• 综述 •
兰明岩, 张秀武, 楚弘宇, 王崇臣. MIL-101(Fe)及其复合物催化去除污染物:合成、性能及机理[J]. 化学进展, 2023, 35(3): 458-474.
Lan Mingyan, Zhang Xiuwu, Chu Hongyu, Wang Chongchen. MIL-101(Fe) and Its Composites for Catalytic Removal of Pollutants: Synthesis Strategies, Performances and Mechanisms[J]. Progress in Chemistry, 2023, 35(3): 458-474.
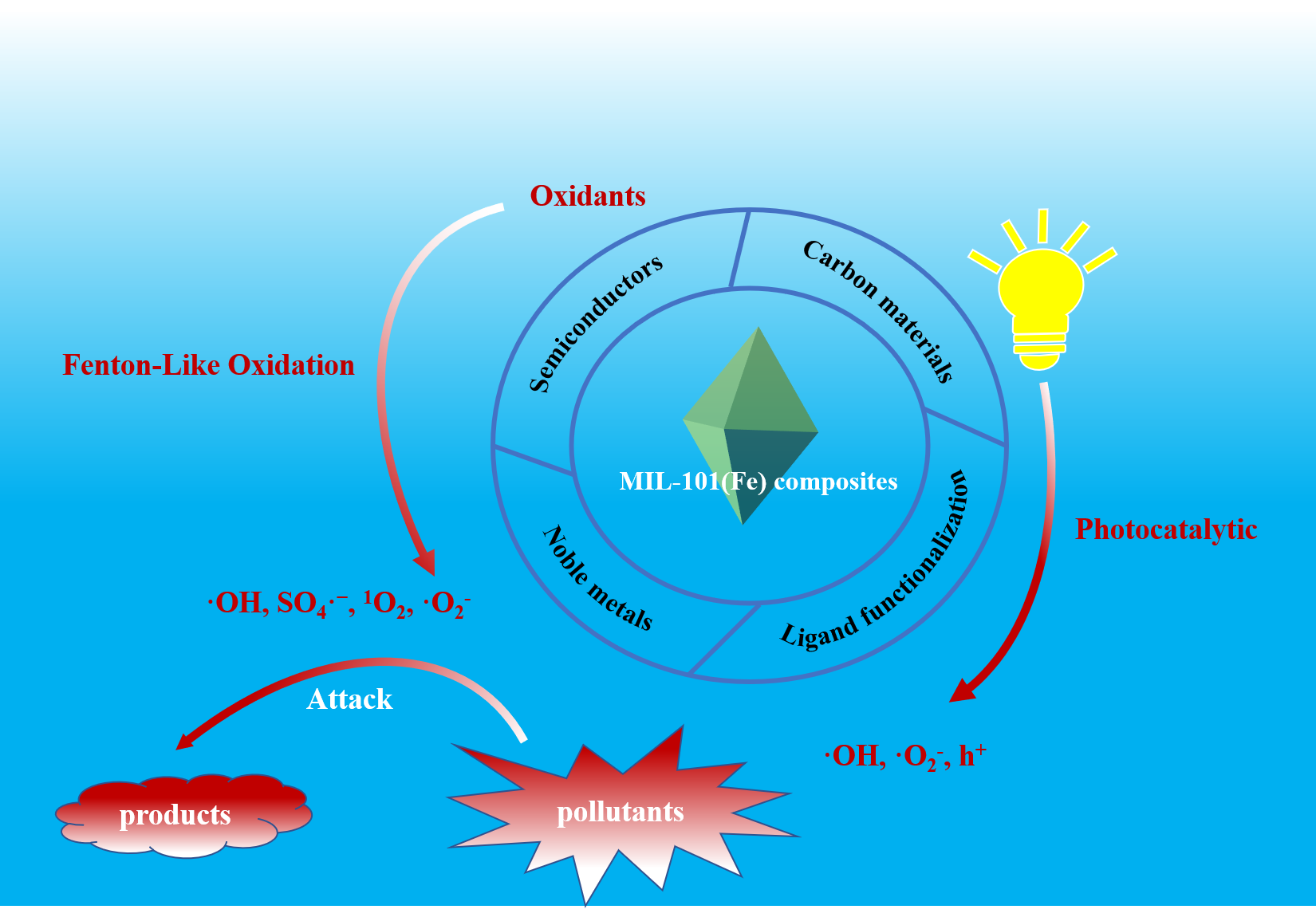
MIL-101(Fe)是一种典型的铁基金属有机框架材料(Fe-MOFs),具有结构灵活、比表面积大、孔隙率大、孔径可调节等优点。近年来,MIL-101(Fe)及其复合物在水污染修复领域得到了广泛的研究,特别是在还原六价铬(Cr(Ⅵ))和高级氧化去除水中有机污染物方面展现出良好的应用前景。通过功能化修饰以及与特定功能材料复合等方法可进一步改善MIL-101(Fe)的水稳定性、增强其光吸收特性和促进载流子分离效率等。本文重点综述了MIL-101(Fe)及其复合物的制备策略及其作为异相催化材料实现光催化还原Cr(Ⅵ)和高级氧化(光催化、活化H2O2和活化过硫酸盐)去除水中有机污染物的研究进展,并对MIL-101(Fe)及其复合物今后的发展予以展望。
分享此文:
| Catalyst/Dosage (g·L-1) | Volume (mL)/ Concentration (mg·L-1)/pH | Light source | Reaction time (min) | Degradation efficiency (%) | ref |
|---|---|---|---|---|---|
| NH2-MIL-101(Fe)/0.5 | 40/80/2 | visible light | 60 | 100 | |
| 150-g-C3N4/NH2-MIL-101(Fe)/0.5 | 40/10/2 | 300 W Xe lamp (λ≥ 400 nm) | 60 | 100 | |
| MIL-101(Fe)/g-C3N4/0.5 | 40/20/5 | 150 W halogen cold light source (λ≥ 420 nm) | 60 | 92.6 | |
| 1%Ag/AgCl/MIL-101(Fe/)1 | 50/10/6 | 300 W Xe lamp (λ≥ 420 nm) | 75 | 100 | |
| Cellulose/NH2-MIL-101(Fe) hybrid foams/1 | 40/20/5 | light intensity: 100 mW/cm2(λ≥ 420 nm) | 180 | 100 | |
| Sand-Cl@NH2-MIL-101(Fe)-50%/0.5 | 100/10/ | 1000 W halogen lamp | 20 | 97.3 | |
| g-C3N4 (150 mg)/ NH2-MIL-101(Fe)/1 | 30/20/2 | solar light (60,000 lux) | 90 | 91 | |
| MIL-101(Fe)-NH2@Al2O3/0.3 | 50/5/3.4 | 300 W Xe lamp | 180 | 100 | |
| TmErNd@Nd(x)@NFM/0.5 | 40/20/2 | 300 W Xe lamp | 50 | 91 |
| Catalyst/dosage (g·L-1) | Polluant/Volume (mL)/ Concentration (mg·L-1)/pH | Light source | Reaction time (min) | Degradation efficiency (%) | ref |
|---|---|---|---|---|---|
| MIL-101(Fe)/0.5 | tetracycline/100/50/- | 300 W Xe lamp (λ≥ 420 nm) | 180 | 96.6 | |
| V2O5/NH2-MIL-101(Fe)-10/0.5 | tetracycline/100/-/- | ultraviolet-visible light from a 300 W xenon lamp | 120 | 88.3 | |
| NH2-MIL-101(Fe)/Cu2O-2/1 | rhodamine B/100/4.8/- | 300 W Xe lamp (λ≥ 420 nm) | 90 | 92 | |
| Electrospun graphene oxide/MIL-101(Fe)/poly (acrylonitrile-co-maleic acid) nanofiber/2 | rhodamine B/20/-/- | ultraviolet lamp (16 W) | 20 | 93.7 | |
| carbon fibers/TiO2/MIL-101(Fe)/2 | 17β-estradiol/100/3/-;tetracycline/100/20/- | visible light | 60 | 87.4 (17β-estradiol)/94.2 (tetracycline) | |
| m-MIL-101-1.0/0.5 | tetracycline/20/20/- | 300 W Xe lamp (λ≥ 420 nm) | 60 | 85.41 | |
| Magnetic MIL-101(Fe)/TiO2/1 | tetracycline/50/20/7 | solar light | 10 | 92.76 | |
| 5-Bi2MoO6/MIL-101(Fe)/0.3 | rhodamine B/100/15/6.5 | blue light LED | 83.2 | 90 | |
| MIL-101(Fe)/gC3N4/0.5 | bisphenol A/40/10/6.8 | 150 W halogen cold light source (λ≥ 420 nm) | 240 | 94.8 | |
| 1%Ag/AgCl/MIL-101(Fe/)1 | phenol/50/10/6 | 300 W Xe lamp (λ≥ 420 nm) | 30 | 70 | |
| g-C3N4/NH2-MIL-101(Fe)/1 | 2,6-dichlorophen/30/10/- 2,4,5-trichlorophenol/30/10/- | 300 W Xe-lamp | 180 | 98.7 (2,6- dichlorophen)/ 97.3 (2,4,5- trichlorophenol) | |
| Cu2O/Fe3O4/MIL-101(Fe)/0.5 | ciprofloxacin//20/7 | 500 W Xe lamp | 105 | 99.2 | |
| NCQDs/MIL-101(Fe)/0.5 | tetracycline/100/10/- | 500 W Xe lamp (λ≥ 420 nm) | 180 | 100 | |
| g-C3N4@NiO/Ni-3@MIL-101/0.01 | ibuprofen/30/30/- | 500W Xenon (λ>400 nm) | 120 | 95.6 | |
| Tm@Yb@Y/NMF/0.03 | tetracycline/levofloxacin/ rhodamine B/60/20/- | 500 W Xe lamp | 50 | 47 (tetracycline)/ 70 (levofloxacin)/ 77 (rhodamine B) | |
| NH2-MIL-101(Fe)/Ti3C2Tx/1 | phenol/chlorophenol/100/23.5/- | 300 W Xe lamp (λ≥ 420 nm) | 60 | 99.36 (phenol)/ 99.83 (chlorophenol) |
| Catalyst/dosage (g·L-1) | Polluant/Volume (mL)/ Concentration (mg·L-1)/pH | H2O2 dosage | Light source | Reaction time (min) | Degradation efficiency (%) | ref |
|---|---|---|---|---|---|---|
| MIL-101(Fe)/0.1 | phenol/150/50/4 | 15 mM | in dark | 30 | 62 | |
| Fe3O4/MIL-101(Fe)/0.5 | rhodamine B/100/10/7 | 20 mM | in dark | 30 | 100 | |
| NH2-MIL-101(Fe)/0.1 | rhodamine B/50/0.025 mM/7.22 | 0.5 mL | in dark | 4 | 100 | |
| GA/MIL-101(Fe)/0.1 | phenol/50/0.1 mM/5 | 6 mM | in dark | 40 | 99 | |
| MIL-101(Fe,Cu)/0.1 | ciprofloxacin/100/20/7 | 3 mM | in dark | 30 | 100 | |
| NH2-MIL-101(Fe) -EPU/0.5 | tetrabromobisphenol A/20/1.84 mM/3 | 165 mM | light-emitting diodes (λ≥ 400 nm) | 120 | 120 | |
| MIL-101(Fe,Co)/0.2 | ciprofloxacin/100/20/5 | 5 mM | in dark | 30 | 97.8 | |
| NH2-MIL-101(Fe)/0.2 | bisphenol A/50/50/6 | 10 mM | in dark | 30 | 100 | |
| MIL-101 (Fe)/PANI/Pd/0.05 | methylene Blue/-/25/7 | 1 M | - | 34 | 92 | |
| MoS2@NH2-MIL-101(Fe)/0.2 | rhodamine B/50/50/- bisphenol A/50/20/- | 1.76 mM | 300 W Xe lamp | 10 | 97.4 (RhB) 99.9 (BPA) | |
| Fe/Ce-MIL-101/0.3 | norfloxacin/-/10/7 | 20 mM | in dark | 60 | 94.8 | |
| TiO2@17%NH2-MIL-101(Fe)/1 | methylene Blue/100/50/- | - | 300 W Xe lamp (λ≥ 420 nm) | 30 | 96 | |
| CNT@MIL-101(Fe)/0.5 | ciprofloxacin/100/3.02 μM/3 | 165 mM | white light LEDs, 360-830 nm | 45 | 90 | |
| GO@MIL-101(Fe)/0.5 | tris(2-chloroethyl) phosphate/-/3.51μM/3 | 165 mM | multiple wavelength LEDs | 30 | 95 | |
| AFG@30MIL-101(Fe)/0.4 | diazinon/50/30/9 atrazine/50/30/2 | 1.5 mL | high-pressure mercury- vapor lamp (400 W and λ = 546.8 nm) | 120 | 100 (diazinon) 81 (atrazine) | |
| MIL/Co/(3%)GO/0.2 | direct Red 23/-/100/3 reactive Red 198/-/100/3 | 50 μL | 100 W LED projector | 70 | 99.93 (Direct Red 23) 99.65 (Reactive Red 198) | |
| MIL-101(Fe)@Zn/Co-ZIFs/0.2 | rhodamine B/50/100/5 | 90 mM | 350 W Xe lamp (λ≥ 420 nm) | 180 | 98 | |
| MIL-101(Fe)/Bi2WO6/Fe(Ⅲ)/ 0.5 | methylene Blue/100/20/- | 500 μL | 200 W incandescent lamp | 75 | 86.7 | |
| MIL-101(Fe)-NH2@Al2O3/0.3 | norfloxacin/50/10/- | 15 μL | 350 W Xe lamp | 97.3 | 100 |
| Catalyst/Dosage (g·L-1) | Polluant/Volume (mL)/ Concentration (mg·L-1)/pH | PS dosage | Light source | Reaction time (min) | Degradation efficiency (%) | ref |
|---|---|---|---|---|---|---|
| MIL-101(Fe)/0.625 | acid orange 7/25/80/6.16 | 15 mM | in dark | 120 | 95 | |
| Fe3O4@MIL-101/1 | acid orange 7/10/25/3.58 | 25 mM | in dark | 60 | 98.1 | |
| Quinone-modified NH2-MIL-101(Fe)/0.2 | bisphenol A/25/60/5.76 | 10 mM | in dark | 120 | 97.7 | |
| 6 wt% Co-MIL-101(Fe)/0.2 6 wt% Cu-MIL-101(Fe)/0.2 | acid orange 7/100/0.1 mM/- | 8 mM | in dark | 180 | 92 (6 wt% Co-MIL-101(Fe)) 98 (6 wt% Co-MIL-101(Fe)) | |
| g-C3N4/MIL-101(Fe)/0.5 | bisphenol A/-/10/- | 1 mM | 350 W Xe lamp (λ≥ 400 nm) | 60 | 98 | |
| MIL-101(Fe) via vacuum thermal treatment/0.1 | X-3B/100/100/- | 15 mM | in dark | 180 | 95.7 | |
| MIL-101(Fe)/0.5 | tris(2-chloroethyl) phosphate/20/3.51 μM/- | 500 mg·L-1 | light-emitting diodes (LEDs) with emission peaks | 180 | > 90 | |
| MIL-101(Fe)/TiO2/1 | tetracycline/-/80/7 | 1 g·L-1 | 500 W Xe lamp | 30 | 93.02 | |
| MIL-101(Fe)-NH2/1 | amaranth/200/50/7 | 200 mg·L-1 | 150 W visible light | 30 | 100 | |
| NH2-MIL-101(Fe)/0.02 | bisphenol F/200/20/5 | 1 mM | in dark | 120 | 100 | |
| MIL-101(Fe)/1 | methylene Blue/20/10/7 | 500 mg·L-1 | in dark | 25 | > 90 | |
| N,S:CQD/MIL-101(Fe)/0.4 | bisphenol A/100/20/- | 3 mM | 350 W Xe lamp (λ≥ 400 nm) | 60 | 100 | |
| CuS-modified MIL-101(Fe)/0.1 | E. coli/100/ 107.5 cfu· mL-1/6.5 | 50 μM | white LED lamps (11,000 Lux, 400~700 nm) | 40 | 100 | |
| TiO2@MIL-101(Fe)/1.052 | nitrobenzene/28.5/800 μM/- | 1.6 mM | Xe lamp (λ≥ 420 nm) | 240 | 66.53 | |
| RGO/MIL-101(Fe)/0.5 | trichlorophenol/-/20/3 | 20 mM | in dark | 180 | 92 | |
| MIL-101(Fe)/0.1 | orange G/50/15/3 | 0.05 mM | in dark | 40 | 74 | |
| MIL-101(Fe)/g-C3N4/0.08 | tetracycline hydrochloride/ 50/-/3.5 | 0.85 mM | 30-W LED lamp (λ=410~760 nm) | 40 | 99 | |
| NH2-MIL-101(Fe)-ferrocene/0.2 | bisphenol A/25/60/5.76 | 10 mM | in dark | 40 | 100 | |
| NH2-MIL-101(FeCo)-2/0.005 | orange G/99/0.2 nM/7 | 2 mM | in dark | 45 | 100 | |
| M/Z2/0.01 | 2-chlorophenol/100/100/9 | 300 mg·L-1 | in dark | 10 | 90.3 |
| [1] |
Wang C C, Yi X H, Wang P. Appl. Catal. B Environ., 2019, 247: 24.
doi: 10.1016/j.apcatb.2019.01.091 URL |
| [2] |
Zhou H C, Kitagawa S. Chem. Soc. Rev., 2014, 43(16): 5415.
doi: 10.1039/C4CS90059F URL |
| [3] |
Ren X Y, Wang C C, Li Y, Wang P, Gao S J. J. Hazard. Mater., 2023, 445: 130552.
doi: 10.1016/j.jhazmat.2022.130552 URL |
| [4] |
Zhang Y M, Yuan S, Day G, Wang X, Yang X Y, Zhou H C. Coord. Chem. Rev., 2018, 354: 28.
doi: 10.1016/j.ccr.2017.06.007 URL |
| [5] |
Qian Y T, Zhang F F, Pang H. Adv. Funct. Mater., 2021, 31(37): 2104231.
|
| [6] |
Chu H Y, Wang T Y, Wang C C. Progress in Chemistry, 2022, 34 (12): 2700.
|
|
(楚宏宇, 王天宇, 王崇臣. 化学进展, 2022, 34 (12): 2700.).
|
|
| [7] |
Li X Y, Zhang H C, Wang P Y, Hou J, Lu J, Easton C D, Zhang X W, Hill M R, Thornton A W, Liu J Z. Nat. Commun., 2019, 10(1): 1.
doi: 10.1038/s41467-018-07882-8 |
| [8] |
Chen X R, Tong R L, Shi Z Q, Yang B, Liu H, Ding S P, Wang X, Lei Q F, Wu J, Fang W J. ACS Appl. Mater. Interfaces, 2018, 10(3): 2328.
doi: 10.1021/acsami.7b16522 URL |
| [9] |
Jie B R, Lin H D, Zhai Y X, Ye J Y, Zhang D Y, Xie Y F, Zhang X D, Yang Y Q. Chem. Eng. J., 2023, 454: 139931.
|
| [10] |
Hu Y H, Zhang L. Adv. Mater., 2010, 22(20): E117.
doi: 10.1002/adma.200902096 URL |
| [11] |
Yaghi O M, Li H L. J. Am. Chem. Soc., 1995, 117(41): 10401.
doi: 10.1021/ja00146a033 URL |
| [12] |
FÉrey G, Mellot-Draznieks C, Serre C, Millange F, Dutour J, SurblÉ S, Margiolaki I. Science, 2005, 309(5743): 2040.
doi: 10.1126/science.1116275 URL |
| [13] |
Biswas S, Couck S, Grzywa M, Denayer J F M, Volkmer D, Van Der Voort P. Eur. J. Inorg. Chem., 2012, 2012(15): 2481.
doi: 10.1002/ejic.v2012.15 URL |
| [14] |
Taghizadeh M, Tahami S. Rev. Chem. Eng., 2022,DOI:10.1015/revce-2021-0050.
doi: 10.1015/revce-2021-0050 |
| [15] |
Ravi Y, Prasanthi I, Behera S, Datta K K R. ACS Appl. Nano Mater., 2022, 5(4): 5857.
doi: 10.1021/acsanm.2c01083 URL |
| [16] |
Wang D K, Huang R K, Liu W J, Sun D R, Li Z H. ACS Catal., 2014, 4(12): 4254.
doi: 10.1021/cs501169t URL |
| [17] |
Duan M J, Guan Z Y, Ma Y W, Wan J Q, Wang Y, Qu Y F. Chem. Pap., 2018, 72(1): 235.
doi: 10.1007/s11696-017-0276-7 URL |
| [18] |
Li Z C, Liu X M, Jin W, Hu Q S, Zhao Y P. J. Colloid Interface Sci., 2019, 554: 692.
doi: 10.1016/j.jcis.2019.07.046 URL |
| [19] |
Yang M, Tang J, Ma Q Q, Zheng N N, Tan L. J. Porous Mater., 2015, 22(5): 1345.
doi: 10.1007/s10934-015-0011-0 URL |
| [20] |
Wang F X, Wang C C. Research of Environmental Sciences, 2021, 34(12): 2924.
|
|
(王茀学, 王崇臣. 环境科学研究, 2021, 34(12): 2924.).
|
|
| [21] |
Taylor-Pashow K M L, Della Rocca J, Xie Z G, Tran S, Lin W B. J. Am. Chem. Soc., 2009, 131(40): 14261.
doi: 10.1021/ja906198y pmid: 19807179 |
| [22] |
Dong Y N, Hu T D, Pudukudy M, Su H Y, Jiang L H, Shan S Y, Jia Q M. Mater. Chem. Phys., 2020, 251: 123060.
doi: 10.1016/j.matchemphys.2020.123060 URL |
| [23] |
Wu W B, Decker G E, Weaver A E, Arnoff A I, Bloch E D, Rosenthal J. ACS Cent. Sci., 2021, 7(8): 1427.
doi: 10.1021/acscentsci.1c00686 URL |
| [24] |
Huang P P, Yao L L, Chang Q, Sha Y H, Jiang G D, Zhang S H, Li Z. Chemosphere, 2022, 291: 133026.
doi: 10.1016/j.chemosphere.2021.133026 URL |
| [25] |
Zhao X D, Zhang C W, Liu B S, Zhao H F, Gao X L, Wang Y Y, Zhang Y Z, Liu D H, Wang C C. Resour. Conserv. Recycl., 2023, 188: 106647.
doi: 10.1016/j.resconrec.2022.106647 URL |
| [26] |
Zhang L, Wang C Y, Wang C C. Resour. Conserv. Recycl., 2023, 190: 106805.
doi: 10.1016/j.resconrec.2022.106805 URL |
| [27] |
Zhao F P, Liu Y P, Ben Hammouda S, Doshi B, Guijarro N, Min X B, Tang C J, Sillanpää M, Sivula K, Wang S B. Appl. Catal. B Environ., 2020, 272: 119033.
doi: 10.1016/j.apcatb.2020.119033 URL |
| [28] |
Huo Q, Liu G Q, Sun H H, Fu Y F, Ning Y, Zhang B Y, Zhang X B, Gao J, Miao J R, Zhang X L, Liu S Y. Chem. Eng. J., 2021, 422: 130036.
doi: 10.1016/j.cej.2021.130036 URL |
| [29] |
Vu T A, Le G H, Dao C D, Dang L Q, Nguyen K T, Dang P T, Tran H T K, Duong Q T, Nguyen T V, Lee G D. RSC Adv., 2014, 4(78): 41185.
doi: 10.1039/C4RA06522K URL |
| [30] |
Jiang Y N, Wang Z J, Huang J B, Yan F, Du Y, He C S, Liu Y, Yao G, Lai B. Chem. Eng. J., 2022, 439: 135788.
doi: 10.1016/j.cej.2022.135788 URL |
| [31] |
Xu J J, Wang S Y, Yan C Y, Adeel Sharif H M, Yang B. Chemosphere, 2022, 288: 132666.
doi: 10.1016/j.chemosphere.2021.132666 URL |
| [32] |
Gong J Q, Zhang W W, Sen T, Yu Y C, Liu Y C, Zhang J L, Wang L Z. ACS Appl. Nano Mater., 2021, 4(5): 4513.
doi: 10.1021/acsanm.1c00119 URL |
| [33] |
O’Brien T J, Ceryak S, Patierno S R. Mutat. Res., Fundam. Mol. Mech. Mutagen., 2003, 533(1/2): 3.
doi: 10.1016/j.mrfmmm.2003.09.006 URL |
| [34] |
Wang C C, Du X D, Li J, Guo X X, Wang P, Zhang J. Appl. Catal. B Environ., 2016, 193: 198.
doi: 10.1016/j.apcatb.2016.04.030 URL |
| [35] |
Al-Fartusie F S, Mohssan S N. Indian J. Adv. Chem. Sci., 2017, 5(3): 127.
|
| [36] |
Wang C C, Ren X Y, Wang P, Chang C. Chemosphere, 2022, 303: 134949.
|
| [37] |
Wang F X, Yi X H, Wang C C, Deng J G. Chinese Journal of Catalysis., 2017, 38(12): 2141.
doi: 10.1016/S1872-2067(17)62947-4 URL |
|
(王茀学, 衣晓虹, 王崇臣, 邓积光. 催化学报, 2017, 38(12): 2141.).
|
|
| [38] |
Li Y H, Yi X H, Li Y X, Wang C C, Wang P, Zhao C, Zheng W W. Environ. Res., 2021, 201: 111596.
doi: 10.1016/j.envres.2021.111596 URL |
| [39] |
Wang J W, Qiu F G, Wang P, Ge C J, Wang C C. J. Clean. Prod., 2021, 279: 123408.
doi: 10.1016/j.jclepro.2020.123408 URL |
| [40] |
Zhou Y C, Wang P, Fu H F, Zhao C, Wang C C. Chin. Chem. Lett., 2020, 31(10): 2645.
doi: 10.1016/j.cclet.2020.02.048 URL |
| [41] |
Du X D, Yi X H, Wang P, Zheng W W, Deng J G, Wang C C. Chem. Eng. J., 2019, 356: 393.
doi: 10.1016/j.cej.2018.09.084 URL |
| [42] |
Zhao Q, Yi X H, Wang C C, Wang P, Zheng W W. Chem. Eng. J., 2022, 429: 132497.
doi: 10.1016/j.cej.2021.132497 URL |
| [43] |
Doan V D, Huynh B A, Le Pham H A, Vasseghian Y, Le V T. Environ. Res., 2021, 201: 111593.
doi: 10.1016/j.envres.2021.111593 URL |
| [44] |
Shi L, Wang T, Zhang H B, Chang K, Meng X G, Liu H M, Ye J H. Adv. Sci., 2015, 2(3): 1500006.
|
| [45] |
Liu B K, Wu Y J, Han X L, Lv J H, Zhang J T, Shi H Z. J. Mater. Sci. Mater. Electron., 2018, 29(20): 17591.
doi: 10.1007/s10854-018-9862-x |
| [46] |
Liu J, Hao D D, Sun H W, Li Y, Han J L, Fu B, Zhou J C. Ind. Eng. Chem. Res., 2021, 60(33): 12220.
doi: 10.1021/acs.iecr.1c01777 URL |
| [47] |
Sadeghian S, Pourfakhar H, Baghdadi M, Aminzadeh B. Chemosphere, 2021, 268: 129365.
doi: 10.1016/j.chemosphere.2020.129365 URL |
| [48] |
Pattappan D, Kavya K V, Vargheese S, Kumar R T R, Haldorai Y. Chemosphere, 2022, 286: 131875.
doi: 10.1016/j.chemosphere.2021.131875 URL |
| [49] |
Jiang J M, Huang F H, Bai R, Zhang J L, Wang L. J. Environ. Chem. Eng., 2022, 10(3): 107908.
|
| [50] |
Fu Y H, Sun D R, Chen Y J, Huang R K, Ding Z X, Fu X Z, Li Z H. Angew. Chem. Int. Ed., 2012, 51(14): 3364.
doi: 10.1002/anie.v51.14 URL |
| [51] |
Ai L H, Zhang C H, Li L L, Jiang J. Appl. Catal. B Environ., 2014, 148/149: 191.
|
| [52] |
Li Y X, Wang X, Wang C C, Fu H F, Liu Y B, Wang P, Zhao C. J. Hazard. Mater., 2020, 399: 123085.
doi: 10.1016/j.jhazmat.2020.123085 URL |
| [53] |
Li Y X, Fu H F, Wang P, Zhao C, Liu W, Wang C C. Environ. Pollut., 2020, 256: 113417.
doi: 10.1016/j.envpol.2019.113417 URL |
| [54] |
Liu X L, Ma R, Zhuang L, Hu B W, Chen J R, Liu X Y, Wang X K. Crit. Rev. Environ. Sci. Technol., 2021, 51(8): 751.
doi: 10.1080/10643389.2020.1734433 URL |
| [55] |
Wen J Q, Xie J, Chen X B, Li X. Appl. Surf. Sci., 2017, 391: 72.
doi: 10.1016/j.apsusc.2016.07.030 URL |
| [56] |
Li X Y, Pi Y H, Wu L Q, Xia Q B, Wu J L, Li Z, Xiao J. Appl. Catal. B Environ., 2017, 202: 653.
doi: 10.1016/j.apcatb.2016.09.073 URL |
| [57] |
Ndolomingo M J, Bingwa N, Meijboom R. J. Mater. Sci., 2020, 55(15): 6195.
doi: 10.1007/s10853-020-04415-x |
| [58] |
Tzou Y M, Chen K Y, Cheng C Y, Lee W Z, Teah H Y, Liu Y T. Environ. Pollut., 2020, 261: 114024.
|
| [59] |
Wang D B, Jia F Y, Wang H, Chen F, Fang Y, Dong W B, Zeng G M, Li X M, Yang Q, Yuan X Z. J. Colloid Interface Sci., 2018, 519: 273.
doi: 10.1016/j.jcis.2018.02.067 URL |
| [60] |
Huang C, Wang J, Li M J, Lei X F, Wu Q. Solid State Sci., 2021, 117: 106611.
doi: 10.1016/j.solidstatesciences.2021.106611 URL |
| [61] |
Geng J G, Ma J L, Li F, Ma S Q, Zhang D, Ning X F. Ceram. Int., 2021, 47(10): 13291.
doi: 10.1016/j.ceramint.2020.09.239 URL |
| [62] |
Huang Z J, Lai Z, Zhu D Y, Wang H Y, Zhao C X, Ruan G H, Du F Y. J. Colloid Interface Sci., 2021, 597: 196.
doi: 10.1016/j.jcis.2021.04.020 URL |
| [63] |
Zhang Y, Xiong M Y, Sun A R, Shi Z, Zhu B, Macharia D K, Li F, Chen Z G, Liu J S, Zhang L S. J. Clean. Prod., 2021, 290: 125782.
|
| [64] |
Xie L X, Zhang T S, Wang X Y, Zhu W X, Liu Z L, Liu M S, Wang J, Zhang L, Du T, Yang C Y, Zhu M Q, Wang J L. J. Clean. Prod., 2022, 359: 131808.
doi: 10.1016/j.jclepro.2022.131808 URL |
| [65] |
He L, Dong Y N, Zheng Y E, Jia Q M, Shan S Y, Zhang Y Q. J. Hazard. Mater., 2019, 361: 85.
doi: 10.1016/j.jhazmat.2018.08.079 URL |
| [66] |
Hajiali M, Farhadian M, Tangestaninejad S, Khosravi M. Adv. Powder Technol., 2022, 33(5): 103546.
doi: 10.1016/j.apt.2022.103546 URL |
| [67] |
Su S Y, Xing Z P, Zhang S Y, Du M, Wang Y, Li Z Z, Chen P, Zhu Q, Zhou W. Appl. Surf. Sci., 2021, 537: 147890.
doi: 10.1016/j.apsusc.2020.147890 URL |
| [68] |
Xie Y H, Liu C R, Li D J, Liu Y. Appl. Surf. Sci., 2022, 592: 153312.
doi: 10.1016/j.apsusc.2022.153312 URL |
| [69] |
Cong Y Q, Li Y N, Wang X R, Wei X, Che L, Lv S W. Sep. Purif. Technol., 2022, 297: 121531.
doi: 10.1016/j.seppur.2022.121531 URL |
| [70] |
Ren H H, Bai R, Huang F H, Zhang J L, Wang L. Cryst. Growth. Des., 2022, 22(8): 4864.
doi: 10.1021/acs.cgd.2c00346 URL |
| [71] |
Hu C Y, Jiang Z W, Yang C P, Wang X Y, Wang X, Zhen S J, Wang D M, Zhan L, Huang C Z, Li Y F. Chem. A Eur. J., 2022, 28(54): e202201437.
|
| [72] |
Li Y H, Wang P, Wang C C, Liu Y B. Chin. J. Inorg. Chem., 2022, 38(12): 2342.
|
|
(李渝航, 王鹏, 王崇臣, 刘艳彪. 无机化学学报, 2022, 38(12): 2342.).
|
|
| [73] |
Shi K X, Qiu F G, Wang J W, Wang P, Li H Y, Wang C C. Sep. Purif. Technol., 2023, 309: 122991.
doi: 10.1016/j.seppur.2022.122991 URL |
| [74] |
Yang H, Zhao Z C, Yang Y P, Zhang Z, Chen W, Yan R Q, Jin Y X, Zhang J. Sep. Purif. Technol., 2022, 300: 121846.
doi: 10.1016/j.seppur.2022.121846 URL |
| [75] |
Guo W X, Zhang F, Lin C J, Wang Z L. Adv. Mater., 2012, 24(35): 4761.
doi: 10.1002/adma.201201075 URL |
| [76] |
Du C Y, Zhang Y, Zhang Z, Zhou L, Yu G L, Wen X F, Chi T Y, Wang G L, Su Y H, Deng F F, Lv Y C, Zhu H. Chem. Eng. J., 2022, 431: 133932.
doi: 10.1016/j.cej.2021.133932 URL |
| [77] |
Bokare A D, Choi W. J. Hazard. Mater., 2014, 275: 121.
doi: 10.1016/j.jhazmat.2014.04.054 pmid: 24857896 |
| [78] |
Fu H F, Song X X, Wu L, Zhao C, Wang P, Wang C C. Mater. Res. Bull., 2020, 125: 110806.
|
| [79] |
Wang F X, Wang C C, Du X D, Li Y, Wang F, Wang P. Chem. Eng. J., 2022, 429: 132495.
doi: 10.1016/j.cej.2021.132495 URL |
| [80] |
Zhao Q, Wang C C, Wang P. Chin. Chem. Lett., 2022, 33(11): 4828.
doi: 10.1016/j.cclet.2022.01.033 URL |
| [81] |
Wu L, Wang C C, Chu H Y, Yi X H, Wang P, Zhao C, Fu H F. Chemosphere, 2021, 280: 130659.
doi: 10.1016/j.chemosphere.2021.130659 URL |
| [82] |
Chen D D, Yi X H, Ling L, Wang C C, Wang P. Appl. Organomet. Chem., 2020, 34(9): e5795.
|
| [83] |
Gao C, Chen S, Quan X, Yu H T, Zhang Y B. J. Catal., 2017, 356: 125.
doi: 10.1016/j.jcat.2017.09.015 URL |
| [84] |
Zhang Y W, Liu F, Yang Z C, Qian J S, Pan B C. Nano. Res., 2021, 14(7): 2383.
doi: 10.1007/s12274-020-3239-1 |
| [85] |
Zhao C C, Dong P, Liu Z M, Wu G R, Wang S J, Wang Y Q, Liu F. RSC Adv., 2017, 7(39): 24453.
doi: 10.1039/C7RA01883E URL |
| [86] |
Aboueloyoun Taha A, Huang L B, Ramakrishna S, Liu Y. J. Water Process. Eng., 2020, 33: 101004.
doi: 10.1016/j.jwpe.2019.101004 URL |
| [87] |
Liang H, Liu R P, An X Q, Hu C Z, Zhang X W, Liu H J. Chem. Eng. J., 2021, 414: 128669.
doi: 10.1016/j.cej.2021.128669 URL |
| [88] |
Fu J W, Wang L, Chen Y H, Yan D Y, Ou H S. Environ. Sci. Pollut. Res., 2021, 28(48): 68560.
doi: 10.1007/s11356-021-14834-1 |
| [89] |
Liang H, Liu R P, Hu C Z, An X Q, Zhang X W, Liu H J, Qu J H. J. Hazard. Mater., 2021, 406: 124692.
doi: 10.1016/j.jhazmat.2020.124692 URL |
| [90] |
Karami K, Beram S M, Siadatnasab F, Bayat P, Ramezanpour A. J. Mol. Struct., 2021, 1231: 130007.
|
| [91] |
Li Z T, Gu Y F, Li F T. J. Environ. Chem. Eng., 2022, 10(3): 107686.
doi: 10.1016/j.jece.2022.107686 URL |
| [92] |
Bao C S, Zhao J, Sun Y Y, Zhao X L, Zhang X H, Zhu Y K, She X L, Yang D J, Xing B S. Environ. Sci.: Nano, 2021, 8(8): 2347.
|
| [93] |
Ma Y W, Lu Y F, Hai G T, Dong W J, Li R J, Liu J H, Wang G. Sci. Bull., 2020, 65(8): 658.
doi: 10.1016/j.scib.2020.02.001 URL |
| [94] |
Yan D Y, Hu H, Gao N Y, Ye J S, Ou H S. Appl. Surf. Sci., 2019, 498: 143836.
doi: 10.1016/j.apsusc.2019.143836 URL |
| [95] |
Lin J L, Hu H, Gao N Y, Ye J S, Chen Y J, Ou H S. J. Water Process. Eng., 2020, 33: 101010.
doi: 10.1016/j.jwpe.2019.101010 URL |
| [96] |
Fakhri H, Farzadkia M, Boukherroub R, Srivastava V, Sillanpää M. Sol. Energy, 2020, 208: 990.
doi: 10.1016/j.solener.2020.08.050 URL |
| [97] |
Bagherzadeh S B, Kazemeini M, Mahmoodi N M. J. Colloid Interface Sci., 2021, 602: 73.
doi: 10.1016/j.jcis.2021.05.181 URL |
| [98] |
Li Y C, Wang X Y, Duan Z Y, Yu D H, Wang Q, Ji D D, Liu W X. Sep. Purif. Technol., 2022, 293: 121099.
doi: 10.1016/j.seppur.2022.121099 URL |
| [99] |
Song R T, Yao J, Yang M, Ye Z B, Xie Z, Zeng X. Nanoscale, 2022, 14(18): 7055.
doi: 10.1039/D1NR07915H URL |
| [100] |
Lin H D, Jie B R, Ye J Y, Zhai Y X, Luo Z J, Shao G J, Chen R Z, Zhang X D, Yang Y Q. Surf. Interfaces, 2023, 36: 102564.
|
| [101] |
Zheng L, Gu Y F, Hua B L, Fu J R, Li F T. Chemosphere, 2022, 307: 135728.
doi: 10.1016/j.chemosphere.2022.135728 URL |
| [102] |
Wang J L, Tang J T. J. Mol. Liq., 2021, 332: 115755.
doi: 10.1016/j.molliq.2021.115755 URL |
| [103] |
Wu Q S, Yang H P, Kang L, Gao Z, Ren F F. Appl. Catal. B Environ., 2020, 263: 118282.
doi: 10.1016/j.apcatb.2019.118282 URL |
| [104] |
Jiang S T, Zhao Z Y, Chen J F, Yang Y, Ding C Y, Yang Y Q, Wang Y X, Liu N, Wang L, Zhang X D. Surf. Interfaces, 2022, 30: 101843.
|
| [105] |
Zhang X W, Lan M Y, Wang F, Yi X H, Wang C C. J. Environ. Chem. Eng., 2022, 10(3): 107997.
doi: 10.1016/j.jece.2022.107997 URL |
| [106] |
Zhang X W, Lan M Y, Wang F, Wang C C, Wang P, Ge C J, Liu W. Chem. Eng. J., 2022, 450: 138082.
doi: 10.1016/j.cej.2022.138082 URL |
| [107] |
Yi X H, Wang C C. Progress in Chemistry, 2021, 33(03): 471.
|
|
(衣晓虹, 王崇臣. 化学进展, 2021, 33(03): 471.).
|
|
| [108] |
Li X H, Guo W L, Liu Z H, Wang R Q, Liu H. Appl. Surf. Sci., 2016, 369: 130.
doi: 10.1016/j.apsusc.2016.02.037 URL |
| [109] |
Yue X X, Guo W L, Li X H, Zhou H H, Wang R Q. Environ. Sci. Pollut. Res., 2016, 23(15): 15218.
doi: 10.1007/s11356-016-6702-5 URL |
| [110] |
Li X H, Guo W L, Liu Z H, Wang R Q, Liu H. J. Hazard. Mater., 2017, 324: 665.
doi: 10.1016/j.jhazmat.2016.11.040 URL |
| [111] |
Gong Y, Yang B, Zhang H, Zhao X. J. Mater. Chem. A, 2018, 6(46): 23703.
doi: 10.1039/C8TA07915C URL |
| [112] |
Yang J Y, Zeng Z Q, Huang Z G, Cui Y. Catalysts, 2019, 9(11): 906.
doi: 10.3390/catal9110906 URL |
| [113] |
Hu H, Zhang H X, Chen Y, Chen Y J, Zhuang L, Ou H S. Chem. Eng. J., 2019, 368: 273.
doi: 10.1016/j.cej.2019.02.190 URL |
| [114] |
He L, Zhang Y Q, Zheng Y E, Jia Q M, Shan S Y, Dong Y N. J. Porous Mater., 2019, 26(6): 1839.
doi: 10.1007/s10934-019-00778-y |
| [115] |
Zhang M W, Lin K Y A, Huang C F, Tong S P. Sep. Purif. Technol., 2019, 227: 115632.
|
| [116] |
Liu Z, Su R D, Sun X, Zhou W Z, Gao B Y, Yue Q Y, Li Q. Sci. Total Environ., 2020, 741: 140464.
doi: 10.1016/j.scitotenv.2020.140464 URL |
| [117] |
Xiao Z Y, Li Y, Fan L, Wang Y X, Li L. J. Colloid Interface Sci., 2021, 589: 298.
doi: 10.1016/j.jcis.2020.12.123 URL |
| [118] |
Jiang H, Zhong Y, Tian K X, Pang H L, Hao Y Y. Appl. Surf. Sci., 2022, 577: 151902.
doi: 10.1016/j.apsusc.2021.151902 URL |
| [119] |
Xu Y Y, Wang Y, Wan J Q, Ma Y W. Chemosphere, 2020, 240: 124849.
doi: 10.1016/j.chemosphere.2019.124849 URL |
| [120] |
Moazeni M, Hashemian S M, Sillanpää M, Ebrahimi A, Kim K H. J. Environ. Manag., 2022, 303: 113897.
doi: 10.1016/j.jenvman.2021.113897 URL |
| [121] |
Bi H, Liu C Z, Li J Y, Tan J. J. Solid State Chem., 2022, 306: 122741.
|
| [122] |
Wang Y, Guo W L, Li X H. RSC Adv., 2018, 8(64): 36477.
doi: 10.1039/C8RA07007E URL |
| [123] |
Li H X, Lou Y C, Zheng J T, Su L Y, Lu S, Xu C, Huang J G, Zhou Q W, Tang J H, Huang M Z. J. Environ. Chem. Eng., 2022, 10(5): 108272.
doi: 10.1016/j.jece.2022.108272 URL |
| [124] |
Gu A T, Wang P, Chen K W, Djam Miensah E, Gong C H, Jiao Y, Mao P, Chen K, Jiang J L, Liu Y, Yang Y. Sep. Purif. Technol., 2022, 298: 121461.
doi: 10.1016/j.seppur.2022.121461 URL |
| [125] |
Ezzatahmadi N, Ayoko G A, Millar G J, Speight R, Yan C, Li J H, Li S Z, Zhu J X, Xi Y F. Chem. Eng. J., 2017, 312: 336.
doi: 10.1016/j.cej.2016.11.154 URL |
| [126] |
Xu L J, Wang J L. Appl. Catal. B Environ., 2012, 123/124: 117.
doi: 10.1016/j.apcatb.2012.04.028 URL |
| [127] |
Jabbari V, Veleta J M, Zarei-Chaleshtori M, Gardea-Torresdey J, Villagrán D. Chem. Eng. J., 2016, 304: 774.
doi: 10.1016/j.cej.2016.06.034 URL |
| [128] |
Guo H S, Su S N, Liu Y, Ren X H, Guo W L. Environ. Sci. Pollut. Res., 2020, 27(14): 17194.
doi: 10.1007/s11356-020-08316-z |
| [129] |
Ali Khan U, Liu J J, Pan J B, Ma H C, Zuo S L, Yu Y C, Ahmad A, Ullah S, li B S. Ind. Eng. Chem. Res., 2019, 58(1): 79.
doi: 10.1021/acs.iecr.8b04627 URL |
| [130] |
Li H T, Kang Z H, Liu Y, Lee S T. J. Mater. Chem., 2012, 22(46): 24230.
doi: 10.1039/c2jm34690g URL |
| [131] |
Wang Q J, Wang G L, Liang X F, Dong X L, Zhang X F. Appl. Surf. Sci., 2019, 467/468: 320.
doi: 10.1016/j.apsusc.2018.10.165 URL |
| [132] |
Qin X T, Qiang T T, Chen L, Wang S T. Microporous Mesoporous Mater., 2021, 315: 110889.
|
| [133] |
Qu D, Sun Z C, Zheng M, Li J, Zhang Y Q, Zhang G Q, Zhao H F, Liu X Y, Xie Z G. Adv. Opt. Mater., 2015, 3(3): 360.
doi: 10.1002/adom.v3.3 URL |
| [134] |
Wang L J, Li X T, Yang B, Xiao K, Duan H B, Zhao H Z. Chem. Eng. J., 2022, 450: 138215.
doi: 10.1016/j.cej.2022.138215 URL |
| [135] |
Burtch N C, Jasuja H, Walton K S. Chem. Rev., 2014, 114(20): 10575.
doi: 10.1021/cr5002589 URL |
| [136] |
Kuznicki A, Lorzing G R, Bloch E D. Chem. Commun., 2021, 57(67): 8312.
doi: 10.1039/D1CC02104D URL |
| [137] |
Ettlinger R, Lächelt U, Gref R, Horcajada P, Lammers T, Serre C, Couvreur P, Morris R E, Wuttke S. Chem. Soc. Rev., 2022, 51(2), 464.
|
| [138] |
Tamames-Tabar C, Cunha D, Imbuluzqueta E, Ragon F, Serre C, Blanco-Prieto M J, Horcajada P. J. Mater. Chem. B, 2014, 2(3): 262.
doi: 10.1039/c3tb20832j pmid: 32261505 |
| [139] |
Ruyra À, Yazdi A, Espín J, CarnÉ-Sánchez A, Roher N, Lorenzo J, Imaz I, Maspoch D. Chem. Eur. J., 2015, 21(6): 2508.
doi: 10.1002/chem.201405380 URL |
| [1] | 马云华, 邵晗, 蔺腾龙, 邓钦月. 基于多齿钯化合物的磁性纳米颗粒催化材料的设计合成及应用[J]. 化学进展, 2023, 35(9): 1369-1388. |
| [2] | 申小雨, 杜中田, 郭百睿, 郭忠旭, 梁长海. 1,6-己二醇选择氧化内酯化制备ε-己内酯[J]. 化学进展, 2023, 35(8): 1191-1198. |
| [3] | 刘文亮, 王宇琦, 李晓晗, 张轩瑜, 王继乾. 手性等离子体核壳纳米结构的设计及应用[J]. 化学进展, 2023, 35(8): 1168-1176. |
| [4] | 赵晨妍, 孙宇翔, 杨莉莉, 郑明辉, 刘书廷, 刘国瑞. 六氯丁二烯的排放源及环境污染特征[J]. 化学进展, 2023, 35(7): 1040-1052. |
| [5] | 王男, 魏迎旭, 刘中民. 甲醇制烯烃反应中的凝聚态化学[J]. 化学进展, 2023, 35(6): 839-860. |
| [6] | 王海, 王成涛, 周航, 王亮, 肖丰收. 小分子催化转化中的凝聚态化学[J]. 化学进展, 2023, 35(6): 861-885. |
| [7] | 肖丰收, 吴勤明, 王成涛. 分子筛催化反应中的凝聚态化学[J]. 化学进展, 2023, 35(6): 886-903. |
| [8] | 秦学涛, 周子乔, 马丁. 金属/金属氧化物催化剂的SMSI效应[J]. 化学进展, 2023, 35(6): 928-939. |
| [9] | 王远, 于聿律, 谭心. 二氧化碳氢化制多碳化合物金属纳米簇催化[J]. 化学进展, 2023, 35(6): 918-927. |
| [10] | 李庆贺, 乔波涛, 张涛. 单原子催化中的凝聚态化学[J]. 化学进展, 2023, 35(6): 821-838. |
| [11] | 李帅, 朱娜, 程扬健, 陈缔. NH3选择性催化还原NOx的铜基小孔分子筛耐硫性能及再生研究[J]. 化学进展, 2023, 35(5): 771-779. |
| [12] | 李佳烨, 张鹏, 潘原. 在大电流密度电催化二氧化碳还原反应中的单原子催化剂[J]. 化学进展, 2023, 35(4): 643-654. |
| [13] | 邵月文, 李清扬, 董欣怡, 范梦娇, 张丽君, 胡勋. 多相双功能催化剂催化乙酰丙酸制备γ-戊内酯[J]. 化学进展, 2023, 35(4): 593-605. |
| [14] | 王丹丹, 蔺兆鑫, 谷慧杰, 李云辉, 李洪吉, 邵晶. 钼酸铋在光催化技术中的改性与应用[J]. 化学进展, 2023, 35(4): 606-619. |
| [15] | 徐怡雪, 李诗诗, 马晓双, 刘小金, 丁建军, 王育乔. 表界面调制增强铋基催化剂的光生载流子分离和传输[J]. 化学进展, 2023, 35(4): 509-518. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||