INTRODUCTION
Compound 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone is a derivative of chalcone, a secondary metabolite belonging to the flavonoid group. Chalcone has several biological activities, including antiplatelet, antibacterial, immunomodulatory, antihyperglycemic, and anti-inflammatory [1]. Several studies reported that 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone demonstrated similar pharmaceutical effects to its parent compound, such as anti-inflammatory and anticancer. Its anti-inflammatory activity has been explored previously by Wibowo [2], and its effect was similar to ibuprofen. In addition, the cytotoxic activity of 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone toward cancer cells was determined using WiDr colon cancer cells, and the result suggested that it inhibited the growth of the cells with IC50 value of 16 μM [3]. This compound has low water solubility, which is one of the major obstacles in developing and administering new chemical entities in medical therapy, specifically for oral delivery drugs. Therefore, finding solutions to overcome the poor solubility of 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone is expected to increase its application through oral, as the most preferred route of drug administration.
Several approaches have been applied to enhance the oral bioavailability of poorly water-soluble materials or lipophilic drugs such as a nano-based delivery system that includes polymeric, lipid-based nanoparticles, nanoemulsion, inorganic particles, carbon nanotubes, self-nano emulsifying drug delivery system (SNEDDS), and so forth [4–9]. SNEDDS has become a promising strategy for the efficient delivery of lipophilic drugs. It is a nanocarrier consisting of a mixture of oil, surfactants, and cosurfactants that forms an oil-in-water nanoemulsion spontaneously and encapsulates the poorly soluble drug when in contact with gastrointestinal fluid. The nanoemulsion produced has a globule size of <100 nm, thereby providing a larger interfacial area that enhances the absorption of insoluble drugs [10,11]. SNEDDS also provide better enzymatic and chemical stability and have been reported to increase intracellular permeability by increasing the lipid fluidity of enterocytes` membranes as well as inhibiting efflux pumps to improve oral bioavailability [12]. This indicates that the encapsulation of 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone using this method can enhance its probability for further development in medical applications. Therefore, this study aims to develop an SNEDDS for 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone as well as to assess its physicochemical properties and in vitro cytotoxic effect on MCF7 cells.
MATERIALS AND METHODS
Materials
The materials used were 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone which was synthesized at Universitas Muhammadiyah Yogyakarta, Indonesia, without a solvent using the microwave irradiation method by reacting 2,5-dihydroxy acetophenone (CAS 490-78-8) and pyridine-2-carbaldehyde (CAS 1121-60-4), which were catalyzed by K2CO3 (CAS 584-08-7). The process led to the production of a reddish–brown colored crystal that was practically insoluble in water and only soluble in dimethyl sulfoxide (DMSO) (CAS 67-68-5). Other materials include olive oil (Sasso, Italia), tween 80 (Bratachem, Indonesia), polyethylene glycol 400 (Bratachem, Indonesia), aquadest, MCF-7 breast cancer cells, [3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium-bromide] (MTT/CAS 298-93-1) (Biobasic, NY, USA), Dulbecco’s Modified Eagle Medium (DMEM) high glucose culture medium (Gibco, CA, USA), fetal bovine serum (FBS) 10% CAS 9014-81-7 (Sigma, MA, USA), 1% MEM nonessential amino acids (Sigma, MA, USA), pen-strep 2% CAS 8025-06-7 (Gibco, CA, USA), Fungizone 0,5% CAS 1397-89-3 (Gibco, CA, USA), Tripsin Ethylenediamine tetraacetic acid/Tripsin EDTA CAS 9002-07-7 (Gibco, CA, USA), 96-well plate (Nunc, MA, USA), and DMSO CAS 67-68-5 (Merck KGaA, Darmstadt, Germany). Furthermore, the equipment used was Zetasizer Pro® particle size analyzer (PSA) (Malvern, Germany); Tecan Spark® (Tecan Trading AG, Switzerland), software GraphPad Prism 7 (GraphPad Software, CA, USA).
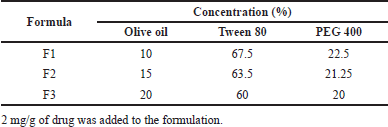 | Table 1. Composition of 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone SNEDDS. [Click here to view] |
Methods
SNEDDS formulation
SNEDDS formulation of 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone was carried out using the composition in Table 1. Furthermore, the drug (2 mg/g SNEDDS) was mixed with olive oil by vortexing for 5 seconds, followed by the addition of a mixture of Tween 80 and PEG 400 based on the formula. All three formulations were subjected to physical characteristic evaluation to select the optimal formula. The optimal formula was then checked for loading capacity by adding 3–6 mg of drug per 1 g SNEDDS until drug precipitation was visible after 24 hours. The formula which produces a transparent solution upon dilution and has the maximum amount of drug dissolved in the system was determined as the loading capacity of the SNEDDS formulation.
Determination of globule size, size distribution, and zeta potential
The globule size and zeta potential were determined using the PSA. Subsequently, 100 μl of SNEDDS samples were mixed with 5 ml of distilled water, and placed in a cuvette for analysis [13].
Emulsification time
Emulsification time was observed on SNEDDS for 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone in 100 ml distilled water. Subsequently, mixing was carried out using a magnetic stirrer at 37°C with a speed of 120 rpm. A total of 1 ml of SNEDDS was then quickly dripped into the media. The complete dissolution of SNEDDS in the media indicated that the nanoemulsion was well formed [14].
Cytotoxic activity
The cytotoxicity of 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone SNEDDS in cancer cells was examined against human breast carcinoma cell line MCF-7 using an MTT assay modified from Ali et al. [15]. The MTT assay is a colorimetric assay based on the intracellular reduction of MTT a yellow tetrazolium salt to a violet formazan product. Human breast cancer cells (MCF-7 cells) were collected from the Integrated Research and Testing Laboratory (LPPT), Universitas Gadjah Mada, Indonesia. Cells were grown in DMEM high glucose (Gibco, CA, USA) liquid culture medium containing 10% FBS (Sigma, MA, USA), 1% MEM NEAA (Sigma, MA, USA), Pen Strep 2% (Gibco, CA, USA), and Fungizone 0,5% (Gibco, CA, USA).
The cells were observed under the inverted microscope. Cells can be harvested if the conditions are 80% confluent. The media was thrown into the flask. PBS was added to the flask as much as 3–4 ml, shaken the flask as the lid closed to wash the residue of the media. PBS was thrown once. The EDTA Tripsin (Gibco, CA, USA, 0.25%), 0.5–1 ml was added and incubated for 4 minutes in a CO2 incubator. The flask should be removed from the incubator and shaken to release cells from the artificial matrix flask. The complete media was added as much as 10 ml to inactivate the EDTA Tripsin 0.25%. The flask should be washed to clean the residue of the cells. The compound was resuspended and transferred to the 15 ml conical flask and centrifugated at 2,500 rpm for 5 minutes. The supernatant was eliminated. The complete media was added as much as 1 ml and then resuspended until homogeny. The cell suspension was taken as much as 1 ml and applied to the hemocytometer by volumetric pipette and observed under a microscope. Finally, the cell was counted by the counter.
The plate 96-well was prepared and filled with 100 μl cell suspension with the number of cells 2 × 104 for each well and then incubated for 24 hours. Cells were treated with SNEDDS 0.375, 0.625, 1.25, 2.5, 5, and 10 ug/ml and incubated depending on the treatment (24 hours). The media in each well was thrown and washed by PBS once, and the MTT reagent (Biobasic, NY, USA) with 0.5 mg/ml concentration was prepared and placed on each well as much as 100 μl. The well was incubated for 4 hours and stopped by DMSO (Merck KGA, Darmstadt, Germany) as much as 100 μl/well. The absorbance was read by Tecan Spark® (Tecan Trading AG, Switzerland) on 570 nm. Finally, the data were analyzed. The average value of cell viability (Eq. 1) with SNEDDS was counted by Microsoft Excel and one way ANOVA with GraphPad Prism 7 (GraphPad Software, CA, USA) software.
RESULTS AND DISCUSSION
Formulation and evaluation of SNEEDS
Several studies revealed that 1-(2,5-dihydroxyphenyl)-3-pyridine-2-yl-propenone, a chalcone derivate, has very low solubility. Therefore, incorporation into SNEDDS is a suitable option to increase its functionality in medical therapy, specifically in the development of oral dosage form, which is still the first choice of drug delivery route. SNEDDS components in this study including olive oil, Tween 80, and PEG 400, were selected based on low cost and availability. This combination of components has also been used as a carrier for several lipophilic drugs that produce a stable and clear emulsified form [16,17]. SNEDDS is an isotropic mixture of oil, surfactant, and or cosurfactant with molecularly soluble drugs. Visual appearance was often used as an early assessment in evaluating the quality of a product [18]. An ideal SNEDDS often has a clear homogenous system with no apparent precipitation or phase separation either in the emulsified or original form, and this indicates the solubility of the incorporated drug [19]. Figure 1 shows the clear yellowish appearance of 1-(2,5-dihydroxyphenyl)-3-pyridine-2-yl-propenone SNEDDS with no visible sedimentation. However, the diluted form produced an optically different emulsion system, which was assessed with a globule size measurement using a PSA. Tables 1 and 2 show that F2 and F3 with a higher concentration of oil had larger globule sizes compared to F1. Although in the original form they have a clear and homogenous system, these formulas produced a turbid emulsion with a globule size above 500 nm after dilution with water. The results showed that diluted F1 was still able to maintain a clear appearance (globule size 10–11 nm).
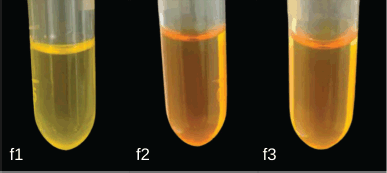 | Figure 1. Visual appearance of SNEDDS 1-(2,5-dihydroxyphenyl)-3-pyridine-2-yl-propenone (F1-F3). [Click here to view] |
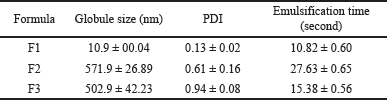 | Table 2. Screening of optimal SNEDDS composition. [Click here to view] |
The droplet size of emulsified SNEDDS can directly affect its performance in terms of dissolution, stability, and absorptivity. These findings indicated that characterization is a crucial parameter in its development. Aside from mean droplet size measurements, the size distribution of oil droplets, which was expressed as polydispersity index (PDI) is also an important factor that must be considered. PDI is the ratio between the SD and the mean droplet size, and it indicates the degree of nonuniformity during the production process. Low PDI indicated the uniformity and narrow size distribution of the polydispersed phase [20]. The results showed that F1, F2, and F3 had values of 0.13 ± 0.02, 0.61 ± 0.16, and 0.94 ± 0.08, respectively, as shown in Table 2. F2 and F3 with higher amounts of the oil phase have large PDI values (>0.5), showing that the emulsion produced has a large droplet size distribution. For a lipid-based carrier, a PDI of 0.3 and below was considered to be acceptable because a highly polydisperse droplet size can lead to aggregation and phase separation upon storage [21].
SNEDDS is a formulation that spontaneously forms a fine oil-in-water emulsion. This attribute was examined by measuring the length of time required for the formula to form a homogenous dispersion in aqueous media, such as gastrointestinal fluids with gentle agitation [22,23]. Furthermore, this phenomenon occurred due to low and negative or positive free energy initiated when the change in entropy causing dispersion of the system was bigger than the force required to increase the surface area of the dispersion phase [24]. SNEDDS with good self-emulsification capacity often requires a short time of <1 minute to emulsify in the medium [25]. In this study, all the formulations tend to emulsify spontaneously with gentle agitation, whereas F1 with the lowest amount of oil phase had the fastest emulsification time, as shown in Table 2. In summary, F1 was categorized as grade A SNEDDS and selected as the optimal formula. This was because it can spontaneously form a fine and homogenous oil-in-water emulsion with a droplet size of <200 nm [26]. The particle size and emulsification capacity of SNEDDS were influenced by its composition. An increase in surfactant and co-surfactant proportion played a key role in decreasing the interfacial tension with the oil phase and produced smaller droplet sizes within a short period. Therefore, F1 with the least amount of oil phase provided better attributes of SNEDDS.
The oil phase used in this study consisted of olive oil as a natural edible material rich in oleic acid, a long triglyceride (C18). The majority of natural vegetable oils have low solubility in water, which affects their dispersion capacity, hence, a greater concentration of surfactant is needed. The surfactant mixture in this study was composed of Tween 80 and PEG 400. Tween 80 is a nonionic component with a relatively high hydrophilic-lipophilic balance (HLB) value of 15. For SNEDDS formulation, a surfactant with an HLB value of >12 was required to enable spontaneous oil-in-water emulsion formation upon dilution. Furthermore, the concentration of surfactant has been demonstrated to affect the droplet size of the emulsion produced. Increasing the amount used can lower the interface between oil and water, thereby reducing the free energy for emulsification and fine globule formation. The addition of cosolvent is very important to lower the interfacial tension, specifically when poorly soluble oil is used in the formulation. Cosolvent, such as PEG 400 can cooperate synergically with a surfactant to enhance drug solubility and fluidity of surfactant in the oil, thereby increasing the stability and homogeneity of the emulsion [27]. The optimized SNEDDS (F1) had the highest proportion of surfactant mixture, 67.5% w/w Tween 80 and 22.5% w/w PEG 400 at a fixed amount of oil (10% w/w).
Formula 1 was then further evaluated to assess its drug-loading efficiency through the addition of various concentrations of 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone. Globule size, PDI, and zeta potential were then used to determine the influence of drug concentration in the formula. Furthermore, the highest amount of drug, which can be added to the formula without the formation of precipitation and significant change in SNEDDS characteristic was determined as drug loading capacity. Table 3 and Figure 2 showed that F1 can solubilize up to 6 mg of drug per 1 g SNEDDS and precipitation was visible after the amount of drug was increased to 7 mg, as shown in Figure 2 (f1). This high loading capacity was influenced by the high concentration of surfactant used in the formula. The addition of the drug to the formula had no significant effect on the globule size of the diluted emulsion (droplet size ranged from 10.67 to 11.22 nm) and PDI. F1 with the highest amount of drug (6 mg) was able to produce a fine, nanosized, and highly monodisperse emulsion.
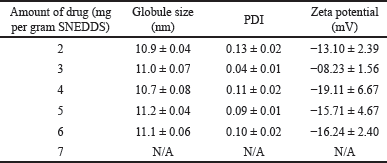 | Table 3. Drug loading capacity of formula 1. [Click here to view] |
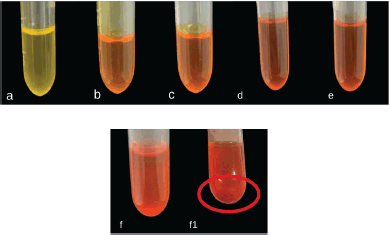 | Figure 2. Visual appearance of optimal SNEDDS formula containing a varied amount of 1-(2,5-dihydroxyphenyl)-3-pyridine-2-yl-propenone the amount of drug from a to f were 2–7 mg, respectively. Sedimentation was found after the addition of 7 mg drug/g SNEDDS (f1). [Click here to view] |
Based on the results, the increase in drug concentration also caused an increment in the zeta potential of the emulsified SNEDDS, as shown in Table 3. This was probably due to the interaction between the functional groups of the compound 1-(2,5-dihydroxyphenyl)-3-pyridine-2-yl-propenone and those of other excipients. Zhang [31] revealed that there was a difference in the zeta potential value between the blank nanoemulsion and the other variant containing curcumin. It was assumed that this was caused by the formation of intermolecular hydrogen bonds between the hydroxyl groups in curcumin and some other related groups containing oxygen or nitrogen atoms in surfactants, cosurfactants, and oils, thereby causing changes in the surface charge of the nanoemulsion globules. Zeta potential is the electrostatic potential found on the surface of the nanoparticles [28]. Furthermore, its value played a significant role in stabilizing nanoformulation. Based on previous findings, values above ±20 mV were acceptable, indicating the presence of a high repulsive force between oil droplets that prevents coalescence and destabilization of the system [29,30]. The zeta potential value of the optimized SNEDDS was lower than 20 mV (−08.23 to −19.11 mV), but it was inaccurate to conclude that the emulsion formed by the optimized SNEDDS was not physically stable. Tween 80, a nonionic surfactant, was provided along with steric without an electrostatic barrier between oil droplets to prevent the system from aggregating, which lowered the zeta potential [31]. The negative value obtained in F1 can be attributed to the presence of some anionic impurities in the surfactant, such as free fatty acids, as well as the adsorption of anionic species from the water, including hydroxyl ions to the droplet surfaces. It was also caused by the charge of encapsulated drug, 1-(2,5-dihydroxyphenyl)-3-pyridine-2-yl-propenone, which has a hydroxyl group in its structure [32,33]. Based on globule size, PDI, and zeta potential, F1 with a maximum loading of 6 mg was selected as the best formula and subjected to anticancer activity evaluation. The summarized physical characteristics of F1 are presented in Table 4.
Effect of SNEDDS treatment on MCF-7 viability assay
The cytotoxicity of 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone SNEDDS in cancer cells was examined against human breast carcinoma cell line MCF-7 using an MTT assay. The cell line was subjected to an increasing concentration of SNEDDS ranging from 0.3125 to 10 μg/ml for 24 hours. The reports showed that the treatment containing 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone decreased the percentage of MCF-7 cell viability in a concentration-dependent manner, as shown in Figure 3. To further measure the anticancer potency, the IC50 values were calculated using the linear regression presented in Figure 3a. Furthermore, the IC50 values of SNEDDS containing 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone was 5.2 μg/ml or 21.57 μM since the molecular weight of the molecule was 241 g/ml. This finding indicated that the treatment showed a potent cytotoxic effect on MCF-7 cells. The observation of cell morphology after SNEDDS treatment in Figure 3b–e also showed a similar result. The cells treated with an increased concentration of 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone SNEDDS experienced morphological changes, such as a decrease in cell volume and shrinkage, which led to the reduction in cell viability percentage. The tested compound 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone was a derivative of 2′, 5′-dihydroxychalcone in which the B ring was replaced by a 2-pyridine ring. The replacement of the B ring of chalcone with a pyridine ring was responsible for its cytotoxicity effect. Xu et al. [34] also reported that the substitution of the compound’s structure with 3,4,5 trimethoxyphenyl moiety in A ring with pyridine ring (an electron donating group) improved the cytotoxic activity by three folds with IC50 values ranging from 0.023 to 0.047 μM. Furthermore, Madhavi et al. [35] revealed that pyridine-incorporated chalcone derivates in the B ring showed potent cytotoxic effects on ACHN, MCF-7, and A549 cell lines with IC50 values ranging from 0.73 to 1.10 μM. Compound 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone has also been investigated for its cytotoxicity effect on other cancer cells, such as WiDr (colon carcinoma) using the MTT assay. The result showed that it had an IC50 value of 16 μM toward the WiDr cell line. Lower values indicated a high cytotoxic effect, showing that this compound was more selective in inhibiting the samples compared to a breast cancer cell [3]. This finding is consistent with Anwar et al. [36] that all four hydroxychalcone derivatives had a higher selectivity index to WiDR compared to T47D breast cancer cells. Apart from the cytotoxic activity of the compound, the use of SNEDDS as a carrier also improved cell cytotoxicity. This was due to SNEDDS composition, which increased the permeability and internalization of the molecularly dissolved drugs into the cancer cells through endocytosis [37]. Based on these results, 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone SNEDDS has the potential to be developed as an anticancer therapy.
 | Table 4. Physical characteristic of optimal SNEDDS 1-(2,5-dihydroxyphenyl)-3-pyridine-2-yl-propenone (drug loading capacity 6 mg/g SNEDDS). [Click here to view] |
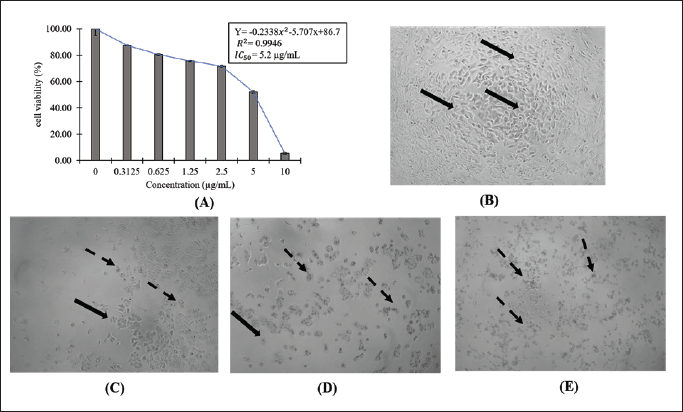 | Figure 3. The cytotoxic effect of 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone SNEDDS on MCF-7 cells. (A) diagram of MCF7 cells viability after 24 hours of treatment and linear regression of IC50 calculation. MCF-7 cells morphology of (B) cell control, (C) after 24 hours of 2.5 μg/ml; (D) 5 μg/ml; (E) 10 μg/ml treatment. The normal cells were indicated with bold arrow ( ) and the changed morphology of cells was shown by dashed arrow ( ). [Click here to view] |
CONCLUSION
To increase the solubility and therapeutic application of a chalcone derivative, particularly in oral dosage form, 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone, a SNEDDS formulation was successfully developed. Furthermore, the optimized SNEDDS was prepared by blending 10% olive oil, 67.5% Tween 80 with 22.5% PEG 400, and this formulation was able to load up to 6 mg drug/g SNEDDS. The product was also able to produce a fine and homogenous emulsion spontaneously upon dilution with low and uniform droplet size, as well as moderate zeta potential. The optimized SNEDDS has moderate cytotoxic activity toward MCF-7 cells and it modified the cells’ morphology, thereby decreasing their viability. Based on these results, 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone SNEDDS had the potential to be developed as an anticancer therapy.
ACKNOWLEDGMENT
The authors are grateful to Lembaga Penelitian and Pengabdian Kepada Masyarakat (LPPM) Universitas Ahmad Dahlan, Lembaga Riset dan Inovasi Universitas Muhammadiyah Yogyakarta, and RisetMu for providing funds for this publication.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJEs) requirements/guidelines.
FINANCIAL SUPPORT
Lembaga Penelitian and Pengabdian kepada Masyarakat (LPPM) Universitas Ahmad Dahlan, Lembaga Riset dan Inovasi Universitas Muhammadiyah Yogyakarta, and RisetMu.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Wibowo AE, Saputra AK, Susidarti RA. Optimization of synthesis of 1-(2,5-dihydroxy phenyl)-3-pyridine-2-yl-propenone, an anti-inflammatoryagent, using NaOH. J Farm Indones. 2018;15(2):204. CrossRef
2. Wibowo AE. Sintesis dan Uji Aktivitas Antiinflamasi Senyawa 1-(2,5-dihidroksifenil)-(3-piridin-2-il) Propenon [Thesis]. Yogyakarta, Indonesia: Fakultas Farmasi Universitas Gadjah Mada; 2013.
3. Ismiyati N, Rini YP, Wibowo AE, Susidarti RA. Cytotoxic activity of 1-(2,5-dihydroxyphenyl)-3-pyridine-2-yl-propenone on colon cancer cell WiDr. Indones J Cancer Chemoprev. 2015;6(1):12–5.
4. Yadollahi R, Vasilev K, Simovic S. Nanosuspension technologies for delivery of poorly soluble drugs. J Nanomater. 2014;2015:216375. CrossRef
5. Sharma M, Sharma R, Jain DK. Nanotechnology based approaches for enhancing oral bioavailability of poorly water soluble antihypertensive drugs. Scientifica (Cairo). 2016;2016:8525679. CrossRef
6. Kumari L, Choudhari Y, Patel P, Gupta GD, Singh D, Rosenholm JM, et al. Advancement in solubilization approaches: a step towards bioavailability enhancement of poorly soluble drugs. Life. 2023;13(5):1099. CrossRef
7. Muqbil I, Masood A, Sarkar FH, Mohammad RM, Azmi AS. Progress in nanotechnology based approaches to enhance the potential of chemopreventive agents. Cancers. 2011;3(1):428–45. CrossRef
8. Wang X, Jiang Y, Wang YW, Huang MT, Ho CT, Huang Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008;108(2):419–24. CrossRef
9. Chopra S, Kohli K, Arora S, Khar RK. In-situ nano-emulsification technique for enhancing oral bioavailability of curcumin and thereby evaluating its anticancer efficacy on human lung adeno-carcinoma epithelial cell line. J Pharm Res. 2011;4:4087–93.
10. Alwadei M, Kazi M, Alanazi FK. Novel oral dosage regimen based on self-nanoemulsifying drug delivery systems for codelivery of phytochemicals—curcumin and thymoquinone. Saudi Pharm J. 2019;27(6):866–6. CrossRef
11. Dokania S, Amita KJ. Self-microemulsifying drug delivery system (SMEDDS)—challanges and road ahead. Drug Deliv. 2015;22(6):675–90. CrossRef
12. Baloch J, Sohail MF, Sarwar HS, Kiani MH, Khan GM, Jahan S, et al. Self-nanoemulsifying drug delivery system (SNEDDS) for improved oral bioavailability of chlorpromazine: in vitro and in vivo evaluation. Medicina (Kaunas). 2019;55(5):210. CrossRef
13. Priani SE, Somantri SY, Aryani R. Formulation and characterization of SNEEDS (self nanoemulsifying drug delivery system) containing black cumin seed oil and olive oil. J Sains Farm Klinis. 2020;7(1):31–8. CrossRef
14. Patel J, Kevin G, Patel A, Raval M, Sheth N. Design and development of a self nanoemulsifying drug delivery system for telmisartan for oral drug delivery. Int J Pharm Investig. 2011;1(2):112–8. CrossRef
15. Ali A, Banerjee S, Kamaal S, Usman M, Das N, Afzal M, et al. Ligand substituent effect on the cytotoxicity activity of two new copper(ii) complexes bearing 8-hydroxyquinoline derivatives: validated by MTT assay and apoptosis in MCF-7 cancer cell line (human breast cancer). RSC Adv. 2021;11(24):14362–73. CrossRef
16. Suryani S. The self-nanoemulsifying drug delivery system formulation of mefenamic acid. Asian J Pharm. 2019;13(4):287. CrossRef
17. Gayathri T, Venkata MR, Rama RN. Formulation and evaluation of self nano emulsifying drug delivery system of raloxifene hydrochloride. JDDT. 2021;11:16–9. CrossRef
18. Ahmed K, Li Y, McClements DJ, Xiao H. Nanoemulsion-and emulsion-based delivery systems for curcumin: encapsulation and release properties. Food Chem. 2012;132(2):799–807. CrossRef
19. Zhang J. Novel emulsion-based delivery systems [Dissertation]. Minneapolis, MN: University of Minnesota; 2011.
20. Chhabra G, Chuttani K, Mishra AK, Pathak K. Design and development of nanoemulsion drug delivery system of amlodipine besilate for improvement of oral bioavailability. Drug Dev Ind Pharm. 2011;37(8):907–16. CrossRef
21. Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. CrossRef
22. Zhao Y, Changguang W, Albert HL, Ke R, Tao G, Zhirong Z, et al. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of zedoary essential oil: formulation and bioavability studies. Int J Pharm. 2010;383(1–2):170–7. CrossRef
23. Zhao Y, Wang C, Chow AH, Ren K, Gong T, Zhang Z, et al. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of zedoary essential oil: formulation and bioavailability studies. Int J Pharm. 2010;383(1–2):170–7. CrossRef
24. Eid AM, El-Enshasy HA, Aziz R, Elmarzugi NA. The preparation and evaluation of self-nanoemulsifying systems containing Swietenia oil and an examination of its anti-inflammatory effects. Int J Nanomed. 2014;9(1):4685–95. CrossRef
25. Makadia HA, Bhatt AY, Parmar RB, Paun JS, Tank HM. Self-nano emulsifying drug delivery system (SNEDDS): future aspects. Asian J Pharm Res. 2013;3(1):21–4.
26. Sagar K, Kendre P, Pande V, Chaundhari V. Design, development, and characterization of self nanoemulsifying drug delivery system (SNEDDS) of nateglinide. World J Pharm Pharm Sci. 2014;3(8):794–811.
27. Buya AB, Beloqui A, Memvanga PB, Préat V. Self-nano-emulsifying drug-delivery systems: from the development to the current applications and challenges in oral drug delivery. Pharmaceutics. 2020;12(12):1194. CrossRef
37. Akhtartavan S, Karimi M, Karimian K, Azarpira N, Khatami M, Heli H. Evaluation of a self-nanoemulsifying docetaxel delivery system. Biomed Pharmacother. 2019;109:2427–33. CrossRef
36. Anwar C, Prasetyo YD, Matsjeh S, Haryadi W, Sholikhah EN, Nendrowati N. Synthesis of chalcone derivatives and their in vitro anticancer test against breast (T47D) and colon (WiDr) cancer cell line. Indones J Chem. 2018;18(1):102–7. CrossRef
35. Madhavi S, Sreenivasulu R, Ansari MY, Ahsan JW, Raju RR. Synthesis, biological evaluation and molecular docking studies of pyridine incorporated chalcone derivatives as anticancer agents. Lett Org Chem. 2016;13(9):682–92. CrossRef
28. Mannuela N. Preparation and evaluation of azithromycin chitosan nanoparticles and test of antibacterial activity against Propionibacterium acnes. J Mahasiswa Farm Fak Kedokt UNTAN. 2016;3(1):1–12.
29. Zanela da Silva Marques T, Santos-Oliveira R, Betzler de Oliveira de Siqueira L, Cardoso VS, Freitas ZMF, Barros RCSA, et al. Development and characterization of a nanoemulsion containing propranolol for topical delivery. Int J Nanomed. 2018;13:2827–37. CrossRef
30. Fitri D, Kiromah NZ, dan Widiastuti TC. Formulasi dan karakterisasi nanopartikel ekstrak etanol daun salam (Syzygium polyanthum) pada berbagai variasi komposisi kitosan dengan metode gelasi ionik. J Pharm Sci Clin Res. 2020;1:61–9. CrossRef
31. Tan TB, Chu WC, Yussof NS, Abas F, Mirhosseini H, Cheah YK, et al. Physicochemical, morphological and cellular uptake properties of lutein nanodispersions prepared by using surfactants with different stabilizing mechanisms. Food Funct. 2016;7(4):2043–51. CrossRef
32. Tiwari SB, Shenoy DB, Amiji MM. Nanoemulsion formulations for improved oral delivery of poorly soluble drugs. TechConnect Briefs. 2006;1:475–8.
33. Tian Y, Chen L, Zhang W. Influence of ionic surfactants on the properties of nanoemulsions emulsified by nonionic surfactants span 80/Tween 80. J Dispers Sci Technol. 2015;37(10):1511–7. CrossRef
34. Xu F, Li W, Shuai W, Yang L, Bi Y, Ma C, et al. Design, synthesis and biological evaluation of pyridine-chalcone derivatives as novel microtubule-destabilizing agents. Eur J Med Chem. 2019;173:1–14. CrossRef