Collagen induces activation of DDR1 through lateral dimer association and phosphorylation between dimers
Figures
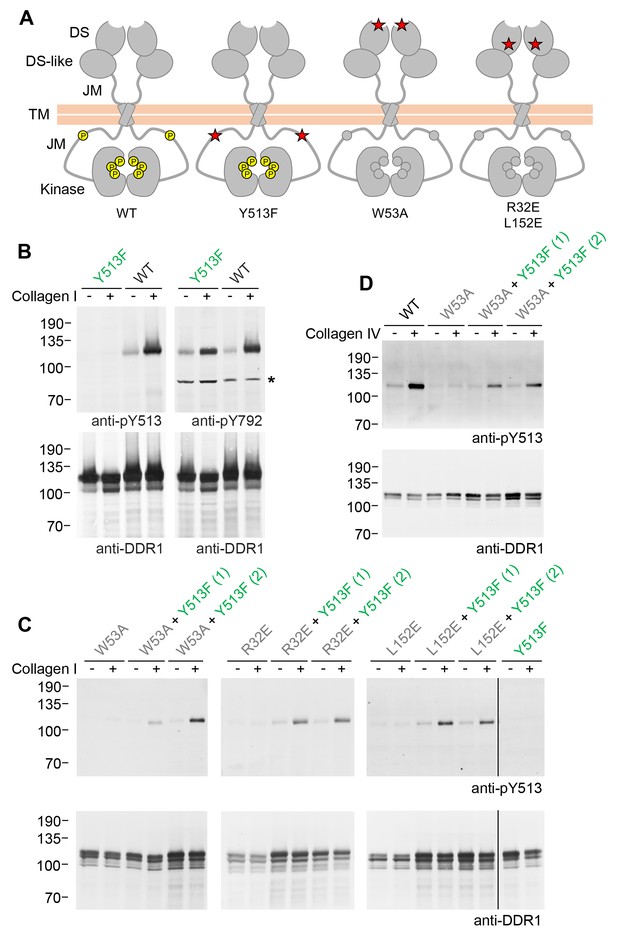
Co-expression of DDR1 donor kinase with signalling-incompetent receiver DDR1 mutants leads to collagen-induced phosphorylation of receiver DDR1.
(A) Schematic diagrams of wild-type and mutant DDR1b. The extracellular region consists of the ligand-binding discoidin (DS) domain, the discoidin-like (DS-like) domain and a flexible juxtamembrane (JM) region. The transmembrane (TM) domain contains a dimerising motif. The cytoplasmic region contains a large unstructured JM region, followed by the catalytic kinase domain. Collagen binding induces phosphorylation of DDR1b on cytoplasmic tyrosine residues; Y513 in the JM region and the three activation loop tyrosines are shown in phosphorylated form for WT DDR1b as yellow circles. DDR1b-Y513F is phosphorylated on the activation loop but cannot be phosphorylated on Y513. Ligand-binding defective DDR1b-W53A and signalling-defective DDR1b-R32E or DDR1b-L152E are DDR1 mutants that are not phosphorylated upon collagen incubation. (B–D) Wild-type or mutant DDR1b constructs were transiently expressed in HEK293 cells, either alone, or co-expressed as indicated. Cells were stimulated with collagen I or collagen IV for 90 min at 37°C. Aliquots of cell lysates were analysed by reducing SDS-PAGE and Western blotting. The blots were probed with phospho-specific Abs, as indicated, and re-probed with anti-DDR1. *, non-specific band. (C, D) Co-expression was performed with different amounts of DDR1b-Y513F expression vector, with the higher amount denoted by (2). The positions of molecular mass markers are indicated on the left (in KDa).
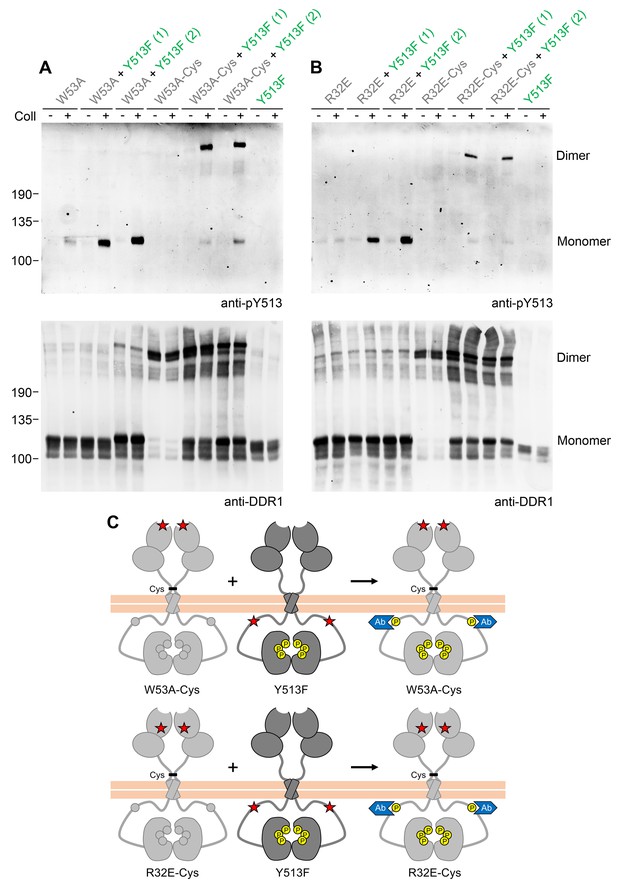
Co-expression with DDR1 donor kinase results in ligand-induced phosphorylation of receiver DDR1 dimers.
(A, B) Ligand-binding defective (W53A or W53A-Cys) or signalling-defective (R32E or R32E-Cys) DDR1b constructs were transiently expressed in HEK293 cells, either alone or co-expressed with DDR1b-Y513F, as indicated. Cells were stimulated with collagen I (Coll) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blots were probed with phospho-specific anti-pY513 (upper blots), and re-probed with anti-DDR1 (lower blots). Co-expression was performed with different amounts of DDR1b-Y513F expression vector, with the higher amount denoted by (2). The positions of molecular mass markers are indicated on the left (in KDa). The positions of cysteine-linked dimeric DDR1 and of mature monomeric DDR1 are indicated on the right. (C) Schematic diagram showing that co-expression of dimeric receiver DDR1b (W53A-Cys or R32E-Cys) with donor DDR1b-Y513F leads to collagen-induced Y513 phosphorylation of receiver dimers. The blue shape denotes anti-pY513 used for Western blotting.
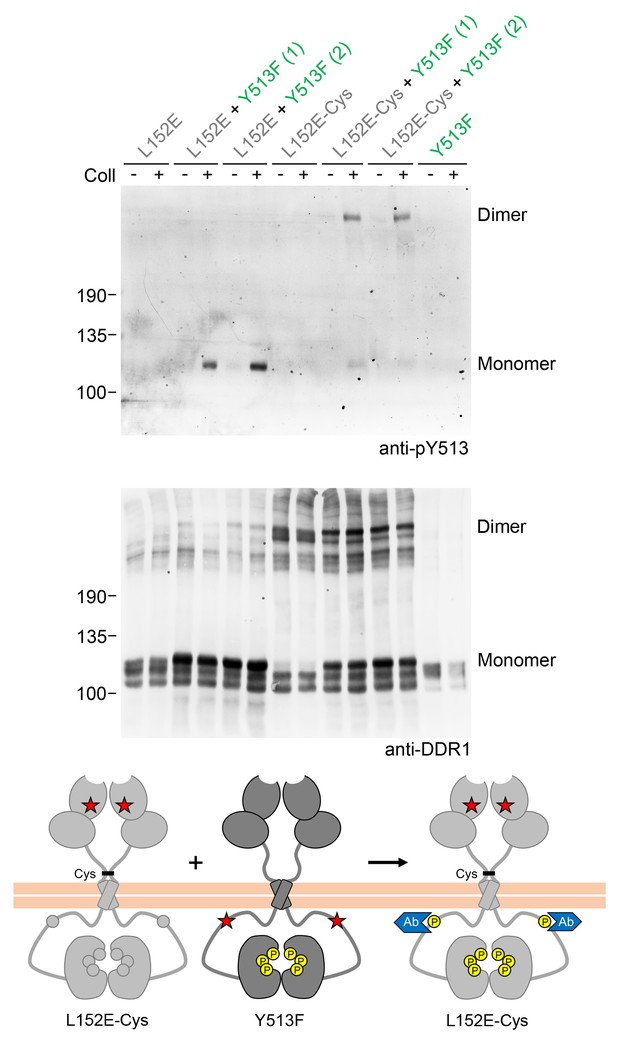
Co-expression with DDR1 donor kinase results in ligand-induced phosphorylation of DDR1-L152E dimers.
(A, B) Signalling defective (L152E or L152E-Cys) DDR1b was transiently expressed in HEK293 cells, either alone or co-expressed with DDR1b-Y513F, as indicated. The cells were stimulated with collagen I (Coll) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blot was probed with phospho-specific anti-pY513 (upper blot), and re-probed with anti-DDR1 (lower blot). Co-expression was performed with different amounts of DDR1b-Y513F expression vector, with the higher amount denoted by (2). The positions of molecular mass markers are indicated on the left (in KDa). The positions of cysteine-linked dimeric DDR1 and of mature monomeric DDR1 are indicated on the right. (C) Schematic diagram showing that co-expression of dimeric receiver DDR1b-L152E-Cys with donor DDR1b-Y513F leads to collagen-induced Y513 phosphorylation of DDR1b-L152E-Cys dimers. The blue shape denotes anti-pY513 used for Western blotting.
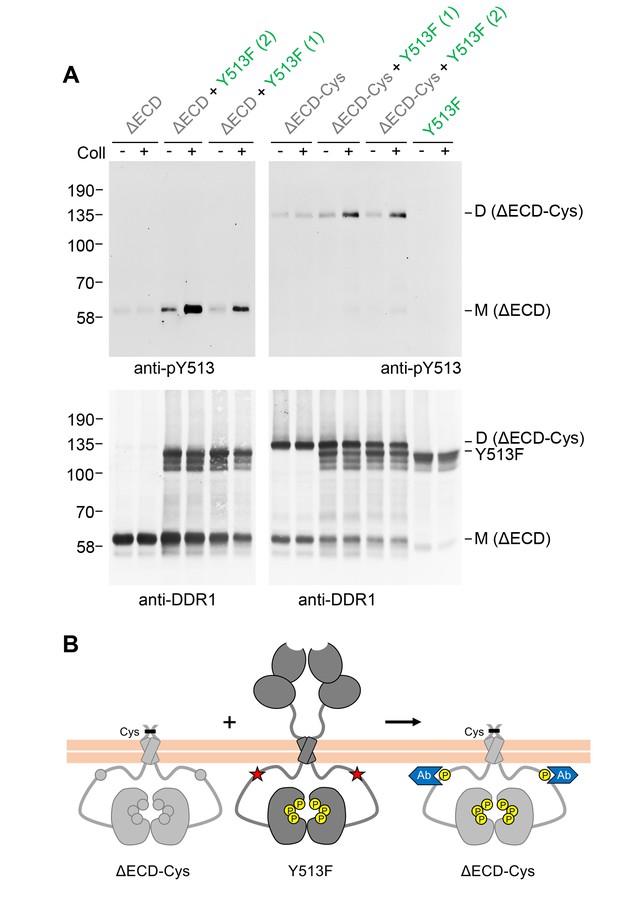
Signalling between DDR1 dimers is independent of ectodomain contacts.
(A) Ectodomain-deletion constructs (DDR1b-ΔECD or DDR1b-ΔECD-Cys) were transiently expressed in HEK293 cells, either alone or co-expressed with DDR1b-Y513F, as indicated. The cells were stimulated with collagen I (Coll) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blots were probed with phospho-specific anti-pY513 (upper blots), and re-probed with anti-DDR1 (lower blots). Co-expression was performed with different amounts of DDR1b-Y513F expression vector, with the higher amount denoted by (2). The positions of molecular mass markers are indicated on the left (in KDa). The positions of cysteine-linked dimeric DDR1b-ΔECD-Cys, DDR1b-Y513F and mature monomeric DDR1b-ΔECD are indicated on the right. (B) Schematic diagram showing that co-expression of ectodomain-deleted dimeric DDR1b with donor DDR1b-Y513F leads to collagen-induced Y513 phosphorylation of ectodomain-deletion dimers. The blue shape denotes anti-pY513 used for Western blotting.
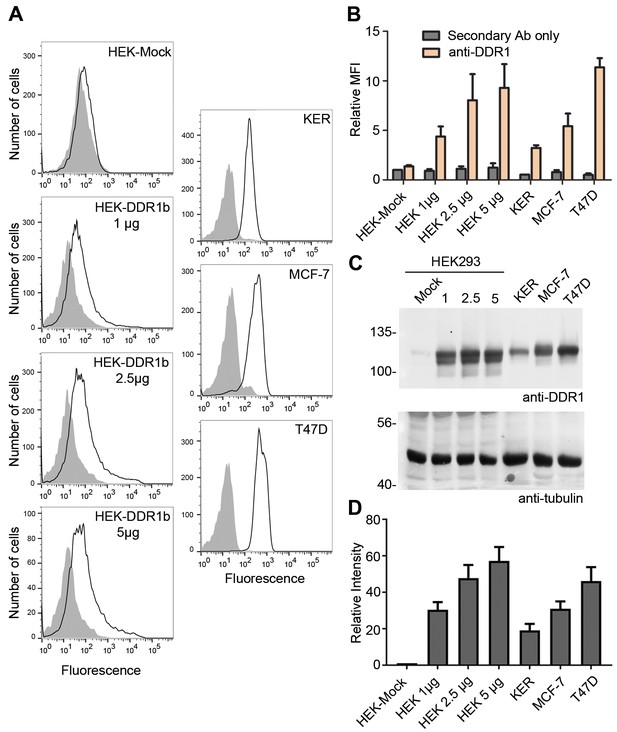
Cell surface and total protein DDR1 expression in DDR1-overexpressing HEK293 cells, primary keratinocytes and cell lines.
(A). HEK293 cells transiently transfected with the indicated amounts of DDR1b cDNA (in total volume of 400 μl), primary dermal keratinocytes (KER), MCF-7 and T47D cells were stained on ice with mouse anti-DDR1 mAb 7A9, followed by anti-mouse FITC conjugated secondary Ab and flow cytometry. Filled gray histograms, secondary Ab only; open histograms, anti-DDR1. (B) Quantification of mean fluorescence intensity (MFI), expressed relative to mock secondary Ab only (n = 3). (C) HEK293 cells transiently transfected with the indicated amounts of DDR1b cDNA (in μg), primary keratinocytes (KER), MCF-7 and T47D cells were lysed and aliquots of cell lysates were analysed by reducing SDS-PAGE followed by Western blotting. The blot was probed with anti-DDR1 (upper blot) and anti-tubulin (lower blot) Abs. The positions of molecular mass markers are indicated on the left (in KDa). (D) Quantitation of DDR1 signals, normalised to respective tubulin signals, expressed as relative band intensity with respect to the lowest signals on the blots (HEK293- Mock; n = 3).
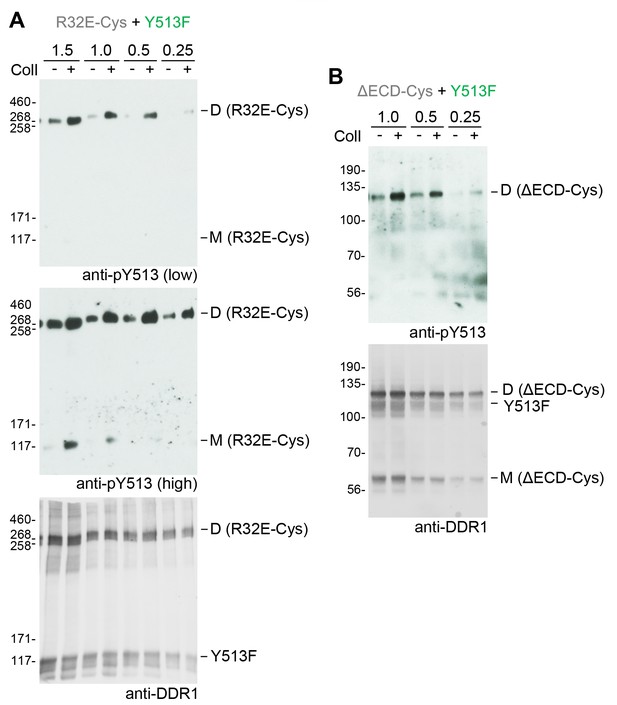
Co-expression with DDR1 donor kinase results in ligand-induced phosphorylation of receiver DDR1 dimers even at low expression levels.
(A, B). Signalling-defective (R32E-Cys) or ectodomain-deletion (ΔECD-Cys) DDR1b constructs were transiently co-expressed in HEK293 cells with DDR1b-Y513F. Co-expression was performed with different amounts of R32E-Cys and DDR1b-Y513F expression vectors, as indicated. Numbers refer to μg of DNA used (1:1 ratio of expression plasmids). Note that cells were transfected in total volume of 100 μl. Cells were stimulated with collagen I (Coll) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blots were probed with phospho-specific anti-pY513 (upper blots and middle blot in A), and the signals were detected with Amersham ECL Select Western blotting detection reagent and X-ray film. The middle blot in A shows a longer exposure. The blots were re-probed with anti-DDR1 (lower blots), followed by detection on a Typhoon FLA 9500 imager. The positions of molecular mass markers are indicated on the left (in KDa). The positions of cysteine-linked dimeric DDR1 and of mature monomeric DDR1 are indicated on the right.
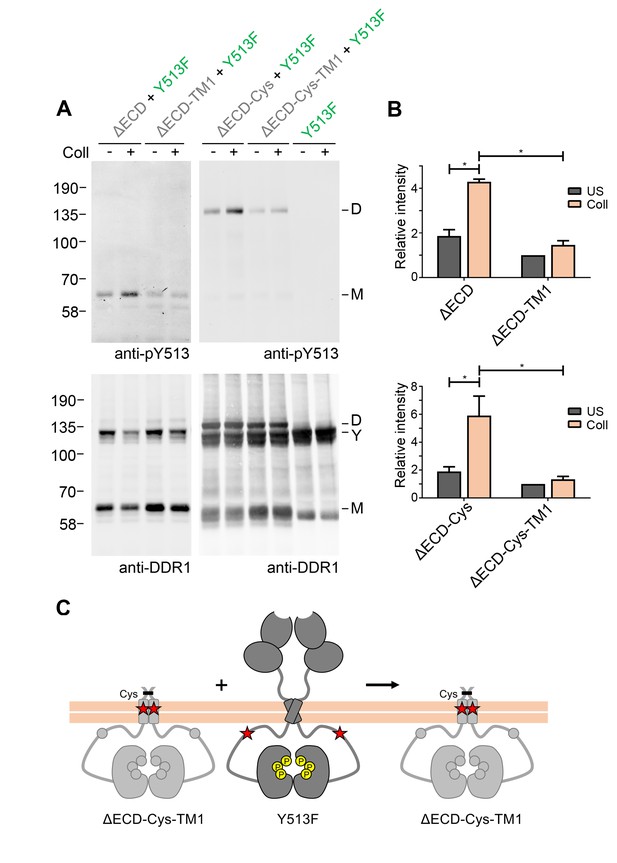
Signalling between DDR1 dimers is dependent on transmembrane domain contacts.
(A) Ectodomain-deletion constructs (DDR1b-ΔECD, DDR1b-ΔECD-TM1, DDR1b-ΔECD-Cys or DDR1b-ΔECD-Cys-TM1) were transiently expressed in HEK293 cells, either alone or co-expressed with DDR1b-Y513F, as indicated. The cells were stimulated with collagen I (Coll) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blots were probed with phospho-specific anti-pY513 (upper blots), and re-probed with anti-DDR1 (lower blots). The positions of molecular mass markers are indicated on the left (in KDa). The positions of cysteine-linked dimeric DDR1b-ΔECD-Cys (D), of DDR1b-Y513F (Y) and of mature monomeric DDR1b-ΔECD (M) are indicated on the right. (B) Quantitation of receiver DDR1 pY513 signals (co-expressed with Y513F), normalised to respective DDR1 signals, expressed as relative band intensity with respect to the lowest signals on the blots (unstimulated DDR1b-ΔECD-TM1 or unstimulated DDR1b-ΔECD-Cys-TM1). US, unstimulated; Coll, stimulation with collagen I. *p<0.05; n = 4. (C) Schematic diagram showing that co-expression of ectodomain-deleted dimeric DDR1b with a transmembrane domain mutation and donor DDR1b-Y513F does not result in collagen-induced Y513 phosphorylation of the deletion construct.
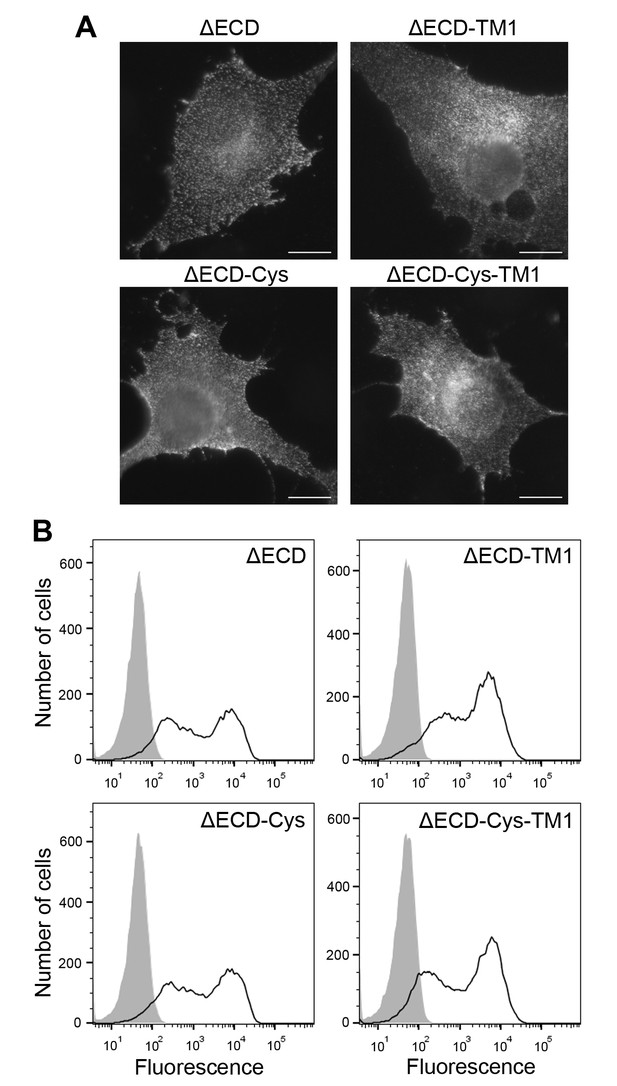
Cell surface expression of ectodomain-deletion constructs.
(A) N-terminally Flag-tagged ectodomain-deletion constructs (DDR1b-ΔECD, DDR1b-ΔECD-TM1, DDR1b-ΔECD-Cys or DDR1b-ΔECD-Cys-TM1) were transiently expressed in Cos-7 cells. Cells were incubated on ice with mouse anti-Flag Ab, before fixation and incubation with anti-mouse Alexa-Fluor-488 Ab. Cells were imaged using a widefield microscope. Scale bars, 20 µm. (B) N-terminally Flag-tagged ectodomain-deletion constructs (DDR1b-ΔECD, DDR1b-ΔECD-TM1, DDR1b-ΔECD-Cys or DDR1b-ΔECD-Cys-TM1) were transiently expressed in HEK293 cells. Cells were stained on ice with rabbit anti-Flag Ab, followed by anti-rabbit Alexa-Fluor-488 conjugated secondary Ab and flow cytometry. Filled gray histograms, secondary Ab only; open histograms, anti-Flag.
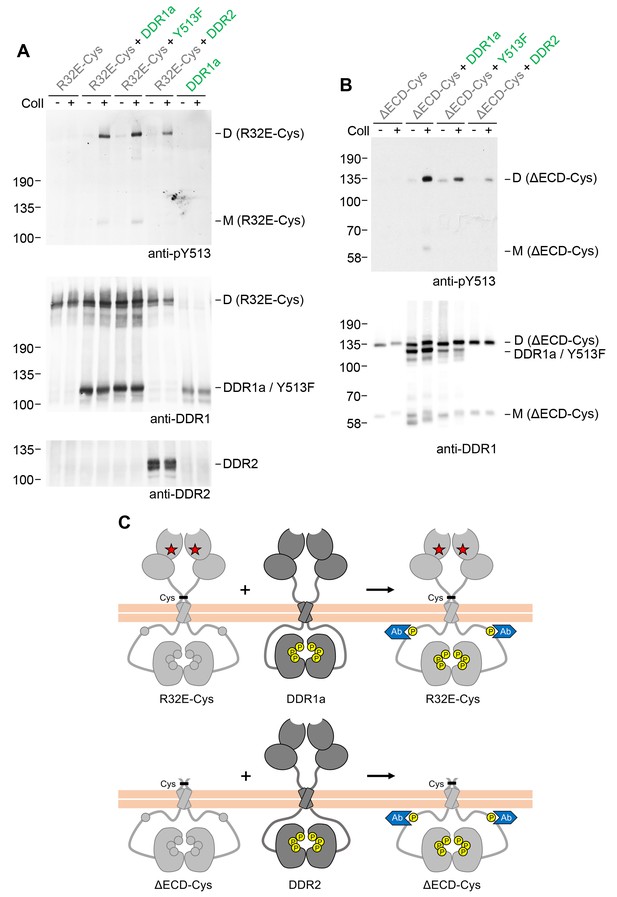
Signalling between dimers does not require identical cytoplasmic regions.
(A, B) Signalling-defective (DDR1b-R32E-Cys) or ectodomain-deletion (DDR1b-ΔECD-Cys) constructs were transiently expressed in HEK293 cells, either alone or co-expressed with DDR1a, DDR1b-Y513F or DDR2, as indicated. The cells were stimulated with collagen I (Coll) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blots were probed with phospho-specific anti-pY513 (upper blots), and re-probed with anti-DDR1 (lower blots). The positions of molecular mass markers are indicated on the left (in KDa). The positions of cysteine-linked dimeric (D) or mature monomeric (M) DDR1 receiver constructs, as well as of DDR1a or DDR1b-Y513F donor DDR1 are indicated on the right. (C) Schematic diagram showing that co-expression of signalling-incompetent dimeric receiver DDR1 constructs with DDR1a (which lacks the region encompassing Y513) or DDR2 as the donor kinases results in collagen-induced Y513 phosphorylation of the receiver dimers. The blue shape denotes anti-pY513 used for Western blotting.
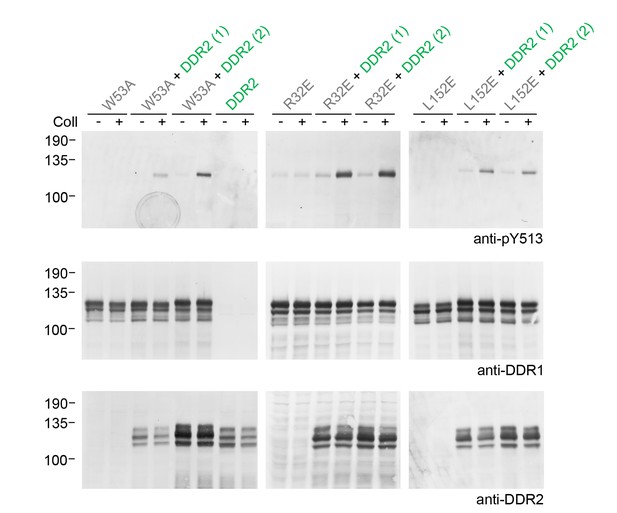
Co-expression of signalling-incompetent receiver DDR1 mutants with DDR2 leads to collagen-induced phosphorylation of receiver mutants.
Ligand-binding defective (DDR1b-W53A) or signalling-defective (DDR1b-R32E, DDR1b-L152E) mutant DDR1b constructs were transiently expressed in HEK293 cells, either alone, or co-expressed with DDR2, as indicated. The cells were stimulated with collagen I (Coll) for 90 min at 37°C, or left untreated. Aliquots of cell lysates were analysed by reducing SDS-PAGE and Western blotting. The blots were probed with phospho-specific anti-pY513 (upper blots), and re-probed with anti-DDR1 (lower blots). Co-expression was performed with different amounts of DDR2 expression vector, with the higher amount denoted by (2). The positions of molecular mass markers are indicated on the left (in KDa).
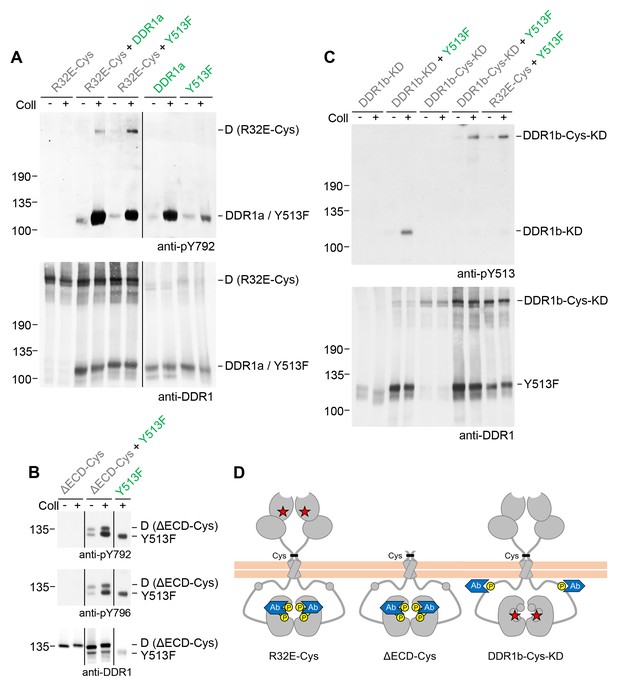
Signalling between dimers leads to activation loop phosphorylation of the receiver kinase but does not require receiver kinase activity.
(A, B) Signalling-defective (DDR1b-R32E-Cys) or ectodomain-deletion (DDR1b-ΔECD-Cys) constructs were transiently expressed in HEK293 cells, either alone or in combination with DDR1a or DDR1b-Y513F, as indicated. (C) Kinase-inactive DDR1 constructs (DDR1b-KD or DDR1b-Cys-KD) were expressed in HEK293 cells, either alone or in combination with DDR1b-Y513F, as indicated. The cells were stimulated with collagen I (Coll) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blots were probed with phospho-specific Abs against activation loop tyrosine-792 or tyrosine-796 (A, B) or juxtamembrane tyrosine-513 (C), as indicated (upper blots), and re-probed with anti-DDR1 (lower blots). The positions of molecular mass markers are indicated on the left (in KDa). The positions of cysteine-linked dimeric (D) DDR1 receiver constructs, as well as DDR1a or DDR1b-Y513F donor DDR1 are indicated on the right. Note that in (A), the latter position includes heterodimers between DDR1b-R32E-Cys and DDR1a/DDR1-Y513F. (D) Schematic diagram showing collagen-induced activation loop (pY792) phosphorylation of DDR1b-R32E-Cys or DDR1b-ΔECD-Cys receiver constructs when co-expressed with donor DDR1, as well as Y513 phosphorylation of DDR1b-Cys-KD when co-expressed with DDR1b-Y513F. The blue shape denotes anti-pY792 or anti-pY513 used for Western blotting.
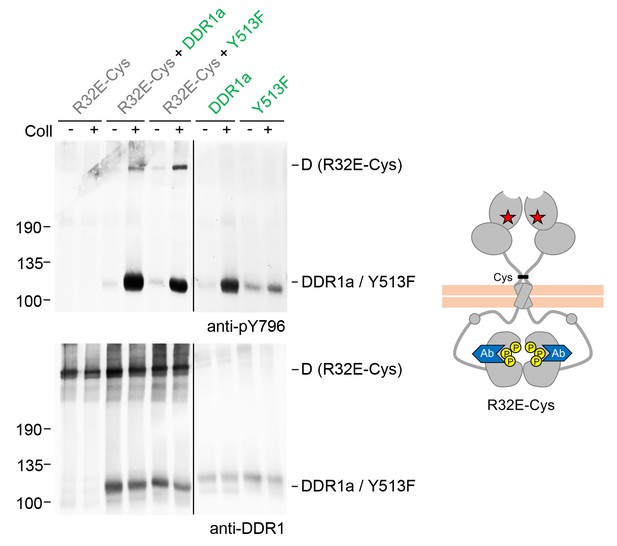
Signalling between dimers leads to activation loop phosphorylation of the receiver kinase.
Signalling-defective DDR1b-R32E-Cys was transiently expressed in HEK293 cells, either alone or in combination with DDR1a or DDR1b-Y513F, as indicated. The cells were stimulated with collagen I (Coll) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blot was probed with a phospho-specific Ab against activation loop tyrosine-796 (upper blot), and re-probed with anti-DDR1 (lower blot). The positions of molecular mass markers are indicated on the left (in KDa). The positions of cysteine-linked dimeric (D) DDR1 receiver constructs, as well as DDR1a or DDR1b-Y513F donor DDR1 are indicated on the right. Note that the latter position includes heterodimers between DDR1b-R32E-Cys and DDR1a/DDR1-Y513F. The schematic diagram shows collagen-induced activation loop (pY796) phosphorylation of DDR1b-R32E-Cys when co-expressed with DDR1a or DDR1b-Y513F. The blue shape denotes anti-pY796 used for Western blotting.
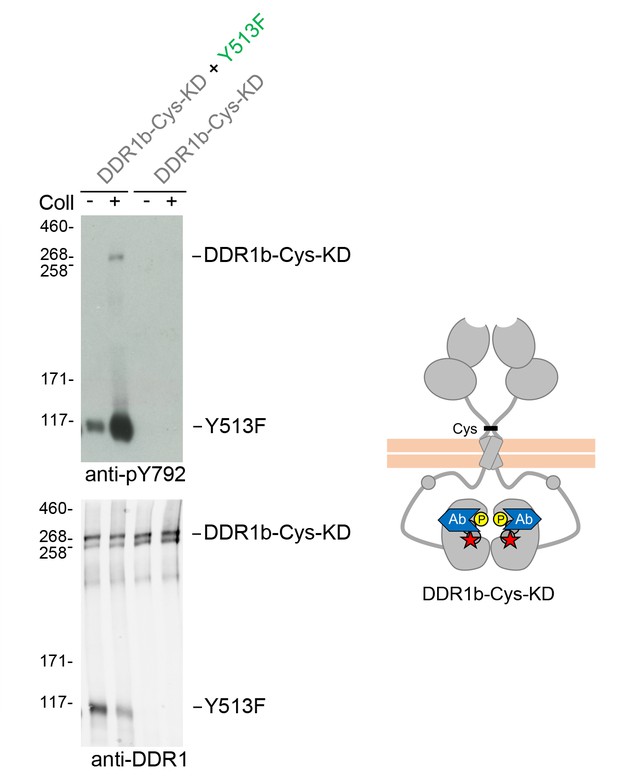
Signalling between dimers leads to activation loop phosphorylation of the receiver kinase independently of receiver kinase activity.
(A) Kinase-inactive DDR1 (DDR1b-Cys-KD) was transiently expressed in HEK293 cells, either alone or co-expressed with DDR1b-Y513F, as indicated. The cells were stimulated with collagen I (Coll) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blot was probed with a phospho-specific Ab against activation loop tyrosine-792 (upper blot), and re-probed with anti-DDR1 (lower blot). The positions of molecular mass markers are indicated on the left (in KDa). The positions of the cysteine-linked dimeric DDR1b-Cys-KD receiver construct, as well as DDR1b-Y513F are indicated on the right. The schematic diagram shows collagen-induced activation loop (pY792) phosphorylation of DDR1b-Cys-KD when co-expressed with DDR1b-Y513F. The blue shape denotes anti-pY792 used for Western blotting.
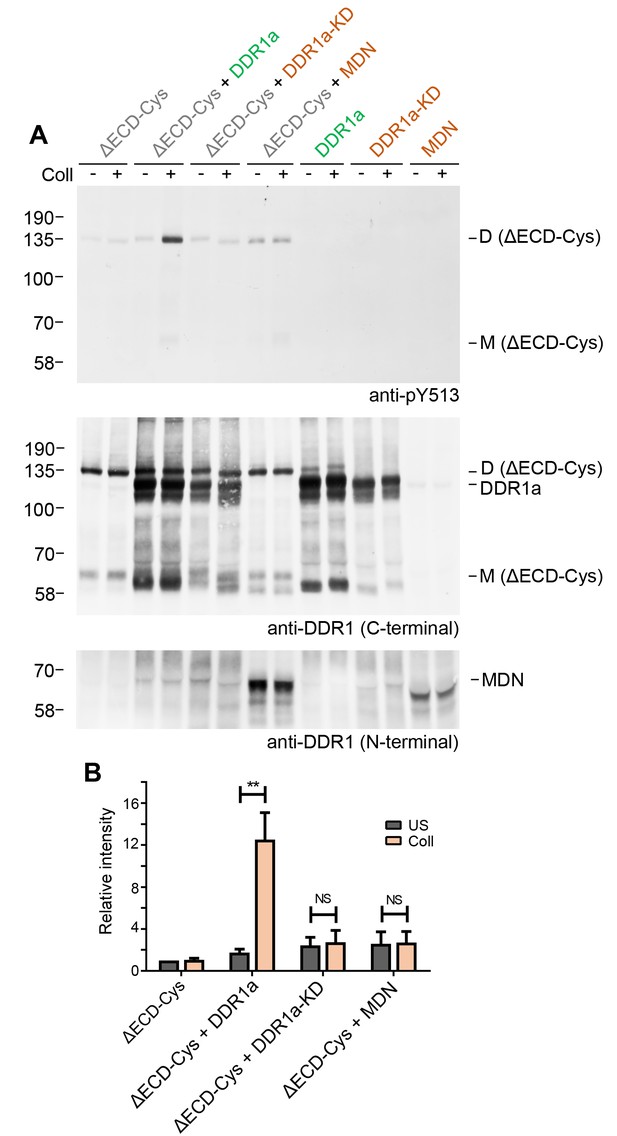
Signalling between dimers requires kinase activity of the donor kinase.
(A) The ectodomain-deletion construct DDR1b-ΔECD-Cys was transiently expressed in HEK293 cells, either alone or co-expressed with DDR1a, kinase-inactive DDR1a (DDR1a-KD) or with a truncated DDR1 construct lacking the kinase domain (DDR1-MDN), as indicated. The cells were stimulated with collagen I (Coll) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blot was probed with phospho-specific anti-pY513 (upper blot), and re-probed with anti-DDR1 (middle blot, C-terminal Ab). Expression of DDR1-MDN was verified by blotting with anti-DDR1 Abs against the ectodomain (lowest blot, N-terminal Ab). The positions of molecular mass markers are indicated on the left (in KDa). The positions of cysteine-linked dimeric DDR1b-ΔECD-Cys (D) and of mature monomeric DDR1b-ΔECD (M) receiver constructs, as well as of DDR1a or DDR1-MDN donor DDR1 are indicated on the right. (B) Quantitation of receiver DDR1 pY513 signals, normalised to respective DDR1 signals, expressed as relative band intensity with respect to the lowest signals on the blots (unstimulated DDR1b-ΔECD-Cys). US, unstimulated; Coll, stimulation with collagen I. **p<0.01; NS, no significance; n = 5.
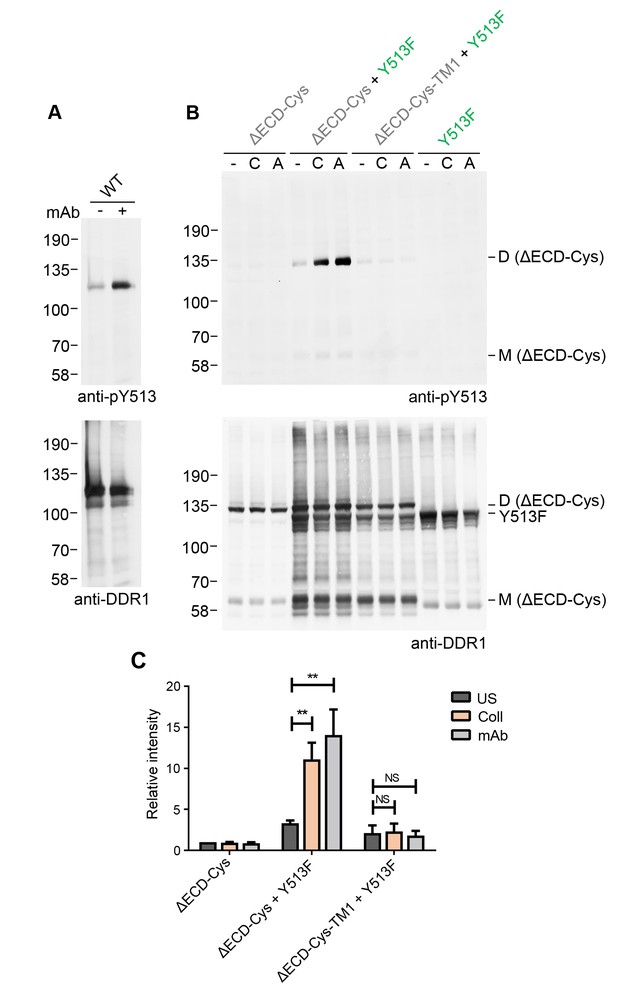
Collagen-independent activation by a multimeric anti-DDR1 Ab leads to signalling between dimers.
(A) Wild-type DDR1b was transiently expressed in HEK293 cells, and the cells were incubated with mAb513 for 90 min at 37°C or left untreated. Cell lysates were analysed by reducing SDS-PAGE followed by Western blotting. (B) The ectodomain-deletion construct DDR1b-ΔECD-Cys was transiently expressed in HEK293 cells, either alone or co-expressed with DDR1b-Y513F, as indicated. The cells were stimulated with collagen I (C) or mAb513 (A) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blots were probed with phospho-specific anti-pY513 (upper blots), and re-probed with anti-DDR1 (lower blots). The positions of molecular mass markers are indicated on the left (in KDa). The positions of cysteine-linked dimeric DDR1b-ΔECD-Cys (D) and mature monomeric DDR1b-ΔECD (M) receiver constructs, as well as DDR1b-Y513F donor DDR1 are indicated on the right. (C) Quantitation of pY513 signals, normalised to respective DDR1 signals, expressed as relative band intensity with respect to the lowest signals on the blots (unstimulated DDR1b-ΔECD-Cys). US, unstimulated; Coll, stimulation with collagen I; mAb, stimulation with mAb513. **p<0.01; NS, no significance; n = 4.
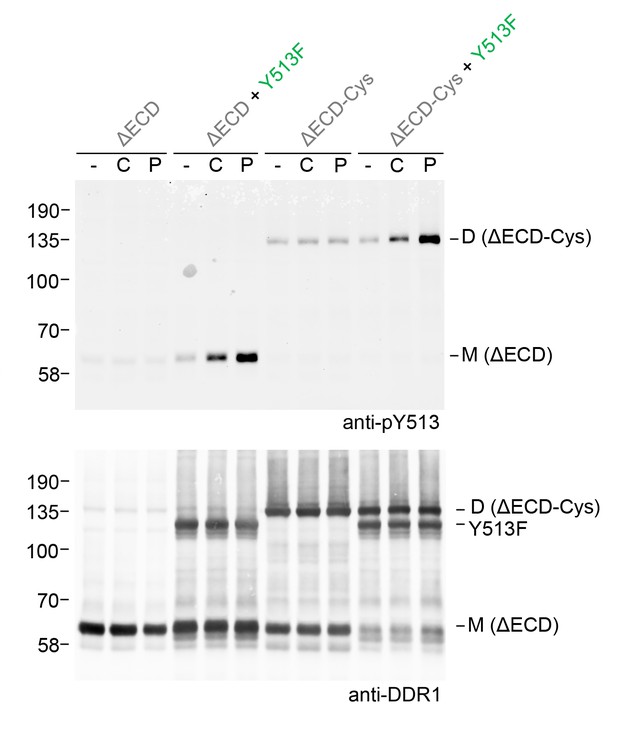
Ligand multivalency is not required for signalling between dimers.
The ectodomain-deletion constructs DDR1b-ΔECD or DDR1b-ΔECD-Cys were transiently expressed in HEK293 cells, either alone or co-expressed with DDR1b-Y513F, as indicated. The cells were stimulated with collagen I (C) or a collagen-mimetic DDR-selective peptide (P) for 90 min at 37°C or left untreated. Cells were lysed in the presence of NEM, and aliquots of cell lysates were analysed by non-reducing SDS-PAGE followed by Western blotting. The blot was probed with phospho-specific anti-pY513 (upper blot), and re-probed with anti-DDR1 (lower blot). The positions of molecular mass markers are indicated on the left (in KDa). The positions of cysteine-linked dimeric DDR1b-ΔECD-Cys (D) and mature monomeric DDR1b-ΔECD (M) receiver constructs, as well as DDR1b-Y513F donor DDR1 are indicated on the right.
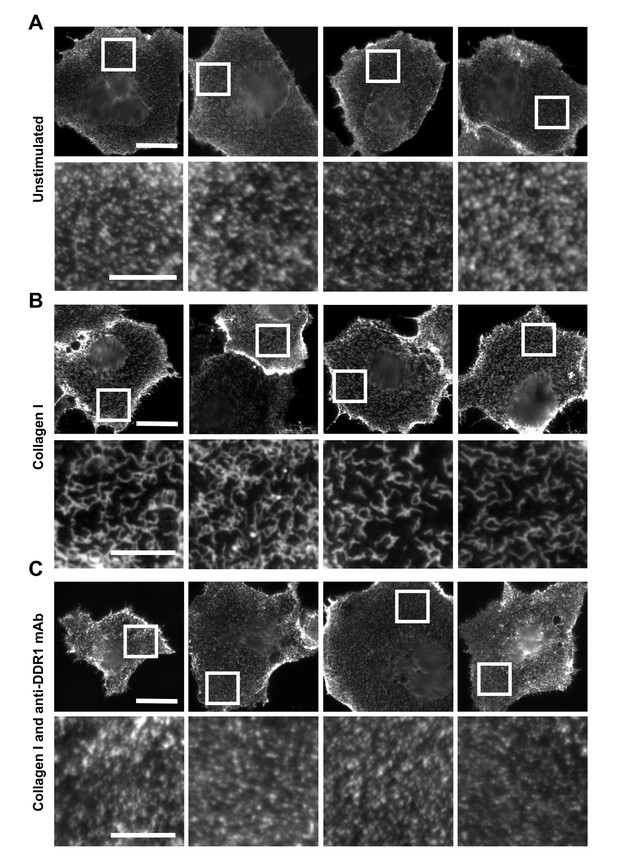
Function-blocking anti-DDR1 mAb inhibits collagen-induced DDR1 clustering.
Wild-type DDR1b was transiently expressed in Cos-7 cells and either left unstimulated (A), stimulated with 10 μg/ml collagen I (B), or stimulated with 10 μg/ml collagen I in the presence of a function-blocking anti-DDR1 mAb at 10 μg/ml (C), for 10 min at 37°C. Cells from all conditions were incubated on ice with a mouse monoclonal Ab against the DDR1 ectodomain, before fixation and incubation with anti-mouse Alexa-Fluor-488 Ab, and mounting. Cells were imaged using a widefield microscope. White boxes in top rows indicate corresponding areas shown at higher magnification in lower rows. Scale bars, 30 μm (top rows) and 10 μm (bottom rows).
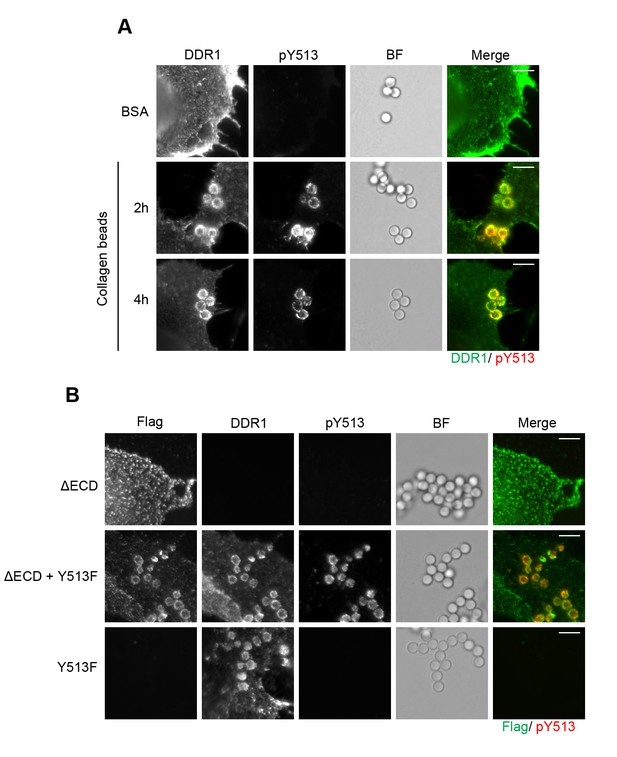
Collagen induces local activation of DDR1 and leads to recruitment of ectodomain-deletion DDR1 into the ligand-binding contact zone.
(A) Wild-type DDR1b was transiently expressed in Cos-7 cells and stimulated with collagen-coated latex beads (3 μm diameter) for 2 or 4 hr at 37°C. Control beads were coated with BSA and incubated for 2 hr at 37°C. Cells were incubated on ice with a mouse monoclonal Ab against the DDR1 ectodomain, before fixation and permeabilisation and incubation with rabbit anti-pY513 Ab. Cells were then incubated with anti-mouse Alexa-Fluor-488 and anti-rabbit Alexa-Fluor-555 secondary Abs. DDR1 (green) and pY513 (red) staining are shown in the merge image (right panel). (B) The ectodomain deletion construct DDR1b-ΔECD (which contains an N-terminal Flag tag) and DDR1b-Y513F were expressed either alone or in combination in Cos-7 cells. Cells were stimulated with collagen-coated latex beads (3 μm diameter) for 2 hr at 37°C. Cells were incubated on ice with mouse anti-Flag IgG1 and mouse anti-DDR1 ectodomain IgG2b Abs, followed by fixation and permeabilisation and incubation with rabbit anti-pY513 Ab. Cells were then incubated with anti-mouse IgG1 Alexa-Fluor-488, anti-mouse IgG2b Alexa-Fluor-555, and anti-rabbit Alexa-Fluor-647 secondary Abs. Flag (green) and pY513 (red) staining are shown in the merge image (right panel). BF, Brightfield. Scale bars, 10 μm.
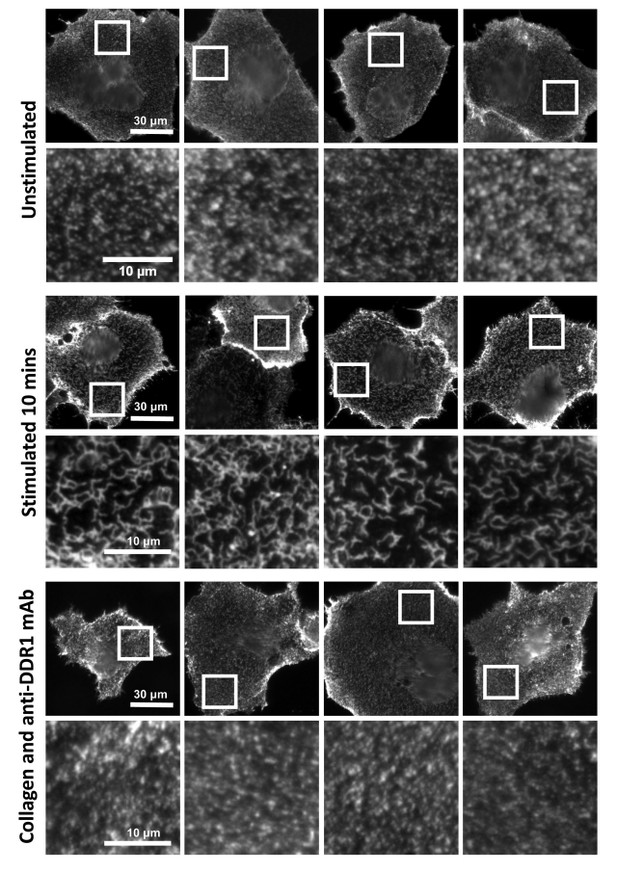
Anti-DDR1 mAbs inhibit collagen-induced DDR1 clustering.
Cos-7 cells expressing DDR1 were incubated with 10 μg/ml collagen I for 10 minutes at 37°C, left unstimulated, or incubated with collagen I in the presence of function-blocking anti-DDR1 mAbs. Cells were incubated on ice with a mouse monoclonal Ab against the DDR1 ectodomain, before fixation and incubation with anti-mouse Alexa-Fluor-488. Cells were imaged on a widefield microscope.





