Abstract
Background:
Vitamin D is a multifunctional hormone essential in the immune system. Vitamin D deficiency may increase the disease burden in patients with COVID-19.Objectives:
We investigated the effect of vitamin D administration as an adjunctive therapy on treating children with COVID-19.Methods:
Children with confirmed or probable COVID-19 who were admitted to 17th Shahrivar Hospital in Rasht from October 2022 to April 2023 were selected by random sampling method. They were divided into two groups by random blocks: the intervention group with vitamin D administration and the placebo-controlled group. The patient data were recorded and analyzed, including age, sex, and the required clinical and laboratory information, including the time required to recover from fever and respiratory distress and the length of hospitalization.Results:
In this study, 60 patients (30 in each group) were evaluated. At the beginning of hospitalization, fever was significantly more observed in the intervention group (90% vs. 53.3%, P = 0.002). The average time required to recover from fever and respiratory distress was less in the intervention group, although this difference was not significant between the two groups (P = 0.591 and P = 0.192, respectively). The hospitalization duration was also similar in both groups, and no complications or death were observed in the two groups.Conclusions:
Administering vitamin D at a dose of 1000 IU daily showed no significant efficacy for recovering children with COVID-19. Further studies are suggested to investigate the high dose of vitamin D supplementation in treating children with COVID-19.Keywords
1. Background
Coronavirus Disease 2019 (COVID-19) was first reported in December 2019 in Wuhan, China. With the increased spread of the disease in different countries, this infection was recognized as one of the most important issues threatening health systems worldwide and suddenly became a health crisis. On January 30, 2020, this viral infection was introduced as a global health emergency by the World Health Organization (WHO) (1). As known, COVID-19 affects the upper and lower respiratory systems. In severe cases (up to 15%), the disease can cause a cytokine storm with severe pneumonia, respiratory distress syndrome, and multi-organ failure, which can be associated with significant mortality (2, 3). Although clinical manifestations such as respiratory symptoms, oxygen saturation (SpO2), shock symptoms, and multiple organ failure are criteria for determining different stages of the disease, laboratory indices such as lymphopenia, increased C-reactive protein (CRP), D-dimer, Interleukin-6 (IL-6), and procalcitonin (PCT) are very important in determining the prognosis of the disease (4).
Nutrition is a crucial factor in maintaining homeostasis and the overall health of various organs and physiological systems in the body, including the proper immune system functioning (5, 6). Inadequate nutrition is a risk factor in viral infection or its exacerbation due to weakness of the immune system, which increases the rate and severity of infections. In addition, viral infections increase the need for several micronutrients such as vitamins A, B, C, D, zinc, and selenium (7-10). Recent observations about the relatively greater impact of COVID-19 in areas with rainy and cloudy weather led to the hypothesis that vitamin D deficiency may increase the burden of disease in vitamin D-deficient COVID-19 patients, especially the elderly (11-13). Vitamin D is a multifunctional hormone whose most obvious effect is on calcium and phosphorus metabolism. However, several experimental and clinical studies have highlighted its essential role in the immune system (14). Vitamin D receptors are present throughout the body in skeletal and smooth muscles and the bladder. Vitamin D contributes to clinical disorders such as asthma, respiratory tract infection, insulin disturbance, autoimmune disorders, metabolic syndrome, fertility, urinary tract infection, overactive bladder syndrome, chronic kidney disease, and pelvic floor muscle disorder. Many studies have indicated that vitamin D may be associated with Lower Urinary Tract Symptoms (LUTS); however, the findings are contradictory (15).
The first reports on the role of vitamin D serum levels in the course of COVID-19 showed that infected patients had lower serum levels of vitamin D than those without COVID-19 (16). Raharusun et al. reported that in 780 patients with COVID-19 in Indonesia, vitamin D status was an independent predictor of mortality (17). Based on a meta-analysis study in Iran in 2018, the rate of vitamin D deficiency in men and women was 55.2% and 64.7%, respectively (18). There are even similar observations in pulmonary infections other than COVID-19. For example, studies have shown that about 67.5% of children with pneumonia in the age group of 3 to 5 years have vitamin D deficiency, or the serum level of vitamin D was lower in pneumonia-affected children in the age group of 24 to 60 months than in healthy children (19). Despite the extensive discussion in the literature about the role of vitamin D in the susceptibility to COVID-19 and the development of severe disease, very few studies have evaluated the effect of this supplement on treatment outcomes and mortality in COVID-19 patients. Most studies have only investigated the vitamin D level in the blood serum of COVID-19 patients.
2. Objectives
This study investigated the effect of vitamin D supplementation on the recovery of hospitalized COVID-19 children.
3. Methods
This clinical trial study randomly selected confirmed or probable patients with COVID-19 in the age range of 3 months to 14 years admitted to 17th Shahrivar Hospital in Rasht from October 2022 to April 2023. The exclusion criteria included patients with a history of hepatitis, malabsorption, taking anticonvulsant drugs and vitamin D in the last 3 months, and patients undergoing chemotherapy. Definite cases included patients with positive RT-PCR of the pharynx and nasopharynx, and probable cases were suspected cases with a confirmatory lung CT scan.
Patients were included in the study based on the inclusion criteria after their parents completed the informed consent form. The method of assigning patients to study groups is given in the consort diagram (Figure 1). Sixty-six affected children were evaluated in terms of eligibility, of whom 6 were excluded from the study due to recently receiving vitamin D. Patients were randomly assigned to two groups, including the intervention group with vitamin D administration and the control group (30 patients in each group), using the random block allocation method. Online software (www.sealedenvelope.com) generated a random sequence according to the desired sample size (30 people in each group) and block size.
Consolidated Standards of Reporting Trials diagram depicting sampling procedure
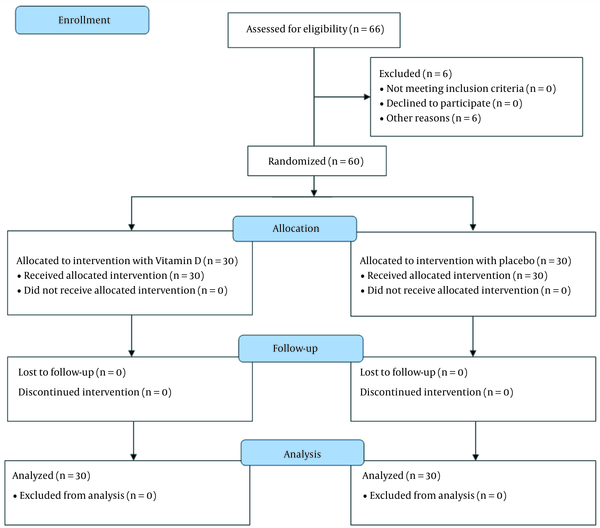
The study groups included the intervention group, receiving vitamin D at a dose of 1000 IU daily for up to one week, and the control group, receiving a placebo. The soft gel of 1000 units of vitamin D3 made by Barij Essence Company was used (Barij Essence Co., batch number: 0106GVD513, 1.10.2024). The content of the soft gel was drawn with a syringe and mixed with a small amount of milk or yogurt for younger children who could not swallow the soft gel. The production of a placebo similar to the original drug was coordinated with the company, and 50 placebos were produced. Hiding the type of drug was also done by using opaque envelopes sealed with a random sequence (envelopes were opaque, sealed, and numbered sequentially). In this method, each random sequence was recorded on a card, and the cards were placed in the envelopes in order. In order to maintain the random sequence, the outer surfaces of the envelopes were numbered in the same order. Finally, the lids of the envelopes were glued and placed in a box. At the time of the registration of the participants, based on the order of entry into the study, one of the envelopes was opened, and the assigned group of that participant was revealed. One of the collaborators on the project did coding, and the physician, evaluator, and patient were blind to it. We had no missing cases in the follow-up; finally, 30 patients in each group were analyzed. Necessary treatments were carried out according to each patient's clinical condition following the national guideline, and the only difference considered was the administration of vitamin D in the intervention group.
The patient information, including age, sex, clinical manifestations, and laboratory findings, was recorded in a checklist. Also, the duration of admission until the recovery from fever and respiratory distress and the duration of hospitalization were the primary outcomes, while short-term complications or death were the secondary outcomes compared between the two groups. Possible short-term complications, including secondary infections such as bacterial pneumonia, convulsions, loss of consciousness, thromboembolic complications, and multisystem inflammatory syndrome, were evaluated daily by a designated pediatric assistant. The assistant, who was unaware of the patient's intervention group, recorded any occurrences of these complications. The disease severity was defined according to the guideline for the diagnosis and treatment of COVID-19 in children and infants (20) as follows:
Moderate respiratory criteria: (1) The presence of lower respiratory symptoms (including shortness of breath, a feeling of pain and pressure in the chest, etc.), (2) fever equal to or more than 38°C, SPO2 between 90% and 93%, and (3) pulmonary involvement less than 50% in lung CT scan.
Severe respiratory criteria: This phase generally has more severe clinical symptoms. The criteria for entering this stage are (1) rapid development of respiratory distress, especially exacerbation of shortness of breath, (2) tachypnea RR > 30, (3) PaO2/SpO2 < 90%, FiO2 ≤ 300 mmHg, and (4) an increase in the A-a gradient and involvement of more than 50% of the lung in CT scan.
All patient information was considered confidential. This study was approved by the Ethics Committee of Guilan University of Medical Sciences (ethics code: IR.GUMS.REC.1401.116 and IRCT registration number: IRCT20090909002438N4). It should be noted that the prescribed daily dose (1000 units per day) was not much more than the required daily dose (600 units per day), and the total dose received by patients was 7000 units, unlikely to cause any risk to patients. Vitamin D poisoning is uncommon in children, as it is caused by total doses of about 240,000 to 4,500,000 units of vitamin D3 (21).
The mean and standard deviation were used to describe quantitative data, and frequency and percentage were used to describe qualitative data. The normality of the quantitative data was assessed using the Kolmogorov-Smirnov test. The comparison of qualitative dependent variables between the groups was done using the chi-square test and, if necessary, Fisher's exact test. An independent t-test was used to compare the 2 groups in the case of normal data distribution, and a Mann-Whitney test was used for non-normal distribution. A P < 0.05 was considered the level of significance. Data analysis was done in SPSS software version 16.
4. Results
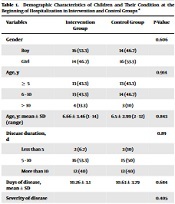
The data of 30 children in the intervention group and 30 children in the control group were analyzed. The mean age of the children in the two groups was 6.58 ± 3.21 years (the youngest child was one year old, and the oldest was 14 years old). The demographic information of the children is shown in Table 1.
Demographic Characteristics of Children and Their Condition at the Beginning of Hospitalization in Intervention and Control Groups a
| Variables | Intervention Group | Control Group | P-Value |
|---|---|---|---|
| Gender | 0.606 | ||
| Boy | 16 (53.3) | 14 (46.7) | |
| Girl | 14 (46.7) | 16 (53.3) | |
| Age, y | 0.914 | ||
| ≥ 5 | 13 (43.3) | 13 (43.3) | |
| 6 - 10 | 13 (43.3) | 14 (46.7) | |
| > 10 | 4 (13.3) | 3 (10) | |
| Age, y: mean ± SD (range) | 6.66 ± 3.46 (1 - 14) | 6.5 ± 2.99 (2 - 12) | 0.843 |
| Disease duration, d | 0.89 | ||
| Less than 5 | 2 (6.7) | 3 (10) | |
| 5 - 10 | 16 (53.3) | 15 (50) | |
| More than 10 | 12 (40) | 12 (40) | |
| Days of disease, mean ± SD | 10.26 ± 3.1 | 10.63 ± 3.79 | 0.684 |
| Severity of disease | 0.405 | ||
| Severe | 8 (26.7) | 11 (36.7) | |
| Moderate | 22 (73.3) | 19 (63.3) | |
| Vitamin D serum levels | 0.592 | ||
| Low/inadequate | 20 (66.7) | 18 (60) | |
| Normal | 10 (33.3) | 12 (40) |
There were no statistically significant differences in the frequency of age, sex, mean duration of illness until admission, categories of duration of illness until admission, severity of illness, and vitamin D serum level on admission among children in the intervention and control groups. The frequency distribution of underlying diseases in children is shown in Table 2, which shows no difference between the two groups.
Frequency Distribution of Underlying Diseases in Children in Two Study Groups
| Variables | Intervention Group, No. (%) | Control Group | P-Value |
|---|---|---|---|
| Underlying disease | 0.559 | ||
| Yes | 9 (30) | 7 (23.3) | |
| No | 21 (70) | 23 (76.7) | |
| Type of underlying disease | 0.276 | ||
| History of seizures | 4 (44.4) | 3 (42.9) | |
| Gastrointestinal diseases- IBS | 2 (22.2) | 1 (14.3) | |
| Thalassemia minor | 1 (11.1) | 0 (0) | |
| History of frequent UTIs | 1 (11.1) | 0 (0) | |
| History of intussusception | 1 (11.1) | 0 (0) | |
| Pulmonary disorders - asthma | 0 (0) | 3 (42.9) |
The frequency of fever, hypoxia, and respiratory distress on the first day of hospitalization in both intervention and control groups is shown in Table 3. Only fever was observed more in the intervention group (P = 0.002).
The Frequency of Fever, Hypoxia, and Respiratory Distress on the First Day of Hospitalization in Intervention and Control Groups
| Variables | Intervention Group, No. (%) | Control Group, No. (%) | P-Value |
|---|---|---|---|
| Hypoxia | 0.573 | ||
| Yes | 8 (26.7) | 10 (33.3) | |
| No | 22 (73.3) | 20 (66.7) | |
| Respiratory distress | 0.176 | ||
| Yes | 8 (26.7) | 13 (43.3) | |
| No | 22 (73.3) | 17 (56.7) | |
| Fever | 0.002 | ||
| Yes | 27 (90) | 16 (53.3) | |
| No | 3 (10) | 14 (46.7) |
The hospitalization period of children is shown in Table 4, which shows no difference between the two groups.
Hospitalization Period in Intervention and Control Groups
| Variables | Intervention Group | Control Group | P-Value | 95% CI of Mean Difference (Lower - Upper) |
|---|---|---|---|---|
| Hospitalization days | 0.513 | - | ||
| Less than 5 days | 12 (40) | 15 (50) | ||
| 5 - 10 days | 12 (40) | 12 (40) | ||
| More than 10 days | 6 (20) | 3 (10) | ||
| Days of hospitalization | 7.36 ± 3.21 | 5.96 ± 3.05 | 0.089 | -0.22 - 3.02 |
None of our patients had a fatal outcome. All children in both groups were discharged from the hospital with recovery, and no side effects were observed.
Eight patients in the intervention group and 13 in the control group had respiratory distress. The average time required for the recovery of their respiratory distress was 1.87 ± 0.64 days in the intervention group and 2.3 ± 0.75 days in the control group, which did not show a significant difference (P = 0.192, 95% CI of mean difference: -1.1 - 0.23).
In addition, 27 patients in the intervention group and 17 in the control group had a fever. The average time required for their body temperature to return to normal in the 2 groups was 2.18 ± 1.07 days and 2.35 ± 0.86 days, respectively, which did not show a significant difference (P = 0.591, 95% CI of mean difference: -0.79 - 0.45).
5. Discussion
This research investigated the efficacy of vitamin D as adjuvant therapy in treating hospitalized children with COVID-19. The frequency of fever was higher in the intervention group, but the average time required to recover from fever was less in the intervention group. This can indicate the effectiveness of vitamin D in the treatment of children with COVID-19, although it should be noted that the difference between the two groups was not statistically significant.
According to a review study by Nikniaz et al. in 2021, intervention with vitamin D supplementation improves clinical conditions and reduces mortality in patients with COVID-19 (22). However, in most previous studies, only the vitamin D level in the blood serum of COVID-19 patients has been investigated. A study on children from one to 16 years old with COVID-19 showed that the incidence of fever was higher in children with low serum levels of vitamin D than in children with normal levels of vitamin D (67% vs. 18.3%) (23). In a study on 40 hospitalized COVID-19 patients, Yılmaz et al. showed that fever was significantly higher in vitamin D-deficient COVID-19 patients than in patients with sufficient vitamin D serum levels (24).
Also, in previous studies, vitamin D deficiency increased the risk of respiratory infections, including respiratory syncytial virus, tuberculosis, and influenza, and was a risk factor for acute respiratory distress syndrome (ARDS) (25). In 2021, Alpcan et al. (26) showed in a study on children aged one to 18 years that respiratory distress was higher in COVID patients with low serum levels of vitamin D (110). In our study, the average time needed for respiratory distress recovery was lower in the intervention group, although the difference between the two groups was insignificant. Patients with a baseline serum vitamin D level < 25 nmol/L who received repeated low doses of vitamin D showed the maximum beneficial effect against respiratory tract infections (27). In a clinical trial study on children aged 6 to 12 years, the results of a 6-month follow-up showed that the rate of overall upper respiratory tract infections decreased by 30% in the intervention group (receiving vitamin D 1,000 IU and calcium 500 mg per day) and by 25% in the placebo group. The difference between the two groups was significant (28).
Although the pathogenic mechanisms related to COVID-19 are not fully elucidated, it is clear that this disease damages organs due to the high affinity of spike protein for the human angiotensin-converting enzyme 2 (ACE2) cell receptor, which is highly expressed in organs such as the lungs, heart, liver, kidneys, brain, and bladder. The disease's clinical severity and mortality rate are generally lower in children than in adults, which could be related to the lower amount of ACE2-expressing AT2 cells and ACE2 protein content in children than in adults (29, 30). Vitamin D also helps protect against this virus in different ways: (1) by stabilizing the intercellular connections in the lung and preventing the penetration and rapid spread of the coronavirus deep in the lung tissue (31), (2) by the differentiation of monocytes into macrophages and increasing the ability of macrophages to phagocytose (32, 33), (3) by binding to the Vitamin D Receptor (VDR) and increasing the expression of antimicrobial peptides such as defensin and cathelicidin (34), (4) by reducing the excessive secretion of inflammatory cytokines and preventing destructive molecular events such as cytokine storm and uncontrolled inflammation in the lung (35, 36), (5) by regulating the renin-angiotensin system and preventing a sharp increase in angiotensin 2, and (6) by protecting tissues against coronavirus, especially the lung (37, 38).
In our study, there were no side effects or death in both vitamin D intervention and control groups. Radujkovic et al. showed a significant relationship between vitamin D deficiency and mortality in patients with COVID-19 (39). Also, Karahan and Katkat found that the serum level of 25 (OH) vitamin D was independently associated with mortality in COVID-19 patients. In their study, vitamin D deficiency was present in 93% of patients with severe COVID-19. Also, the numbers of lymphocytes, white blood cells, and serum albumin were introduced as other independent predictors of mortality (40). The results of the studies on the mortality rate of COVID-19 patients receiving vitamin D are contradictory. Since the statistics of disease centers in different countries are not officially and reliably announced, the exact number of deaths cannot be obtained. In a cross-sectional study investigating the prevalence of vitamin D deficiency and its relationship with mortality in children hospitalized in the intensive care unit, the mortality rate was higher in children with vitamin D deficiency hospitalized in the PICU than in children with normal vitamin D serum levels (41).
Our study also had limitations. The sample size was relatively small. Also, the prescribed dose of vitamin D was not significantly different from the daily required dose, which might be insufficient to treat vitamin D deficiency. Since higher doses of vitamin D are recommended to quickly compensate for vitamin D deficiency and increase its therapeutic effect, studies with higher doses of vitamin D are recommended.
5.1. Conclusions
Vitamin D administration did not significantly affect the treatment of children with COVID-19. More studies are needed to determine the role of vitamin D in treating children with COVID-19.
Acknowledgements
References
-
1.
Sohrabi C, Alsafi Z, O'Neill N, Khan M, Kerwan A, Al-Jabir A, et al. Corrigendum to "World Health Organization declares Global Emergency: A review of the 2019 Novel Coronavirus (COVID-19)" [Int. J. Surg. 76 (2020) 71-76]. Int J Surg. 2020;77:217. [PubMed ID: 32305321]. [PubMed Central ID: PMC7159865]. https://doi.org/10.1016/j.ijsu.2020.03.036.
-
2.
Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, et al. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020;296(2):E15-25. [PubMed ID: 32083985]. [PubMed Central ID: PMC7233368]. https://doi.org/10.1148/radiol.2020200490.
-
3.
Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141-54. [PubMed ID: 33024307]. [PubMed Central ID: PMC7537588]. https://doi.org/10.1038/s41579-020-00459-7.
-
4.
Zhou B, Kojima S, Kawamoto A, Fukushima M. COVID-19 pathogenesis, prognostic factors, and treatment strategy: Urgent recommendations. J Med Virol. 2021;93(5):2694-704. [PubMed ID: 33368358]. https://doi.org/10.1002/jmv.26754.
-
5.
Wu D, Lewis ED, Pae M, Meydani SN. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front Immunol. 2018;9:3160. [PubMed ID: 30697214]. [PubMed Central ID: PMC6340979]. https://doi.org/10.3389/fimmu.2018.03160.
-
6.
Childs CE, Calder PC, Miles EA. Diet and Immune Function. Nutrients. 2019;11(8). [PubMed ID: 31426423]. [PubMed Central ID: PMC6723551]. https://doi.org/10.3390/nu11081933.
-
7.
Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020;12(1). [PubMed ID: 31963293]. [PubMed Central ID: PMC7019735]. https://doi.org/10.3390/nu12010236.
-
8.
Jayawardena R, Sooriyaarachchi P, Chourdakis M, Jeewandara C, Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab Syndr. 2020;14(4):367-82. [PubMed ID: 32334392]. [PubMed Central ID: PMC7161532]. https://doi.org/10.1016/j.dsx.2020.04.015.
-
9.
Jovic TH, Ali SR, Ibrahim N, Jessop ZM, Tarassoli SP, Dobbs TD, et al. Could Vitamins Help in the Fight Against COVID-19? Nutrients. 2020;12(9). [PubMed ID: 32842513]. [PubMed Central ID: PMC7551685]. https://doi.org/10.3390/nu12092550.
-
10.
Messina G, Polito R, Monda V, Cipolloni L, Di Nunno N, Di Mizio G, et al. Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work. Int J Mol Sci. 2020;21(9). [PubMed ID: 32354030]. [PubMed Central ID: PMC7247152]. https://doi.org/10.3390/ijms21093104.
-
11.
Rhodes JM, Subramanian S, Laird E, Kenny RA. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51(12):1434-7. [PubMed ID: 32311755]. [PubMed Central ID: PMC7264531]. https://doi.org/10.1111/apt.15777.
-
12.
Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32(7):1195-8. [PubMed ID: 32377965]. [PubMed Central ID: PMC7202265]. https://doi.org/10.1007/s40520-020-01570-8.
-
13.
Kara M, Ekiz T, Ricci V, Kara O, Chang KV, Ozcakar L. 'Scientific Strabismus' or two related pandemics: coronavirus disease and vitamin D deficiency. Br J Nutr. 2020;124(7):736-41. [PubMed ID: 32393401]. [PubMed Central ID: PMC7300194]. https://doi.org/10.1017/S0007114520001749.
-
14.
Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881-6. [PubMed ID: 21527855]. [PubMed Central ID: PMC3166406]. https://doi.org/10.2310/JIM.0b013e31821b8755.
-
15.
Siroosbakht S. Are Vitamin D Levels Linked to Primary Monosymptomatic Nocturnal Enuresis in Children? Six Years of Experience about a Controversy in Medicine: A Case-control Study. Iran J Pediatr. 2023;33(3). https://doi.org/10.5812/ijp-133755.
-
16.
D'Avolio A, Avataneo V, Manca A, Cusato J, De Nicolo A, Lucchini R, et al. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients. 2020;12(5). [PubMed ID: 32397511]. [PubMed Central ID: PMC7285131]. https://doi.org/10.3390/nu12051359.
-
17.
Raharusun P, Priambada S, Budiarti C, Agung E, Budi C. Patterns of COVID-19 Mortality and Vitamin D: An Indonesian Study. SSRN Electron J. 2020. https://doi.org/10.2139/ssrn.3585561.
-
18.
Tabrizi R, Moosazadeh M, Akbari M, Dabbaghmanesh MH, Mohamadkhani M, Asemi Z, et al. High Prevalence of Vitamin D Deficiency among Iranian Population: A Systematic Review and Meta-Analysis. Iran J Med Sci. 2018;43(2):125-39. [PubMed ID: 29749981]. [PubMed Central ID: PMC5936844].
-
19.
Hashemian H, Heidarzadeh A. Role of Vitamin D [25(OH) D] Deficiency in Development of Pneumonia in Children. Arch Pediatr Infect Dis. 2017;In Press(In Press). https://doi.org/10.5812/pedinfect.57276.
-
20.
National Committee for Children's COVID-19. Coronavirus Disease 2019 (COVID-19) Diagnosis and Treatment Guideline in children and infants. Ministry of Health, Treatment and Medical Education of the Islamic Republic of Iran. 7th ed. National Committee for Children's COVID-19; 2022.
-
21.
Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7(6):4240-70. [PubMed ID: 26035247]. [PubMed Central ID: PMC4488782]. https://doi.org/10.3390/nu7064240.
-
22.
Nikniaz L, Akbarzadeh MA, Hosseinifard H, Hosseini M. The impact of vitamin D supplementation on mortality rate and clinical outcomes of COVID-19 patients: A systematic review and meta-analysis. Pharm Sci. 2021. https://doi.org/10.34172/ps.2021.13.
-
23.
Heidari S, Mohammadi S, Fathi M, Cigary S, Alisamir M, Mirkarimi M, et al. Association of vitamin D status with COVID-19 disease severity in pediatric patients: A retrospective observational study. Health Sci Rep. 2022;5(3). e569. [PubMed ID: 35415272]. [PubMed Central ID: PMC8987118]. https://doi.org/10.1002/hsr2.569.
-
24.
Yilmaz K, Sen V. Is vitamin D deficiency a risk factor for COVID-19 in children? Pediatr Pulmonol. 2020;55(12):3595-601. [PubMed ID: 33017102]. [PubMed Central ID: PMC7675606]. https://doi.org/10.1002/ppul.25106.
-
25.
Marik PE, Kory P, Varon J. Does vitamin D status impact mortality from SARS-CoV-2 infection? Med Drug Discov. 2020;6:100041. [PubMed ID: 32352080]. [PubMed Central ID: PMC7189189]. https://doi.org/10.1016/j.medidd.2020.100041.
-
26.
Alpcan A, Tursun S, Kandur Y. Vitamin D levels in children with COVID-19: A report from Turkey. Epidemiol Infect. 2021;149. e180. [PubMed ID: 34375576]. [PubMed Central ID: PMC8365038]. https://doi.org/10.1017/S0950268821001825.
-
27.
Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356. i6583. [PubMed ID: 28202713]. [PubMed Central ID: PMC5310969]. https://doi.org/10.1136/bmj.i6583.
-
28.
Mandlik R, Mughal Z, Khadilkar A, Chiplonkar S, Ekbote V, Kajale N, et al. Occurrence of infections in schoolchildren subsequent to supplementation with vitamin D-calcium or zinc: a randomized, double-blind, placebo-controlled trial. Nutr Res Pract. 2020;14(2):117-26. [PubMed ID: 32256986]. [PubMed Central ID: PMC7075745]. https://doi.org/10.4162/nrp.2020.14.2.117.
-
29.
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260-3. [PubMed ID: 32075877]. [PubMed Central ID: PMC7164637]. https://doi.org/10.1126/science.abb2507.
-
30.
Silva MG, Falcoff NL, Corradi GR, Di Camillo N, Seguel RF, Tabaj GC, et al. Effect of age on human ACE2 and ACE2-expressing alveolar type II cells levels. Pediatr Res. 2023;93(4):948-52. [PubMed ID: 35739259]. [PubMed Central ID: PMC9219367]. https://doi.org/10.1038/s41390-022-02163-z.
-
31.
Isaia G, Medico E. Associations between hypovitaminosis D and COVID-19: a narrative review. Aging Clin Exp Res. 2020;32(9):1879-81. [PubMed ID: 32705585]. [PubMed Central ID: PMC7376522]. https://doi.org/10.1007/s40520-020-01650-9.
-
32.
BourBour F, Mirzaei Dahka S, Gholamalizadeh M, Akbari ME, Shadnoush M, Haghighi M, et al. Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Arch Physiol Biochem. 2023;129(1):16-25. [PubMed ID: 32644876]. https://doi.org/10.1080/13813455.2020.1791188.
-
33.
Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020;12(4). [PubMed ID: 32340216]. [PubMed Central ID: PMC7230749]. https://doi.org/10.3390/nu12041181.
-
34.
Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020;13(10):1373-80. [PubMed ID: 32605780]. [PubMed Central ID: PMC7305922]. https://doi.org/10.1016/j.jiph.2020.06.021.
-
35.
Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017;27(1). [PubMed ID: 27714929]. https://doi.org/10.1002/rmv.1909.
-
36.
Tsujino I, Ushikoshi-Nakayama R, Yamazaki T, Matsumoto N, Saito I. Pulmonary activation of vitamin D(3) and preventive effect against interstitial pneumonia. J Clin Biochem Nutr. 2019;65(3):245-51. [PubMed ID: 31777427]. [PubMed Central ID: PMC6877402]. https://doi.org/10.3164/jcbn.19-48.
-
37.
Malek Mahdavi A. A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: Implications for a potential treatment for COVID-19. Rev Med Virol. 2020;30(5). e2119. [PubMed ID: 32584474]. [PubMed Central ID: PMC7362103]. https://doi.org/10.1002/rmv.2119.
-
38.
McMullan CJ, Borgi L, Curhan GC, Fisher N, Forman JP. The effect of vitamin D on renin-angiotensin system activation and blood pressure: a randomized control trial. J Hypertens. 2017;35(4):822-9. [PubMed ID: 28033130]. [PubMed Central ID: PMC5893307]. https://doi.org/10.1097/HJH.0000000000001220.
-
39.
Radujkovic A, Hippchen T, Tiwari-Heckler S, Dreher S, Boxberger M, Merle U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients. 2020;12(9). [PubMed ID: 32927735]. [PubMed Central ID: PMC7551780]. https://doi.org/10.3390/nu12092757.
-
40.
Karahan S, Katkat F. Impact of Serum 25(OH) Vitamin D Level on Mortality in Patients with COVID-19 in Turkey. J Nutr Health Aging. 2021;25(2):189-96. [PubMed ID: 33491033]. [PubMed Central ID: PMC7533663]. https://doi.org/10.1007/s12603-020-1479-0.
-
41.
Habibzadeh M, Elahi-Najafi A, Shafa A. Prevalence of Vitamin D Deficiency and its Correlation with Mortality in Pediatric Intensive Care Unit. J Isfahan Med Sch. 2017;35(456):1654-9. https://doi.org/10.22122/jims.v35i456.8743.