World Journal of Emergency Medicine ›› 2023, Vol. 14 ›› Issue (6): 462-470.doi: 10.5847/wjem.j.1920-8642.2023.102
• Original Article • Previous Articles Next Articles
Shuang Xu1, Lang Guo2, Weijing Shao1, Licai Liang3, Tingting Shu4, Yuhan Zhang5, He Huang6, Guangqi Guo1, Qing Zhang7( ), Peng Sun1(
), Peng Sun1( )
)
Received:2023-03-09
Accepted:2023-06-20
Online:2023-11-10
Published:2023-11-01
Contact:
Qing Zhang, Email: Shuang Xu, Lang Guo, Weijing Shao, Licai Liang, Tingting Shu, Yuhan Zhang, He Huang, Guangqi Guo, Qing Zhang, Peng Sun. Vagus nerve stimulation protects against cerebral injury after cardiopulmonary resuscitation by inhibiting inflammation through the TLR4/NF-κB and α7nAChR/JAK2 signaling pathways[J]. World Journal of Emergency Medicine, 2023, 14(6): 462-470.
Add to citation manager EndNote|Ris|BibTeX
URL: http://wjem.com.cn/EN/10.5847/wjem.j.1920-8642.2023.102

Figure 1.
Vagus nerve stimulation (VNS) treatment improved 72-hour survival and neurological recovery in mice after cardiac arrest/cardiopulmonary resuscitation (CA/CPR). (A) experimental procedure. The GTS-21 (α7nAChR agonist) (4 mg/kg) was injected 30 min before CA. The methyllycaconitine citrate (MLA, inhibitor of α7nAChR) (4 mg/kg) was given 30 min before CA. (B) Kaplan-Meier curves of cumulative survival 72 h after CA and CPR in the five groups. VNS significantly improves neurological deficit score (NDS) at 24 h (C), 48 h (D), and 72 h (E) after CA/CPR. Black points indicate values for individual mice; horizontal bars indicate mean with range values (**P < 0.01 vs. Sham group; *P < 0.05 vs. Sham group; #P < 0.05 vs. CPR group). ROSC: return of spontaneous circulation.
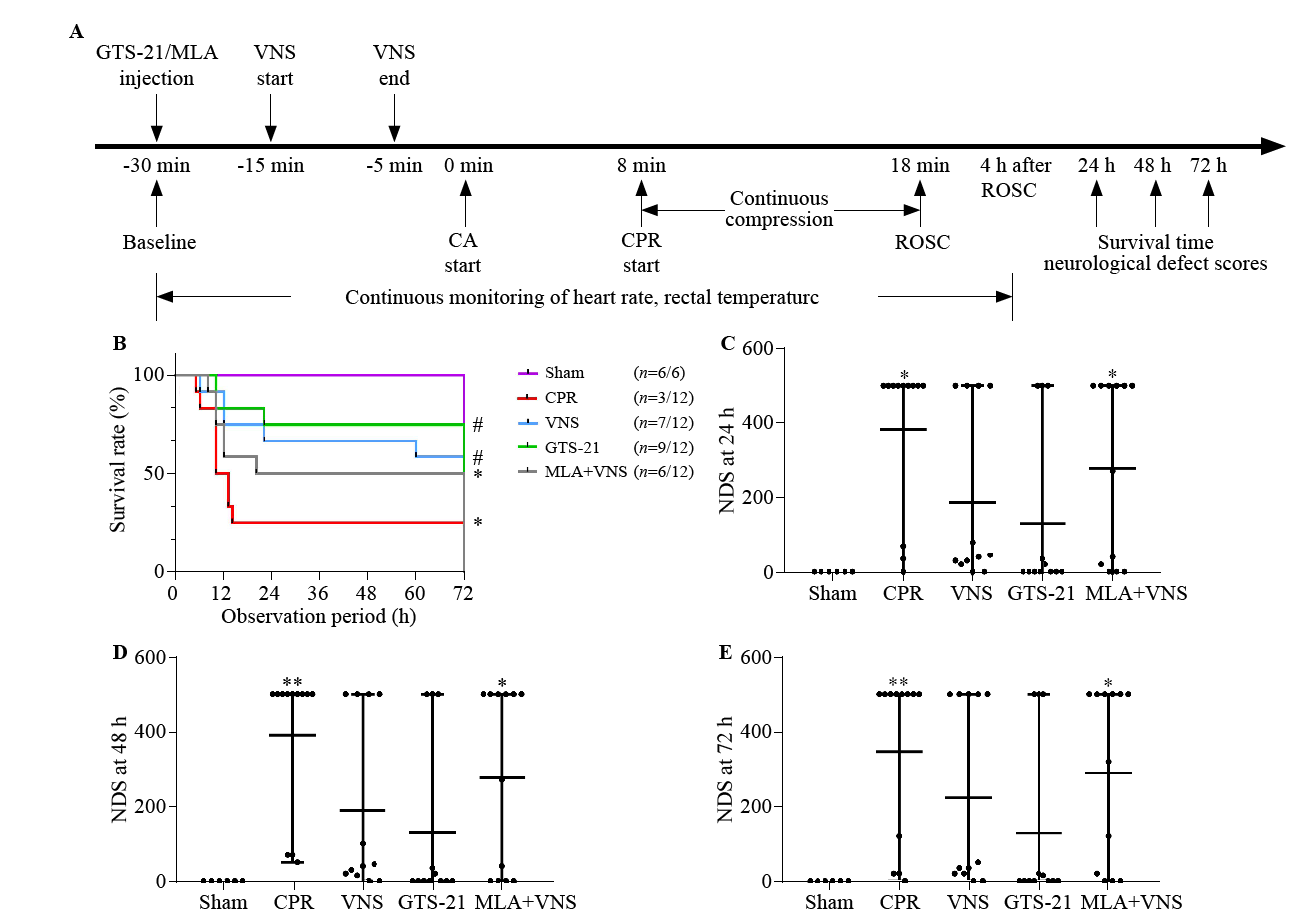
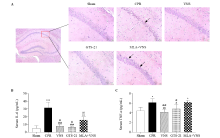
Figure 2.
Vagus nerve stimulation (VNS) markedly reduced pathological damage in mice after cardiac arrest/cardiopulmonary resuscitation (CA/CPR). (A) HE staining in hippocampal CA1 region of mice at the 72 h after return of spontaneous circulation (ROSC). Red square shows the hippocampus CA1 region (magnification 40×). Right panels are 10× magnification photomicrographs from the red square of left panel. Black arrows indicate the nuclear pyknosis. Scale bar is 25 μm. The serum concentrations of interleukin-6 (IL-6) (B), and tumor necrosis factor-α (TNF-α) (C) at 72 h after resuscitation. (n=3, *P<0.05 vs. Sham group, **P<0.01 vs. Sham group, ***P<0.001 vs. Sham group; #P< 0.05 vs. CPR group, ##P<0.01 vs. CPR group, ###P < 0.001 vs. CPR group; &P< 0.05 vs. MLA+VNS group, &&P< 0.01 vs. MLA+VNS group).
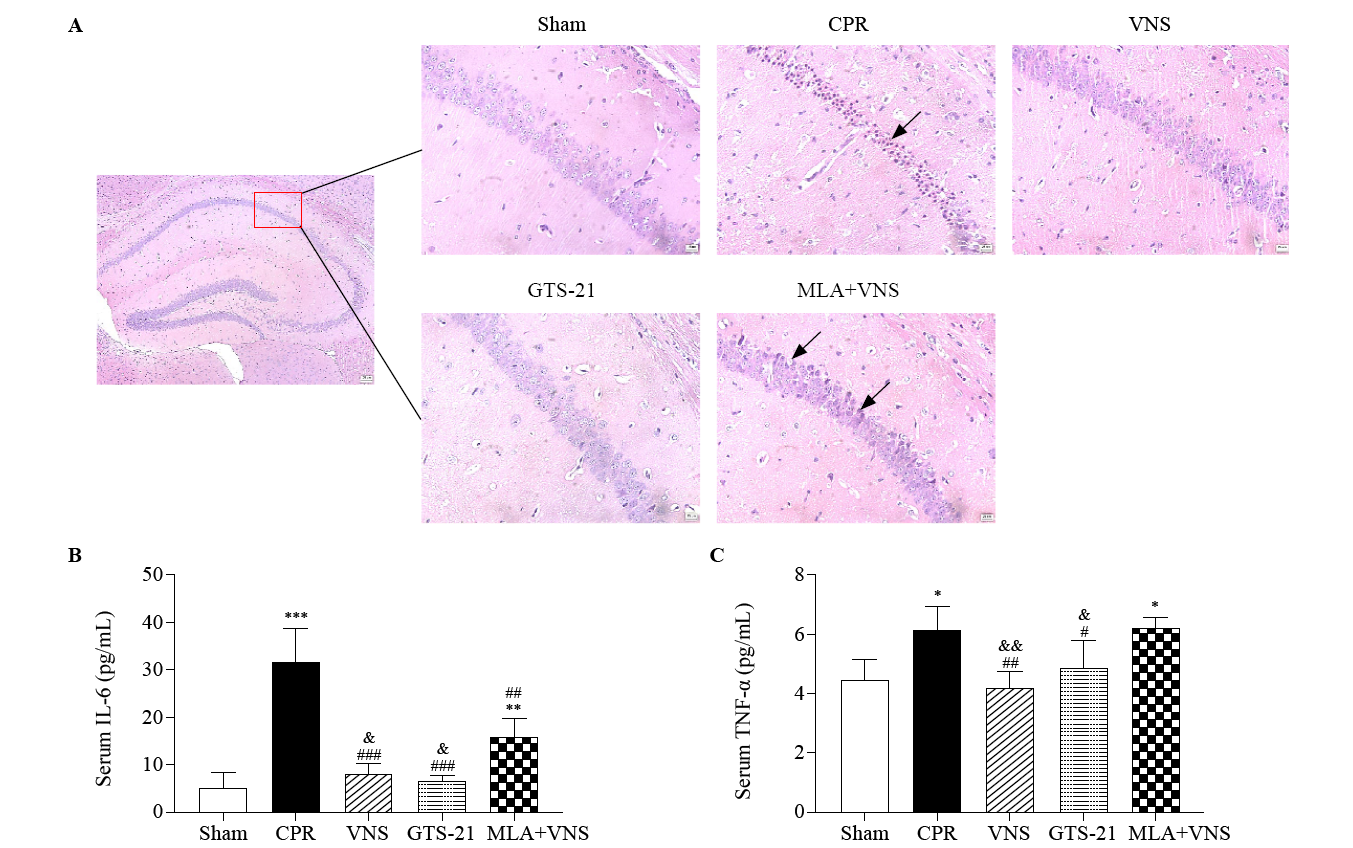
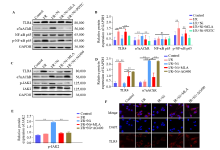
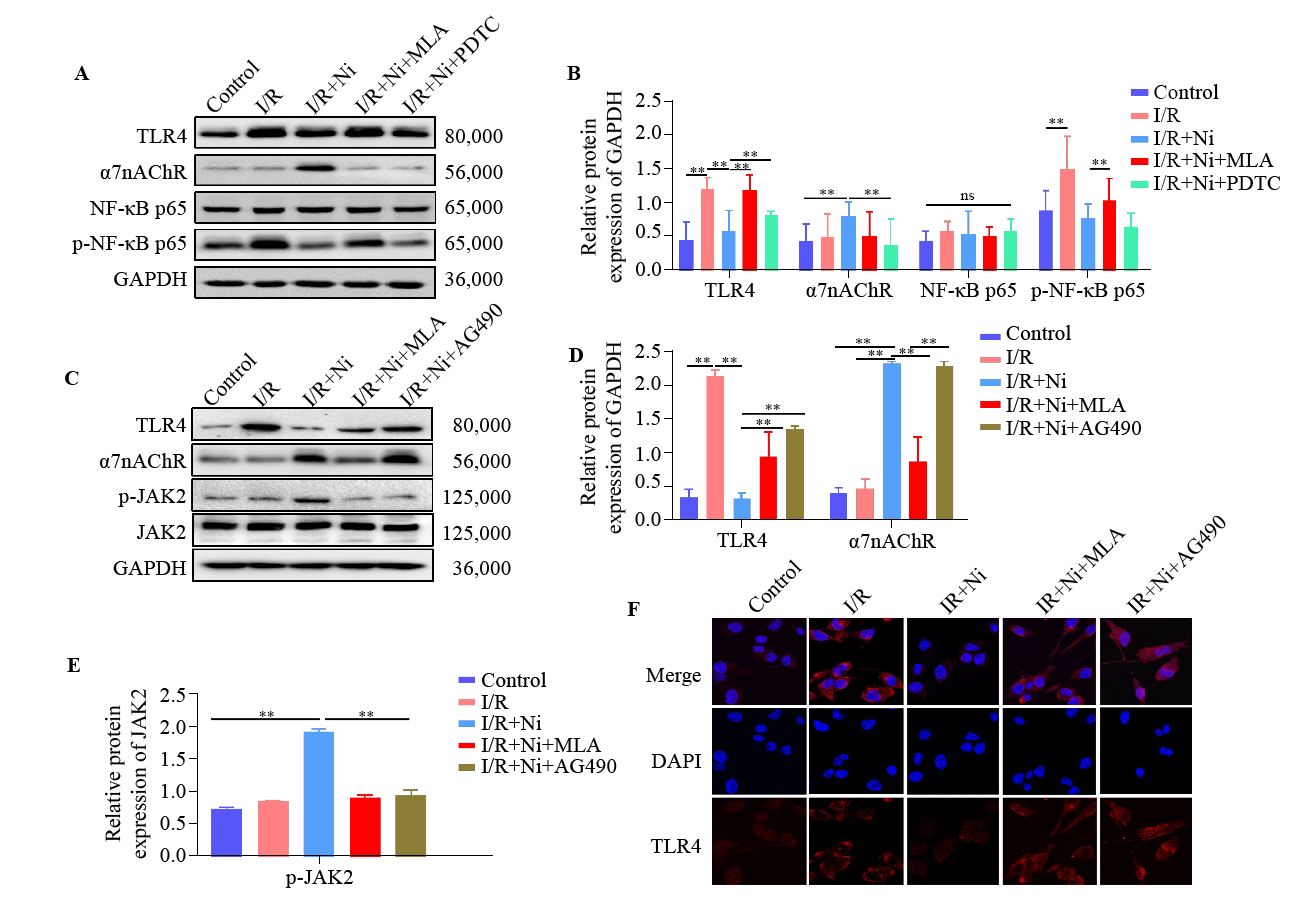
Figure 4.
Nicotine inhibited TLR4/NF-κB pathway by activating α7nAChR/JAK2 axis in BV-2 cell after ischemia/reperfusion (I/R). (A) Representative immunoblots of TLR4, α7nAChR, NF-κB p65, and p-NF-κB p65. (B) Quantitative analysis of TLR4, α7nAChR, NF-κB p65, and p-NF-κB p65 expression. The protein amount was normalized to GAPDH. (C) Representative immunoblots of TLR4, p-JAK2, JAK2, and α7nAChR. Quantitative analysis of TLR4, α7nAChR (D) and p-JAK2 (E) expression. (F) Immunostaining of TLR4 in BV-2 cells. TLR4 (red), DAPI (blue). **P < 0.01; ns: not significantly different.
| 1 |
Kang Y. Management of post-cardiac arrest syndrome. Acute Crit Care. 2019; 34(3): 173-8.
doi: 10.4266/acc.2019.00654 |
| 2 |
Jou C, Shah R, Figueroa A, Patel JK. The role of inflammatory cytokines in cardiac arrest. J Intensive Care Med. 2020; 35(3): 219-24.
doi: 10.1177/0885066618817518 pmid: 30526209 |
| 3 |
Xiang YX, Zhao H, Wang JL, Zhang LT, Liu AC, Chen YG. Inflammatory mechanisms involved in brain injury following cardiac arrest and cardiopulmonary resuscitation. Biomed Rep. 2016; 5(1): 11-7.
doi: 10.3892/br.2016.677 pmid: 27330748 |
| 4 |
Zhang QS, Li G, Xu L, Li Q, Wang QY, Zhang Y, et al. Toll-like receptor 4 contributes to acute kidney injury after cardiopulmonary resuscitation in mice. Mol Med Rep. 2016; 14(4): 2983-90.
doi: 10.3892/mmr.2016.5599 |
| 5 | Liang LC, Shao WJ, Shu TT, Zhang YH, Xu S, Guo L, et al. Xuezhikang improves the outcomes of cardiopulmonary resuscitation in rats by suppressing the inflammation response through TLR4/NF-κB pathway. Biomed Pharmacother. 2019;114: 108817. |
| 6 |
Amani H, Habibey R, Shokri F, Hajmiresmail SJ, Akhavan O, Mashaghi A, et al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep. 2019; 9(1): 6044.
doi: 10.1038/s41598-019-42633-9 pmid: 30988361 |
| 7 |
Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009; 265(6): 663-79.
doi: 10.1111/j.1365-2796.2009.02098.x pmid: 19493060 |
| 8 | Wu YJ, Wang L, Ji CF, Gu SF, Yin Q, Zuo J. The role of α7nAChR-mediated cholinergic anti-inflammatory pathway in immune cells. Inflammation. 2021;44(3): 821-34. |
| 9 |
Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha 7 subunit is an essential regulator of inflammation. Nature. 2003; 421(6921): 384-8.
doi: 10.1038/nature01339 |
| 10 |
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000; 405(6785): 458-62.
doi: 10.1038/35013070 |
| 11 | Akaike A, Shimohama S, Misu Y. Nicotinic acetylcholine receptor signaling in neuroprotection. Singapore: Springer; 2018:1-15. |
| 12 |
Gamage R, Wagnon I, Rossetti I, Childs R, Niedermayer G, Chesworth R, et al. Cholinergic modulation of glial function during aging and chronic neuroinflammation. Front Cell Neurosci. 2020; 14: 577912.
doi: 10.3389/fncel.2020.577912 |
| 13 |
Shao WJ, Shu TT, Xu S, Liang LC, Le Grange JM, Zhou YR, et al. Left-sided vagus nerve stimulation improves cardiopulmonary resuscitation outcomes in rats as effectively as right-sided vagus nerve stimulation. World J Emerg Med. 2021; 12(4): 309.
doi: 10.5847/wjem.j.1920-8642.2021.04.010 |
| 14 |
Sun P, Wang J, Zhao S, Yang Z, Tang Z, Ravindra N, et al. Improved outcomes of cardiopulmonary resuscitation in rats treated with vagus nerve stimulation and its potential mechanism. Shock. 2018; 49(6): 698-703.
doi: 10.1097/SHK.0000000000000962 pmid: 28800036 |
| 15 | Kim TH, Kim SJ, Lee SM. Stimulation of the α7 nicotinic acetylcholine receptor protects against sepsis by inhibiting toll-like receptor via phosphoinositide 3-kinase activation. J Infect Dis. 2014;209(10): 1668-77. |
| 16 |
Deng G, Yonchek JC, Quillinan N, Strnad FA, Exo J, Herson PS, et al. A novel mouse model of pediatric cardiac arrest and cardiopulmonary resuscitation reveals age-dependent neuronal sensitivities to ischemic injury. J Neurosci Methods. 2014; 222: 34-41.
doi: 10.1016/j.jneumeth.2013.10.015 |
| 17 |
Liu HQ, Yu Z, Li Y, Xu B, Yan BH, Paschen W, et al. Novel modification of potassium chloride induced cardiac arrest model for aged mice. Aging Dis. 2018; 9(1): 31-9.
doi: 10.14336/AD.2017.0221 |
| 18 |
Giebelen IA, van Westerloo DJ, LaRosa GJ, de Vos AF, van der Poll T. Stimulation of alpha 7 cholinergic receptors inhibits lipopolysaccharide-induced neutrophil recruitment by a tumor necrosis factor alpha-independent mechanism. Shock. 2007; 27(4): 443-7.
pmid: 17414429 |
| 19 |
Lewis AS, Mineur YS, Smith PH, Cahuzac ELM, Picciotto MR. Modulation of aggressive behavior in mice by nicotinic receptor subtypes. Biochem Pharmacol. 2015; 97(4): 488-97.
doi: S0006-2952(15)00389-5 pmid: 26212554 |
| 20 |
Zhan DY, Du CK, Akiyama T, Morimoto S, Shimizu S, Kawada T, et al. Cardiac vagal control in a knock-in mouse model of dilated cardiomyopathy with a troponin mutation. Auton Neurosci. 2017; 205: 33-40.
doi: 10.1016/j.autneu.2017.03.002 |
| 21 |
Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol. 2016; 594(20): 5781-90.
doi: 10.1113/tjp.2016.594.issue-20 |
| 22 | Chen K, Sun Y, Dong W, Zhang T, Zhou N, Yu W, et al. Activated Α7nachr improves postoperative cognitive dysfunction and intestinal injury induced by cardiopulmonary bypass in rats: inhibition of the proinflammatory response through the Th17 immune response. Cell Physiol Biochem. 2018;46(3): 1175-88. |
| 23 |
Gong MX, Wang GM, Li GD, Liu J, Sun PP, Xu LC, et al. Dysfunction of inflammation-resolving pathways is associated with postoperative cognitive decline in elderly mice. Behav Brain Res. 2020; 386: 112538.
doi: 10.1016/j.bbr.2020.112538 pmid: 32113876 |
| 24 |
Bie B, Wang Z, Chen Y, Sheng L, Li H, You H, et al. Vagus nerve stimulation affects inflammatory response and anti-apoptosis reactions via regulating miR-210 in epilepsy rat model. Neuroreport. 2021; 32(9): 783-91.
doi: 10.1097/WNR.0000000000001655 pmid: 33994524 |
| 25 | Wittebole X, Castanares-Zapatero D, Laterre PF. Toll-like receptor 4 modulation as a strategy to treat sepsis. Mediators Inflamm. 2010; 2010: 568396. |
| 26 | Egea J, Buendia I, Parada E, Navarro E, León R, Lopez MG. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem Pharmacol. 2015;97(4): 463-72. |
| 27 | de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005; 6(8): 844-51. |
| 28 | Lu XX, Hong ZQ, Tan Z, Sui MH, Zhuang ZQ, Liu HH, et al. Nicotinic acetylcholine receptor Alpha7 subunit mediates vagus nerve stimulation-induced neuroprotection in acute permanent cerebral ischemia by α7nAchR/JAK2 pathway. Med Sci Monit. 2017;23: 6072-81. |
| 29 |
Yamauchi S, Ito H, Miyajima A. IkappaBeta, a nuclear IkappaB protein, positively regulates the NF-kappaB-mediated expression of proinflammatory cytokines. Proc Natl Acad Sci USA. 2010; 107(26): 11924-9.
doi: 10.1073/pnas.0913179107 pmid: 20547855 |
| 30 | Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009; 1(6): a001651. |
| 31 | Patel H, McIntire J, Ryan S, Dunah A, Loring R. Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway. J Neuroinflammation. 2017;14(1): 192. |
| 32 | Youssef ME, Abdelrazek HM, Moustafa YM. Cardioprotective role of GTS-21 by attenuating the TLR4/NF-κB pathway in streptozotocin-induced diabetic cardiomyopathy in rats. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(1): 11-31. |
| 33 |
Lv YX, Zhong S, Tang H, Luo B, Chen SJ, Chen L, et al. VEGF-A and VEGF-B coordinate the arteriogenesis to repair the infarcted heart with vagus nerve stimulation. Cell Physiol Biochem. 2018; 48(2): 433-49.
doi: 10.1159/000491775 |
| [1] | Gannan Wang, Zhe Wang, Yi Zhu, Zhongman Zhang, Wei Li, Xufeng Chen, Yong Mei. The neuro-prognostic value of the ion shift index in cardiac arrest patients following extracorporeal cardiopulmonary resuscitation [J]. World Journal of Emergency Medicine, 2023, 14(5): 354-359. |
| [2] | Jia-bao Li, Miao-rong Xie, Mei-li Duan, Ya-nan Yu, Chen-chen Hang, Zi-ren Tang, Chun-sheng Li. Over-expression of programmed death-ligand 1 and programmed death-1 on antigen-presenting cells as a predictor of organ dysfunction and mortality during early sepsis: a prospective cohort study [J]. World Journal of Emergency Medicine, 2023, 14(3): 179-185. |
| [3] | Shi-jiao Yan, Mei Chen, Jing Wen, Wen-ning Fu, Xing-yue Song, Huan-jun Chen, Ri-xing Wang, Mei-ling Chen, Xiao-tong Han, Chuan-zhu Lyu. Global research trends in cardiac arrest research: a visual analysis of the literature based on CiteSpace [J]. World Journal of Emergency Medicine, 2022, 13(4): 290-296. |
| [4] | Mei-jia Shen, Li-chao Sun, Xiao-yu Liu, Meng-chen Xiong, Shan Li, A-ling Tang, Guo-qiang Zhang. Trichostatin A improves the inflammatory response and liver injury in septic mice through the FoxO3a/autophagy signaling pathway [J]. World Journal of Emergency Medicine, 2022, 13(3): 182-188. |
| [5] | Ryan W. Horton, Kian R. Niknam, Viveta Lobo, Kathryn H. Pade, Drew Jones, Kenton L. Anderson. A cadaveric model for transesophageal echocardiography transducer placement training: A pilot study [J]. World Journal of Emergency Medicine, 2022, 13(1): 18-22. |
| [6] | Wei-jing Shao, Ting-ting Shu, Shuang Xu, Li-cai Liang, Jehane Michael Le Grange, Yu-ran Zhou, He Huang, Yu Cai, Qing Zhang, Peng Sun. Left-sided vagus nerve stimulation improves cardiopulmonary resuscitation outcomes in rats as effectively as right-sided vagus nerve stimulation [J]. World Journal of Emergency Medicine, 2021, 12(4): 309-316. |
| [7] | Alexei Birkun, Fatima Trunkwala, Adhish Gautam, Miriam Okoroanyanwu, Adesokan Oyewumi. Availability of basic life support courses for the general populations in India, Nigeria and the United Kingdom: An internet-based analysis [J]. World Journal of Emergency Medicine, 2020, 11(3): 133-139. |
| [8] | Jung Wan Kim, Jin Woong Lee, Seung Ryu, Jung Soo Park, InSool Yoo, Yong Chul Cho, Hong Joon Ahn. Changes in peak inspiratory flow rate and peak airway pressure with endotracheal tube size during chest compression [J]. World Journal of Emergency Medicine, 2020, 11(2): 97-101. |
| [9] | Ye-cheng Liu, Yan-meng Qi, Hui Zhang, Joseph Walline, Hua-dong Zhu. A survey of ventilation strategies during cardiopulmonary resuscitation [J]. World Journal of Emergency Medicine, 2019, 10(4): 222-227. |
| [10] | Israel Olatunji Gabriel, Joel O. Aluko. Theoretical knowledge and psychomotor skill acquisition of basic life support training programme among secondary school students [J]. World Journal of Emergency Medicine, 2019, 10(2): 81-87. |
| [11] | Alexei Birkun, Yekaterina Kosova. Social attitude and willingness to attend cardiopulmonary resuscitation training and perform resuscitation in the Crimea [J]. World Journal of Emergency Medicine, 2018, 9(4): 237-248. |
| [12] | Alexei Birkun, Maksim Glotov, Herman Franklin Ndjamen, Esther Alaiye, Temidara Adeleke, Sergey Samarin. Pre-recorded instructional audio vs. dispatchers’ conversational assistance in telephone cardiopulmonary resuscitation: A randomized controlled simulation study [J]. World Journal of Emergency Medicine, 2018, 9(3): 165-171. |
| [13] | Alexei Birkun, Maksim Glotov. Education in cardiopulmonary resuscitation in Russia: A systematic review of the available evidence [J]. World Journal of Emergency Medicine, 2017, 8(4): 245-252. |
| [14] | Ling Zhou, Hui Li, Hong-yan Wei, Chun-lin Hu, Xiao-li Jing, Hong Zhan, Xiao-xing Liao, Xin Li. Study on the development and usage of a cardiopulmonary resuscitation time point recorder [J]. World Journal of Emergency Medicine, 2017, 8(3): 195-199. |
| [15] | Chennappa Kalvatala Krishna, Hakim Irfan Showkat, Meenakshi Taktani, Vikram Khatri. Out of hospital cardiac arrest resuscitation outcome in North India — CARO study [J]. World Journal of Emergency Medicine, 2017, 8(3): 200-205. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
