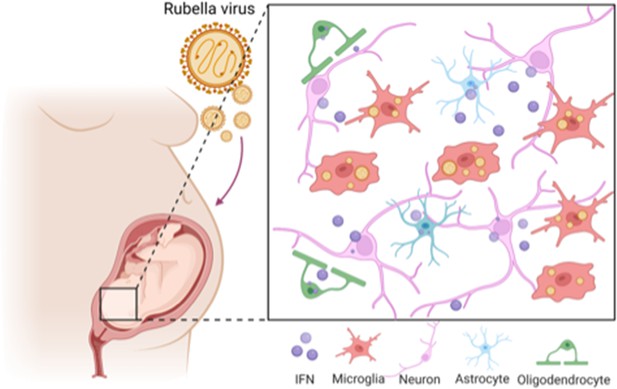
The effect of rubella virus on the brain development.
Rubella viruses (orange circles) target immune cells, called microglia (red), in the brain of the fetus. Popova et al. show that other neighbouring brain cells (pink, green and blue) must be present for infection to take place: it is thought that infection relies on diffusible factors released by these cells. Infection causes the release of large amounts of a signaling protein, called interferon (IFN; purple), which can damage the developing brain.
References
-
Morphology, biochemical analysis and neuraminidase activity of rubella virusArchives of Virology 49:175–186.https://doi.org/10.1007/BF01317536
-
Microglial dynamics during human brain developmentFrontiers in Immunology 9:1014.https://doi.org/10.3389/fimmu.2018.01014
-
Congenital viral infection: traversing the uterine-placental interfaceAnnual Review of Virology 5:273–299.https://doi.org/10.1146/annurev-virology-092917-043236
Article and author information
Author details
Publication history
- Version of Record published: June 16, 2023 (version 1)
Copyright
© 2023, Epifanova and Nguyen
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,003
- views
-
- 67
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
Tissue-clearing and labeling techniques have revolutionized brain-wide imaging and analysis, yet their application to clinical formalin-fixed paraffin-embedded (FFPE) blocks remains challenging. We introduce HIF-Clear, a novel method for efficiently clearing and labeling centimeter-thick FFPE specimens using elevated temperature and concentrated detergents. HIF-Clear with multi-round immunolabeling reveals neuron circuitry regulating multiple neurotransmitter systems in a whole FFPE mouse brain and is able to be used as the evaluation of disease treatment efficiency. HIF-Clear also supports expansion microscopy and can be performed on a non-sectioned 15-year-old FFPE specimen, as well as a 3-month formalin-fixed mouse brain. Thus, HIF-Clear represents a feasible approach for researching archived FFPE specimens for future neuroscientific and 3D neuropathological analyses.
-
- Neuroscience
Pavlovian fear conditioning has been extensively used to study the behavioral and neural basis of defensive systems. In a typical procedure, a cue is paired with foot shock, and subsequent cue presentation elicits freezing, a behavior theoretically linked to predator detection. Studies have since shown a fear conditioned cue can elicit locomotion, a behavior that - in addition to jumping, and rearing - is theoretically linked to imminent or occurring predation. A criticism of studies observing fear conditioned cue-elicited locomotion is that responding is non-associative. We gave rats Pavlovian fear discrimination over a baseline of reward seeking. TTL-triggered cameras captured 5 behavior frames/s around cue presentation. Experiment 1 examined the emergence of danger-specific behaviors over fear acquisition. Experiment 2 examined the expression of danger-specific behaviors in fear extinction. In total, we scored 112,000 frames for nine discrete behavior categories. Temporal ethograms show that during acquisition, a fear conditioned cue suppresses reward seeking and elicits freezing, but also elicits locomotion, jumping, and rearing - all of which are maximal when foot shock is imminent. During extinction, a fear conditioned cue most prominently suppresses reward seeking, and elicits locomotion that is timed to shock delivery. The independent expression of these behaviors in both experiments reveal a fear conditioned cue to orchestrate a temporally organized suite of behaviors.






