Corrosion and protection of aluminum current collector in lithium-ion batteries
-
GRAPHICAL ABSTRACT

-
PUBLIC SUMMARY
-
Aluminum current collector as carrier for electrode plays a crucial role in affecting electrochemical performance.
Aluminum suffers from chemical and electrochemical corrosions, reducing the electrochemical performance.
The effective protection strategies are presented to suppress the corrosion.
-
-
ABSTRACT
Aluminum (Al) current collector, an important component of lithium-ion batteries (LIBs), plays a crucial role in affecting electrochemical performance of LIBs. In both working and calendar aging of LIBs, Al suffers from severe corrosion issue, resulting in the decay of electrochemical performance. However, few efforts are devoted to the research of Al compared to anode and cathode materials, electrolyte, and even separators in LIBs. Here, the recent research advance in Al corrosion and protection is reviewed. We first briefly overview Al corrosion mechanism and its affecting factors. Then, the advanced technologies used to evaluate the electrochemical, morphology and chemical properties of Al are summarized in order to uncover the Al corrosion mechanism in LIBs. Next, we review the Al protection strategies in Al, electrolyte, and inhibitors with function mechanism, materials selection and their structural design. Finally, we outlook the future research direction in Al corrosion and protection. This review provides experimental and theoretical supports in understanding Al corrosion and development of Al anticorrosion, which will be beneficial to the research communities including corrosions, advanced materials, and energy storage devices. -

-
REFERENCES
[1] Li, M., Lu, J., Chen, Z., and Amine, K. (2018). 30 Years of lithium-ion Batteries. Adv. Mater. 30, 1800561. [2] Yoshino, A. (2012). The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 51, 5798-5800. [3] Zhao, Y., Zhou, T., Ashirov, T., et al. (2022). Fluorinated ether electrolyte with controlled solvation structure for high voltage lithium metal batteries. Nat. Commun. 13, 2575. [4] Schmuch, R., Wagner, R., Hörpel, G., et al. (2018). Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 3, 267-278. [5] Wang, F., Harindintwali, J.D., Yuan, Z., et al. (2021). Technologies and perspectives for achieving carbon neutrality. The Innovation 2, 100180. [6] Xie, J., and Lu, Y.C. (2020). A retrospective on lithium-ion batteries. Nat. Commun. 11, 2499. [7] Boyle, D.T., Huang, W., Wang, H., et al. (2021). Corrosion of lithium metal anodes during calendar ageing and its microscopic origins. Nat. Energy 6, 487-494. [8] Yoon, M., Dong, Y., Huang, Y., et al. (2023). Eutectic salt-assisted planetary centrifugal deagglomeration for single-crystalline cathode synthesis. Nat. Energy 8, 482-491. [9] Wu, F., and Yushin, G. (2017). Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 10, 435-459. [10] Huang, J., Ouyang, B., Zhang, Y., et al. (2023). Inhibiting collective cation migration in Li-rich cathode materials as a strategy to mitigate voltage hysteresis. Nat. Mater. 22, 353-361. [11] Zhang, Z., Zhao, S., Wang, B., and Yu, H. (2020). Local redox reaction of high valence manganese in Li2MnO3-based lithium battery cathodes. Cell Rep. Phys. Sci. 1, 100061. [12] Wang, C., Wang, X., Zhang, R., et al. (2023). Resolving complex intralayer transition motifs in high-Ni-content layered cathode materials for lithium-ion batteries. Nat. Mater. 22, 235-241. [13] Wang, C.Y., Liu, T., Yang, X.G., et al. (2022). Fast charging of energy-dense lithium-ion batteries. Nature 611, 485-490. [14] Li, Z., Sami, I., Yang, J., et al. (2023). Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium–sulfur batteries. Nat. Energy 8, 84-93. [15] Sun, J., Zhang, S., Li, J., et al. (2022). Robust transport: an artificial solid electrolyte interphase design for anode-free lithium-metal batteries. Adv. Mater. 35, 2209404. [16] Lu, B., Li, W., Cheng, D., et al. (2022). Suppressing chemical corrosions of lithium metal anodes. Adv. Energy Mater. 12, 2202012. [17] Shi, X., Qiao, Y., Xing, C., et al. (2022). High-performance lithium metal battery realized by regulating Li+ flux distribution on artificial-solid-electrolyte-interphase functionalized 3D carbon framework-Li anode. Mater. Today Phys. 24, 100672. [18] Yi, T.-F., Mei, J., Peng, P.-P., and Luo, S. (2019). Facile synthesis of polypyrrole-modified Li5Cr7Ti6O25 with improved rate performance as negative electrode material for Li-ion batteries. Compos. Pt. B-Eng. 167, 566-572. [19] Yi, T.f., Shi, L., Han, X., et al. (2020). Approaching high‐performance lithium storage materials by constructing hierarchical CoNiO2@CeO2 nanosheets. Energy Environ. Mater. 4, 586-595. [20] Yi, T.-F., Sari, H.M.K., Li, X., et al. (2021). A review of niobium oxides based nanocomposites for lithium-ion batteries, sodium-ion batteries and supercapacitors. Nano Energy 85, 105955. [21] Wei, T.-T., Peng, P., Ji, Y.-R., et al. (2022). Rational construction and decoration of Li5Cr7Ti6O25@C nanofibers as stable lithium storage materials. J. Energy Chem. 71, 400-410. [22] Liu, J., Bao, Z., Cui, Y., et al. (2019). Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180-186. [23] Acebedo, B., Morant‐Miñana, M.C., Gonzalo, E., et al. (2023). Current status and future perspective on lithium metal anode production methods. Adv. Energy Mater. 13, 2203744. [24] Li, W., Erickson, E.M., and Manthiram, A. (2020). High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 5, 26-34. [25] Lv, H., Li, C., Zhao, Z., et al. (2021). A review: Modification strategies of nickel-rich layer structure cathode (Ni ≥ 0.8) materials for lithium ion power batteries. J. Energy Chem. 60 , 435-450. [26] Lin, D., Liu, Y., and Cui, Y. (2017). Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194-206. [27] Li, J., Kong, Z., Liu, X., et al. (2021). Strategies to anode protection in lithium metal battery: A review. InfoMat 3, 1333-1363. [28] Tan, S., Shadike, Z., Li, J., et al. (2022). Additive engineering for robust interphases to stabilize high-Ni layered structures at ultra-high voltage of 4.8 V. Nat. Energy 7 , 484-494. [29] Haregewoin, A.M., Wotango, A.S., and Hwang, B.-J. (2016). Electrolyte additives for lithium ion battery electrodes: progress and perspectives. Energy Environ. Sci. 9, 1955-1988. [30] Feng, Y., Zhou, L., Ma, H., et al. (2022). Challenges and advances in wide-temperature rechargeable lithium batteries. Energy Environ. Sci. 15, 1711-1759. [31] Wang, Z., Sun, Z., Li, J., et al. (2021). Insights into the deposition chemistry of Li ions in nonaqueous electrolyte for stable Li anodes. Chem. Soc. Rev. 50, 3178-3210. [32] Xia, L., Miao, H., Zhang, C., et al. (2021). Review—recent advances in non-aqueous liquid electrolytes containing fluorinated compounds for high energy density lithium-ion batteries. Energy Storage Mater. 38, 542-570. [33] McOwen, D.W., Seo, D.M., Borodin, O., et al. (2014). Concentrated electrolytes: decrypting electrolyte properties and reassessing Al corrosion mechanisms. Energy Environ. Sci. 7, 416-426. [34] Tateishi, K., Waki, A., Ogino, H., et al. (2012). Formation of Al2O3 film and AlF3 containing Al2O3 film by an anodic polarization of aluminum in ionic liquids. Electrochemistry 80, 556-560. [35] Gabryelczyk, A., Ivanov, S., Bund, A., and Lota, G. (2021). Corrosion of aluminium current collector in lithium-ion batteries: A review. J. Energy Storage 43, 103226. [36] Ma, T., Xu, G.L., Li, Y., et al. (2017). Revisiting the corrosion of the aluminum current collector in lithium-ion batteries. J. Phys. Chem. Lett. 8, 1072-1077. [37] Meister, P., Qi, X., Kloepsch, R., et al. (2017). Anodic behavior of the aluminum current collector in imide-based electrolytes: influence of solvent, operating temperature, and native oxide-layer thickness. ChemSusChem 10, 804-814. [38] Nakajima, T., Mori, M., Gupta, V., et al. (2002). Effect of fluoride additives on the corrosion of aluminum for lithium ion batteries. Solid State Sci. 4, 1385-1394. [39] Zhang, X., and Devine, T.M. (2006). Factors that influence formation of AlF3 passive film on aluminum in Li-ion battery electrolytes with LiPF6. J. Electrochem. Soc. 153, B375. [40] Yoon, E., Lee, J., Byun, S., et al. (2022). Passivation failure of Al current collector in LiPF6‐based electrolytes for lithium‐ion batteries. Adv. Funct. Mater. 32, 2200026. [41] Chidiac, J., Timperman, L., and Anouti, M. (2022). Small dissymmetry, yet large effects on the transport properties of electrolytes based on imide salts: Consequences on performance in Li-ion batteries. J. Energy Chem. 65, 352-366. [42] Park, B.K., Jeong, Y.K., Yang, S.Y., et al. (2021). Deterioration behavior of aluminum pouch film used as packaging materials for pouch-type lithium-ion batteries. J. Power Sources 506, 230222. [43] Du, P., Liu, D., Chen, X., et al. (2023). Research progress towards the corrosion and protection of electrodes in energy-storage batteries. Energy Storage Mater. 57, 371-399. [44] Kolesnikov, A., Kolek, M., Dohmann, J.F., et al. (2020). Galvanic corrosion of lithium‐powder‐based electrodes. Adv. Energy Mater. 10, 2000017. [45] Bizot, C., Blin, M.-A., Guichard, P., et al. (2021). Aluminum current collector for high voltage Li-ion battery. Part II: Benefit of the En’ Safe® primed current collector technology. Electrochem. Commun. 126, 107008. [46] Birbilis, N., and Buchheit, R.G. (2008). Investigation and discussion of characteristics for intermetallic phases common to aluminum alloys as a function of solution pH. J. Electrochem. Soc. 155, C117. [47] Stich, M., Göttlinger, M., Kurniawan, M., et al. (2018). Hydrolysis of LiPF6 in carbonate-based electrolytes for lithium-ion batteries and in aqueous media. J. Phys. Chem. Lett. C 122, 8836-8842. [48] Liu, M., Vatamanu, J., Chen, X., et al. (2021). Hydrolysis of LiPF6-containing electrolyte at high Voltage. ACS Energy Lett. 6, 2096-2102. [49] Lux, S.F., Lucas, I.T., Pollak, E., et al. (2012). The mechanism of HF formation in LiPF6 based organic carbonate electrolytes. Electrochem. Commun. 14, 47-50. [50] Hoffmann, J., Milien, M.S., Lucht, B.L., and Payne, M. (2018). Investigation of gas evolution from Li4Ti5O12 anode for lithium ion batteries. J. Electrochem. Soc. 165, A3108-A3113. [51] Geng, Z., Lu, J., Li, Q., et al. (2019). Lithium metal batteries capable of stable operation at elevated temperature. Energy Storage Mater. 23, 646-652. [52] Chen, X., Xu, W., Engelhard, M.H., et al. (2014). Mixed salts of LiTFSI and LiBOB for stable LiFePO4-based batteries at elevated temperatures. J. Mater. Chem. A 2, 2346. [53] Matsumoto, K., Inoue, K., Nakahara, K., et al. (2013). Suppression of aluminum corrosion by using high concentration LiTFSI electrolyte. J. Power Sources 231, 234-238. [54] Bizot, C., Blin, M.-A., Guichard, P., et al. (2021). Aluminum current collector for high voltage Li-ion battery. Part I: A benchmark study with statistical analysis. Electrochem. Commun. 126, 107013. [55] Cai, Y., Zhang, H., Cao, Y., et al. (2022). Synthesis, application and industrialization of LiFSI: A review and perspective. J. Power Sources 535, 231481. [56] Wu, X., and Du, Z. (2021). Study of the corrosion behavior of LiFSI based electrolyte for Li-ion cells. Electrochem. Commun. 129, 107088. [57] Li, C.-l., Zeng, S.-w., Wang, P., et al. (2021). Mechanism of aluminum corrosion in LiFSI-based electrolyte at elevated temperatures. Trans. Nonferrous Met. Soc. China 31, 1439-1451. [58] Chen, Y., Yu, Z., Rudnicki, P., et al. (2021). Steric effect tuned ion solvation enabling stable cycling of high-voltage lithium metal battery. J. Am. Chem. Soc. 143, 18703-18713. [59] Sayed, F.N., Rodrigues, M.F., Kalaga, K., et al. (2017). Curious case of positive current collectors: corrosion and passivation at high temperature. ACS Appl. Mater. Interfaces 9, 43623-43631. [60] Michailidou, E., Visser, P., Mol, J.M.C., et al. (2023). The effect of pH on the corrosion protection of aluminum alloys in lithium-carbonate-containing NaCl solutions. Corrosion Sci. 210, 110851. [61] Wang, M., Tang, M., Chen, S., et al. (2017). Graphene-armored aluminum foil with enhanced anticorrosion performance as current collectors for lithium-ion battery. Adv. Mater. 29,1703882. [62] Li, Y., and Fedkiw, P.S. (2007). Effect of gel electrolytes containing silica nanoparticles on aluminum corrosion. Electrochim. Acta 52, 2471-2477. [63] Rehnlund, D., Valvo, M., Edström, K., and Nyholm, L. (2014). Electrodeposition of vanadium oxide/manganese oxide hybrid thin films on nanostructured aluminum substrates. J. Electrochem. Soc. 161, D515-D521. [64] Tekaligne, T.M., Merso, S.K., Yang, S.-C., et al. (2022). Corrosion inhibition of aluminum current collector by a newly synthesized 5-formyl-8-hydroxyquinoline for aqueous-based battery. J. Power Sources 550, 232142. [65] Yang, H., Kwon, K., Devine, T.M., and Evans, J.W. (2000). Aluminum corrosion in lithium batteries an investigation using the electrochemical quartz crystal microbalance. J. Electrochem. Soc. 147, 4399. [66] Zhang, L., Chai, L., Zhang, L., et al. (2014). Synergistic effect between lithium bis(fluorosulfonyl)imide (LiFSI) and lithium bis-oxalato borate (LiBOB) salts in LiPF6-based electrolyte for high-performance Li-ion batteries. Electrochim. Acta 127, 39-44. [67] Jeong, C.U., Lee, S.-Y., Kim, J., et al. (2018). Embossed aluminum as a current collector for high-rate lithium cathode performance. J. Power Sources 398, 193-200. [68] Yang, S., Li, S., Meng, Y., et al. (2021). Corrosion inhibition of aluminum current collector with molybdate conversion coating in commercial LiPF6-esters electrolytes. Corrosion Sci. 190, 109632. [69] Nardeli, J.V., Snihirova, D.V., Fugivara, C.S., et al. (2016). Localised corrosion assessement of crambe-oil-based polyurethane coatings applied on the ASTM 1200 aluminum alloy. Corrosion Sci. 111, 422-435. [70] Yang, S., Li, S., Du, Z., et al. (2022). MXene-Ti3C2 armored layer for aluminum current collector enable stable high-voltage lithium-ion battery. Adv. Mater. Interfaces 9, 2200856. [71] Doberdò, I., Löffler, N., Laszczynski, N., et al. (2014). Enabling aqueous binders for lithium battery cathodes-carbon coating of aluminum current collector. J. Power Sources 248, 1000-1006. [72] Zhang B., Ma X. L. (2019). A review pitting corrosion initiation investigated by TEM. J. Mater. Sci. Technol. 7,1455-1465. [73] Scharf, J., Chouchane, M., Finegan, D.P., et al. (2022). Bridging nano- and microscale X-ray tomography for battery research by leveraging artificial intelligence. Nat. Nanotechnol. 17, 446-459. [74] Rahe, C., Kelly, S.T., Rad, M.N., et al. (2019). Nanoscale X-ray imaging of ageing in automotive lithium ion battery cells. J. Power Sources 433, 126631. [75] das Neves, E.C., do Nascimento, E.G., Sacilotto, D.G., et al. (2022). Nondestructive analysis of corrosion in ageing hardened AA6351 aluminium alloys. Mater. Chem. Phys. 291, 126664. [76] Cao, M., Liu, L., Yu, Z., et al. (2019). Electrochemical corrosion behavior of 2A02 Al alloy under an accelerated simulation marine atmospheric environment. J. Mater. Sci. Technol. 35, 651-659. [77] Wang, H., Yang, W., Zhang, B., et al. (2022). Effect of crystal orientation on the corrosion behavior of as-cast pure aluminum anodes in air batteries. J. Mater. Eng. Perform. 31, 3584-3593. [78] Parle, J.K., Beni, A., Dhanak, V.R., et al. (2013). STM and XPS investigation of the oxidation of the Al4(Cr,Fe) quasicrystal approximant. Appl. Surf. Sci. 283, 276-282. [79] Li, L., Zhang, N., Su, Y., et al. (2021). Fluorine dissolution-Induced capacity degradation for fluorophosphate-based cathode materials. ACS Appl. Mater. Interfaces 13, 23787-23793. [80] Kim, S.Y., Song, Y.I., Wee, J.-H., et al. (2019). Few-layer graphene coated current collectors for safe and powerful lithium ion batteries. Carbon 153, 495-503. [81] Cho, E.-C., Chang-Jian, C.-W., Wu, Y.-J., et al. (2021). Modification of aluminum current collectors with laser-scribed graphene for enhancing the performance of lithium ion batteries. J. Power Sources 506, 230060. [82] Deng, S., Jiang, M., Rao, A., et al. (2022). Fast‐charging halide‐based all‐solid‐state batteries by manipulation of current collector interface. Adv. Funct. Mater. 32, 2200767. [83] Yu, Y., and Li, Y. (2020). New insight into the negative difference effect in aluminium corrosion using in-situ electrochemical ICP-OES. Corrosion Sci. 168, 108568. [84] Brachhold, N., König, A.S., Brendler, E., and Aneziris, C. (2021). Investigation of the synthesis and the alkali corrosion of potassium aluminosilicates by XRD and NMR (29Si, 27Al). Ceram. Int. 47, 33596-33605. [85] Li, X., Deng, S., Banis, M.N., et al. (2019). Suppressing corrosion of aluminum foils via highly conductive graphene-like carbon coating in high-performance lithium-based batteries. ACS Appl. Mater. Interfaces 11, 32826-32832. [86] Bunker, B.C., Nelson, G.C., Zavadil, K.R., et al. (2002). Hydration of passive oxide films on aluminum. J. Phys. Chem. Lett. B 106, 4705-4713. [87] Cao, L., Li, L., Xue, Z., et al. (2020). The aluminum current collector with honeycomb-like surface and thick Al2O3 film increased durability and enhanced safety for lithium-ion batteries. J. Porous Mat. 27, 1677-1683. [88] Huang, F., Ma, G., Wen, Z., et al. (2018). Enhancing metallic lithium battery performance by tuning the electrolyte solution structure. J. Mater. Chem. A 6, 1612-1620. [89] Gao, H., Ma, T., Duong, T., et al. (2018). Protecting Al foils for high-voltage lithium-ion chemistries. Mater. Today Energy 7, 18-26. [90] Bolloli, M., Kalhoff, J., Alloin, F., et al. (2015). Fluorinated carbamates as suitable solvents for LiTFSI-based lithium-ion electrolytes: Physicochemical Properties and Electrochemical Characterization. J. Phys. Chem. Lett. C 119, 22404-22414. [91] Teucher, G., Van Gestel, T., Krott, M., et al. (2016). Processing of Al-doped ZnO protective thin films on aluminum current collectors for lithium ion batteries. Thin Solid Films 619, 302-307. [92] Vicentini, R., Nunes, W.G., Costa, L.H., et al. (2019). Highly stable nickel-aluminum alloy current collectors and highly defective multi-walled carbon nanotubes active material for neutral aqueous-based electrochemical capacitors. J. Energy Storage 23, 116-127. [93] Qiao, L., Oteo, U., Martinez-Ibanez, M., et al. (2022). Stable non-corrosive sulfonimide salt for 4-V-class lithium metal batteries. Nat. Mater. 21, 455-462. [94] Han, H., Guo, J., Zhang, D., et al. (2011). Lithium (fluorosulfonyl) (nonafluorobutanesulfonyl) imide (LiFNFSI) as conducting salt to improve the high-temperature resilience of lithium-ion cells. Electrochem. Commun. 13, 265-268. [95] Menne, S., Kühnel, R.S., and Balducci, A. (2013). The influence of the electrochemical and thermal stability of mixtures of ionic liquid and organic carbonate on the performance of high power lithium-ion batteries. Electrochim. Acta 90, 641-648. [96] Kramer, E., Passerini, S., and Winter, M. (2012). Dependency of aluminum collector corrosion in lithium ion batteries on the electrolyte solvent. ECS Electrochem. Lett. 1, C9-C11. [97] Zhang, X., and Devine, T.M. (2006). Passivation of aluminum in lithium-ion battery electrolytes with LiBOB. J. Electrochem. Soc. 153, B365. [98] Yamada, Y. (2017). Developing new functionalities of superconcentrated electrolytes for lithium-ion batteries. Electrochemistry 85, 559-565. [99] Jiang, G., Li, F., Wang, H., et al. (2021). Perspective on high‐concentration electrolytes for lithium metal batteries. Small Struct. 2, 2000122. [100] Zheng, J., Lochala, J.A., Kwok, A., et al. (2017). Research progress towards understanding the unique interfaces between concentrated electrolytes and electrodes for energy storage applications. Adv. Sci. 4, 1700032. [101] Suo, L., Xue, W., Gobet, M., et al. (2018). Fluorine-donating electrolytes enable highly reversible 5-V-class Li metal batteries. Proc. Natl. Acad. Sci. 115, 1156-1161. [102] Yamada, Y., Chiang, C.H., Sodeyama, K., et al. (2015). Corrosion prevention mechanism of aluminum metal in superconcentrated electrolytes. ChemElectroChem 2, 1687-1694. [103] Flamme, B., Rodriguez Garcia, G., Weil, M., et al. (2017). Guidelines to design organic electrolytes for lithium-ion batteries: environmental impact, physicochemical and electrochemical properties. Green Chem. 19, 1828-1849. [104] Zheng, Q., Yamada, Y., Shang, R., et al. (2020). A cyclic phosphate-based battery electrolyte for high voltage and safe operation. Nat. Energy 5, 291-298. [105] Zhao, Y., Zhou, T., Mensi, M., et al. (2023). Electrolyte engineering via ether solvent fluorination for developing stable non-aqueous lithium metal batteries. Nat. Commun. 14 , 299. [106] Wang, Q., Zhao, C., Wang, J., et al. (2023). High entropy liquid electrolytes for lithium batteries. Nat. Commun. 14,440. [107] Zhang, G., Chang, J., Wang, L., et al. (2023). A monofluoride ether-based electrolyte solution for fast-charging and low-temperature non-aqueous lithium metal batteries. Nat. Commun. 14, 1081. [108] Wu, H., Song, Z., Wang, X., et al. (2022). N,N-dimethyl fluorosulfonamide for suppressed aluminum corrosion in lithium bis(trifluoromethanesulfonyl)imide-based electrolytes. Nano Res. 16, 8269-8280. [109] Kanamura, K., Umegaki, T., Shiraishi, S., et al. (2002). Electrochemical behavior of Al current collector of rechargeable lithium batteries in propylene carbonate with LiCF3SO3, Li(CF3SO2)2N, or Li(C4F9SO2)(CF3SO2)N. J. Electrochem. Soc. 149, A185. [110] Louis, H., Lee, Y.-G., Kim, K.M., et al. (2013). Suppression of Aluminum Corrosion in Lithium Bis(trifluoromethanesulfonyl)imide-based Electrolytes by the Addition of Fumed Silica. Bull. Korean Chem. Soc. 34, 1795-1799. [111] Zheng, J., Engelhard, M.H., Mei, D., et al. (2017). Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2,17012. [112] Zhuang, Y., Du, F., Zhu, L., et al. (2018). Trimethylsilyl (trimethylsiloxy) acetate as a novel electrolyte additive for improvement of electrochemical performance of lithium-rich Li1.2Ni0.2Mn0.6O2 cathode in lithium-ion batteries. Electrochim. Acta 290 , 220-227. [113] Zhang, Q., Liu, K., Ding, F., et al. (2019). Enhancing the high voltage interface compatibility of LiNi0.5Co0.2Mn0.3O2 in the succinonitrile-based electrolyte. Electrochim. Acta 298 , 818-826. [114] Chen, J., Bai, Z., Li, X., et al. (2022). Reduced graphene oxide-modified aluminum foils as highly conductive and corrosion-resistant cathode current collectors for Li-ion batteries. Appl. Surf. Sci. 606, 155002. [115] Cho, K., Baek, J., Balamurugan, C., et al. (2022). Corrosion study of nickel-coated copper and chromate-coated aluminum for corrosion-resistant lithium-ion battery lead-tab. J. Ind. Eng. Chem. 106, 537-545. [116] Kuenzel, M., Bresser, D., Kim, G.-T., et al. (2019). Unveiling and amplifying the benefits of carbon-coated aluminum current collectors for sustainable LiNi0.5Mn1.5O4 cathodes. ACS Appl. Energ. Mater. 3 , 218-230. [117] Wennig, S., Langklotz, U., Prinz, G.M., et al. (2015). The influence of different pre-treatments of current collectors and variation of the binders on the performance of Li4Ti5O12 anodes for lithium ion batteries. J. Appl. Electrochem. 45, 1043-1055. [118] Ding, K., Chen, J., Zhang, D., et al. (2021). Electrochemical deposition of leaf stalk-shaped polyaniline doped with sodium dodecyl sulfate on aluminum and its use as a novel type of current collector in lithium ion batteries. Synth. Met. 278, 116837. [119] Bohm, S. (2014). Graphene against corrosion. Nat. Nanotechnol. 9, 741-742. [120] Cui, C., Lim, A.T.O., and Huang, J. (2017). A cautionary note on graphene anti-corrosion coatings. Nat. Nanotechnol. 12, 834-835. [121] Xie, Y., Meng, X., Chang, Y., et al. (2022). Heteroatom modification enhances corrosion durability in high-mechanical-performance graphene-reinforced aluminum matrix composites. Adv. Sci. 9, e2104464. [122] Richard Prabakar, S.J., Hwang, Y.-H., Bae, E.G., et al. (2013). Graphene oxide as a corrosion inhibitor for the aluminum current collector in lithium ion batteries. Carbon 52, 128-136. [123] Zhang, G., Lin, K., Qin, X., et al. (2020). Electrosprayed robust graphene layer constructing ultra stable electrode interface for high-voltage lithium-ion batteries. ACS Appl. Mater. Interfaces 12, 37034-37046. [124] Wang, R., Li, W., Liu, L., et al. (2019). Carbon black/graphene-modified aluminum foil cathode current collectors for lithium ion batteries with enhanced electrochemical performances. J. Electroanal. Chem. 833, 63-69. [125] Wang, S., and Xu, S. (2020). Ti/Cr(III) conversion coating on aluminium foil for lithium-ion battery package. Surf. Eng. 37, 365-372. [126] Heckmann, A., Krott, M., Streipert, B., et al. (2017). Suppression of aluminum current collector dissolution by protective ceramic coatings for better high-voltage battery performance. ChemPhysChem 18, 156-163. [127] Piao, N., Wang, L., Anwar, T., et al. (2019). Corrosion resistance mechanism of chromate conversion coated aluminium current collector in lithium-ion batteries. Corrosion Sci. 158, 108100. [128] Lepage, D., Savignac, L., Saulnier, M., et al. (2019). Modification of aluminum current collectors with a conductive polymer for application in lithium batteries. Electrochem. Commun. 102, 1-4. [129] Richard Prabakar, S.J., and Pyo, M. (2012). Corrosion protection of aluminum in LiPF6 by poly(3,4-ethylenedioxythiophene) nanosphere-coated multiwalled carbon nanotube. Corrosion Sci. 57, 42-48. [130] Deyab, M.A., Mele, G., Bloise, E., and Mohsen, Q. (2021). Novel nanocomposites of Ni-Pc/polyaniline for the corrosion safety of the aluminum current collector in the Li-ion battery electrolyte. Sci. Rep. 11, 12371. [131] Ye, Y., Chou, L.-Y., Liu, Y., et al. (2020). Ultralight and fire-extinguishing current collectors for high-energy and high-safety lithium-ion batteries. Nat. Energy 5, 786-793. [132] Liu, Z., Dong, Y., Qi, X., et al. (2022). Stretchable separator/current collector composite for superior battery safety. Energy Environ. Sci. 15, 5313-5323. [133] Zhao, Y., Yang, J., Ma, J., et al. (2021). Highly reduced graphene assembly film as current collector for lithium ion batteries. ACS Sustain. Chem. Eng. 9, 8635-8641. [134] Konarov, A., Kim, H.J., Yashiro, H., and Myung, S.-T. (2019). Passivation of aluminum current collectors in non-aqueous carbonate solutions containing sodium or potassium hexafluorophosphate salts. J. Mater. Chem. A 7, 13012-13018. [135] Otaegui, L., Goikolea, E., Aguesse, F., et al. (2015). Effect of the electrolytic solvent and temperature on aluminium current collector stability: A case of sodium-ion battery cathode. J. Power Sources 297, 168-173. [136] Liu, P., Wang, Y., Hao, H., et al. (2020). Stable Potassium metal anodes with an all-aluminum current collector through improved electrolyte wetting. Adv. Mater. 32, 2002908. [137] Zhang, X., Chen, J., Xu, Z., et al. (2022). Aqueous electrolyte with moderate concentration enables high-energy aqueous rechargeable lithium ion battery for large scale energy storage. Energy Storage Mater. 46, 147-154. [138] Abdisattar, A., Yeleuov, M., Daulbayev, C., et al. (2022). Recent advances and challenges of current collectors for supercapacitors. Electrochem. Commun. 142, 107373. -
ABOUT THIS ARTICLE
Cite this article:
Shi X., Zhang H., Zhang Y., et al., (2023). Corrosion and protection of aluminum current collector in lithium-ion batteries. The Innovation Materials 1(2), 100030. https://doi.org/10.59717/j.xinn-mater.2023.100030
-
Figure 1.
Factors of corrosion and corresponding protection strategy.
-
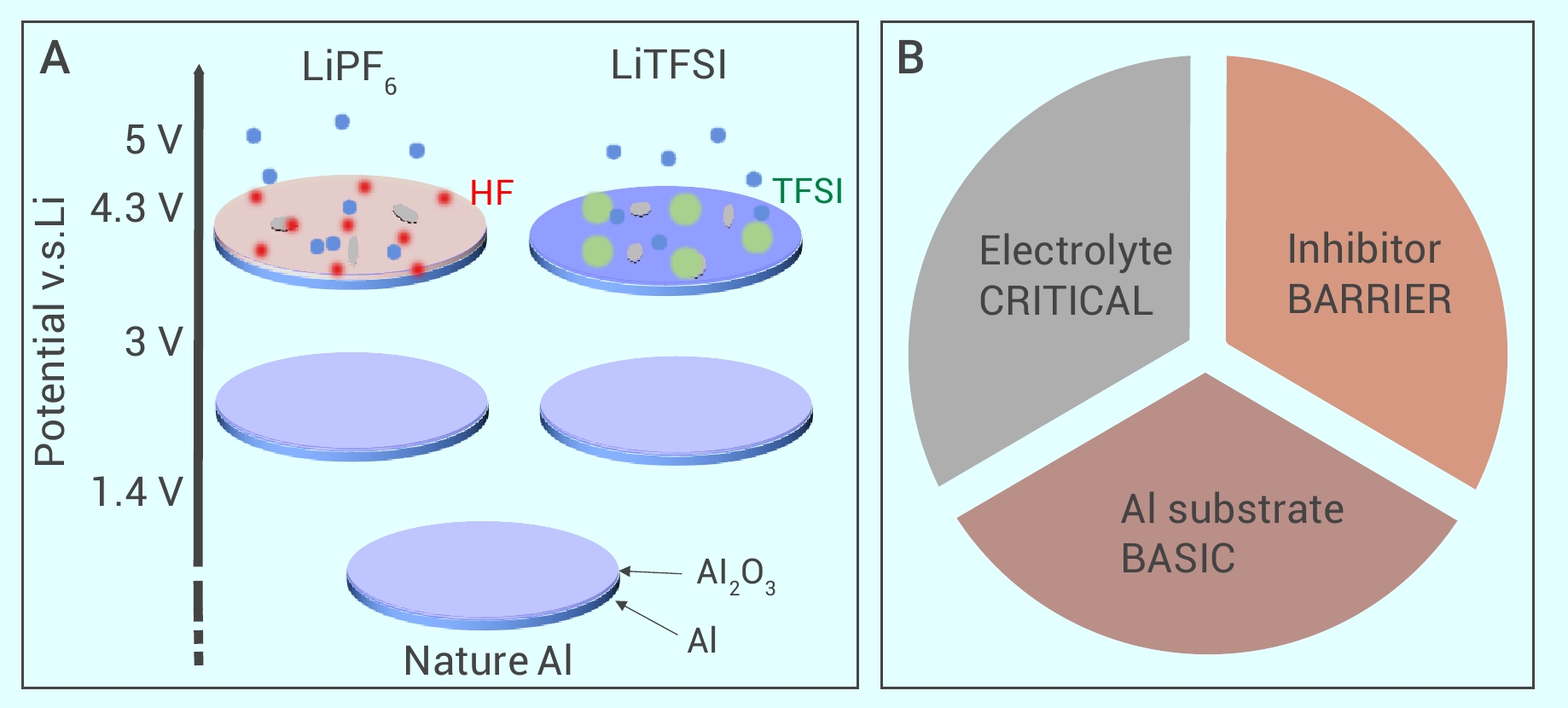
Figure 2.
Corrosion mechanism for LiPF6 and LiTFSI based electrolytes
-
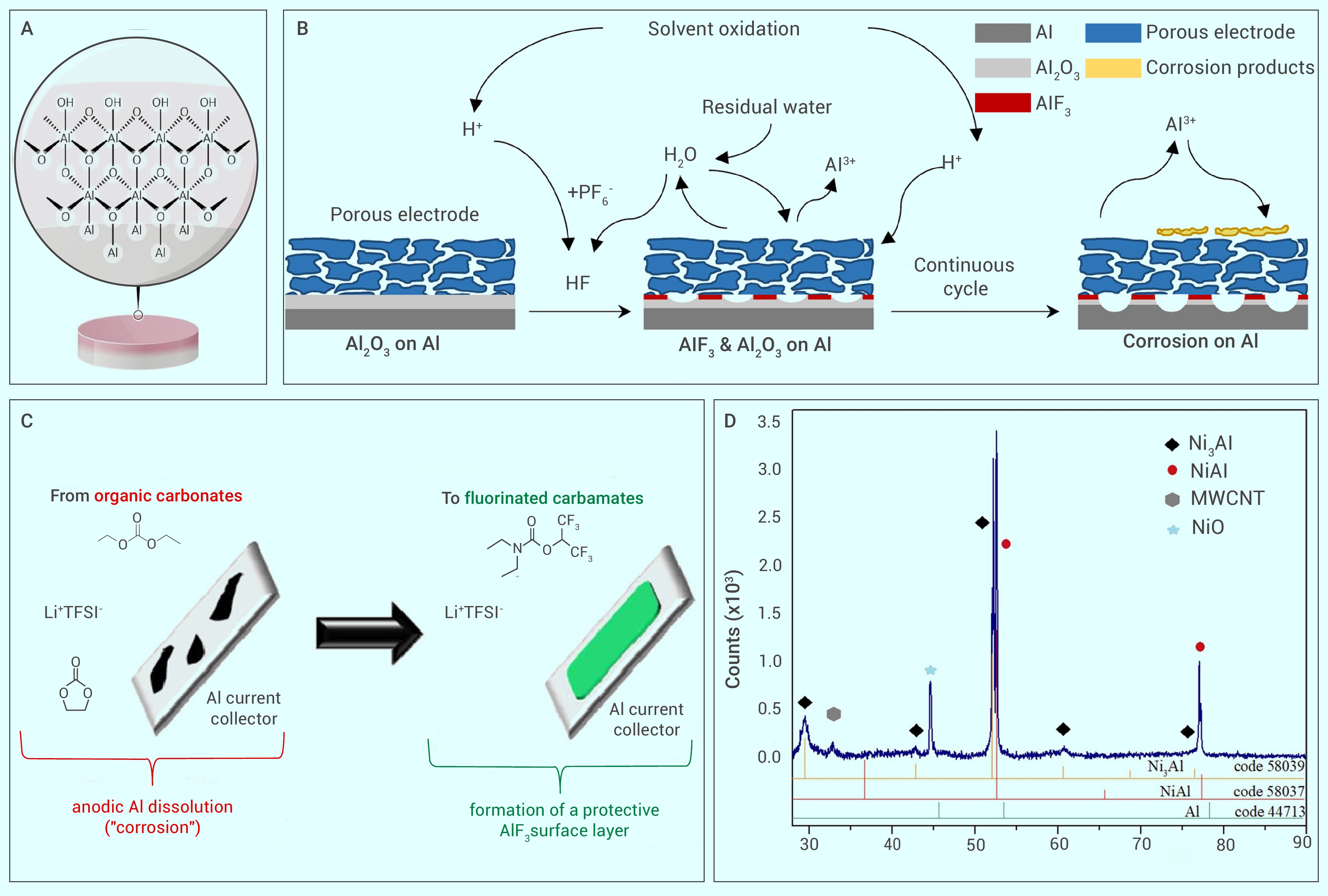
Figure 3.
Multiscale characterization for corrosion and protection including of electrochemical property, morphology and chemical property
-
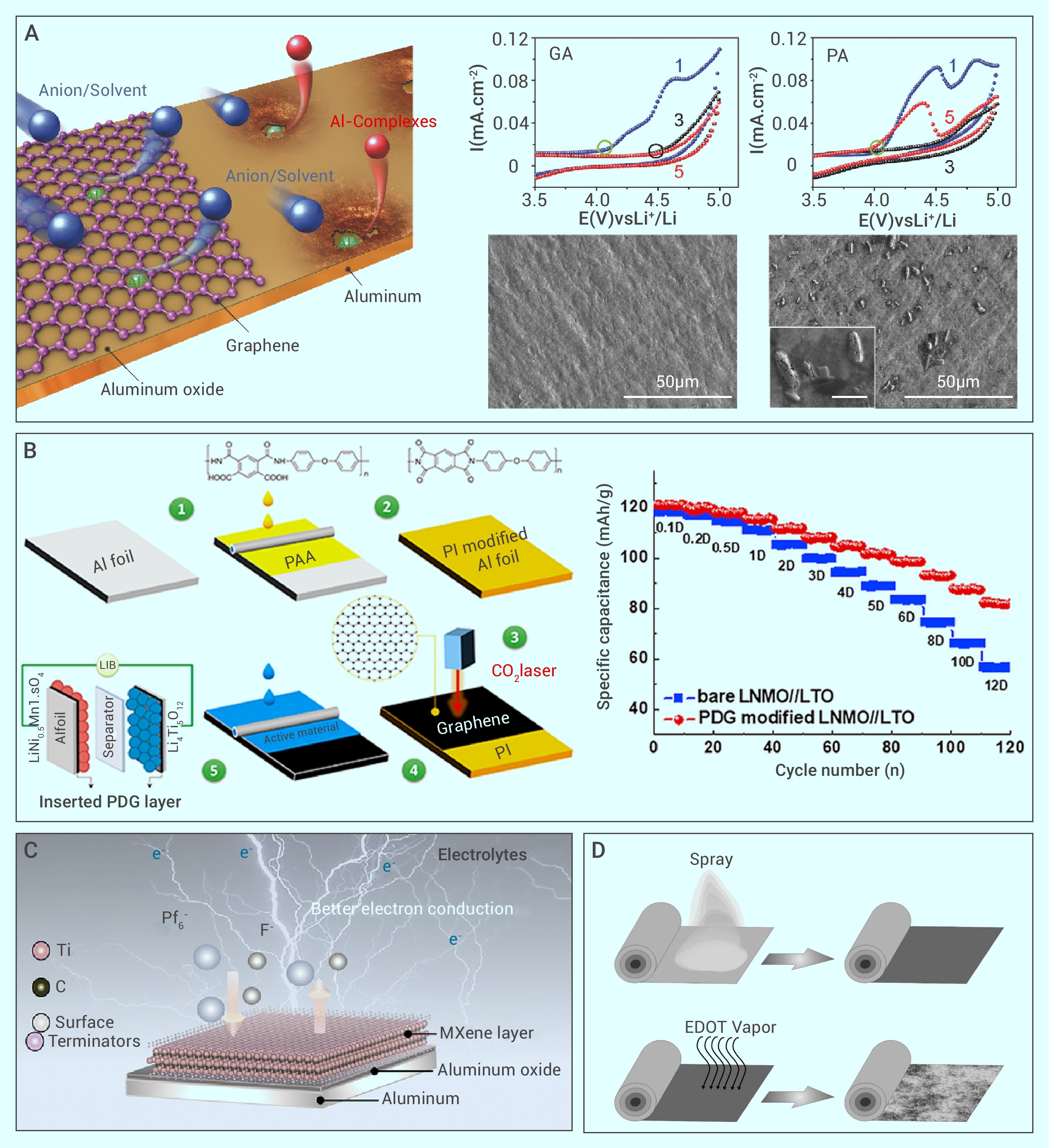
Figure 4.
The protection strategies of Al corrosion in three aspects of Al surface condition, the recipes of electrolyte, and inhibitors in LIBs.
-
Figure 5.
The corrosion mechanism and relevant protection method for Al surface
-
Figure 6.
The composition of electrolyte recipes (lithium salt, solvents, additive)
-
Figure 7.
The inhibitor for Al current collector
-
Figure 8.
The others of Al current collectors
-
Figure 9.
The schematic of Al corrosion and protection strategies


 DownLoad:
DownLoad:





 Twitter
Twitter