Abstract
Background:
CD28 expression is correlated with malignancy development in long-term survivors after liver transplantation. Immune cell activation is mediated by the interaction of CD28 with CD4 and CD8.Objectives:
In this study, we attempted to investigate the expression level and prognostic value of CD28 in hepatocellular carcinoma (HCC).Methods:
A total of 54 HCC patients with complete clinical information were examined. The expression level of CD28 in HCC tissues was detected by immunohistochemistry. The correlations of CD28 expression with clinical characteristics, CD4+/CD8+ T-cells, and prognosis in HCC were analyzed. The expression profile of CD28 and survival time of HCC patients were retrieved from the TCGA database, followed by survival analysis.Results:
The positive expression rate of CD28 in HCC tissues was 70.73%. The CD28 expression was significantly higher in the positive expression group (area: 659174.9 ± 670060, IOD: 123348.3 ± 106348.6) than in the negative expression group (area: 8405.7 ± 9983.3, IOD: 1959.6 ± 2117.7) (P < 0.01). The CD4+ and CD8+ cell counts were 526.13 ± 258.17 cells/µL and 383.93 ± 223.39 cells/µL, respectively. The expression level of CD28 was significantly related to the degree of differentiation and the number of CD4+ and CD8+ T-cells (P < 0.05). The survival time of patients was longer in the positive CD28 expression group than in the negative expression group. Based on the CD28 expression profiles of 406 HCC patients retrieved from the TCGA database, patients with high CD28 expression showed a better prognosis than those with low expression (P < 0.05).Conclusions:
CD28 may play a vital role in the occurrence, development, and prognosis of HCC by interacting with CD4+ and CD8+ T-cells. Thus, CD28 could be suggested as the immune checkpoint target for HCC treatment.Keywords
CD28 Expression Hepatocellular Carcinoma Immunohistochemistry Prognosis TCGA Database
1. Background
Hepatocellular carcinoma (HCC) is the most frequent form of liver cancer, accounting for more than 90% of all liver cancers (1). The etiology of HCC is complicated, which can be generally explained by the combination of environmental factors, aging, unhealthy lifestyle, and genetic factors (2). Due to its asymptomatic feature in the early stage, HCC is usually diagnosed at an advanced stage. Despite advancements in early diagnosis, the prognosis remains extremely poor in HCC patients. The patients’ prognosis exhibits a global variation. It is reported that the median survival time of HCC patients in North America, Europe, and China is 33, 24, and 23 months, respectively (3). Thus, HCC remains a global public health concern, and novel curative therapies are urgently needed.
Antitumor immune response mediated by immune cells is an essential natural barrier to cancer development and progression (4). The dysregulation of the interaction between tumor and immune cells contributes to tumor cells’ evasion from the immune system. T-cells constitute a significant group of immune cells involved in tumor immune response. Cell activity is mediated by the interaction of the T-cell receptor with transmembrane receptor (5, 6). CD28 is a glycoprotein expressed by most T-cells. Evidence shows that CD28 polymorphism is associated with the risk of breast cancer and cervical cancer (7, 8). It was reported that treatment with CD28 antagonists conferred long-term tolerance of mice with organ graft (6), proving the critical role of CD28 in inducing immune responses. However, the role of CD28 in the HCC prognosis has not been clarified.
Cytotoxic T-lymphoma (CTL) exerts immunologically specific cytotoxicity in tumor cells by interacting with antigenic molecules and T-cell receptor-mediated MHC/peptide complexes. Besides, CD28 blocks the interaction of T-cell receptors (including CD4 and CD8) with the MHC complex (9). CD28 expression has been suggested as the potential marker for malignancies in survivors of liver transplantation (10). Currently, little is known about the relationship of CD28 with CD4+ and CD8+ immune cells in HCC. Given that CD28 plays a regulatory role in tumor immunity by interacting with CD4 and CD8 (11), we speculated that CD28 might be involved in the progression of HCC, and its expression is correlated with the prognosis of HCC patients.
2. Objectives
We investigated the expression level of CD28 in HCC and its correlation with the prognosis of HCC patients. We also investigated the correlation of CD28 with CD4 and CD8. We hope that our study will have some guiding significance for HCC treatment.
3. Methods
3.1. Clinical Tumor Sample Collection
From January 2014 to July 2019, HCC patients were recruited from the First Affiliated Hospital of Henan University of Traditional Chinese Medicine. Hepatocellular carcinoma was determined in patients based on the pathological diagnosis. Other inclusion criteria included patients with complete clinical information and a history of hepatitis B. All the eligible patients had not received any treatment before this study. On the other hand, patients with a history of HBV infection and incomplete clinical information were excluded.
Finally, 54 HCC patients (44 males and 10 females), with an average age of 54.85 ± 11.14 years, were examined. The basic information of patients is depicted in Table 1. Pathological tumor tissues were collected under surgery or puncture with informed consent. A total of 54 HCC tissues were staged according to the Barcelona Clinical Liver Cancer (BCLC) staging system (12), with stage A in 27 cases, stage B in 13 cases, and stage C in 14 cases. The blood samples of the included patients were collected at diagnosis, and the serum AFP level was detected by chemiluminescence immunoassay kit (Mlbio, Shanghai, China) with an automatic chemiluminescence analyzer (DX1800, Beckman Coulter, Inc., Fullerton, CA).
Baseline Characteristics of Included Patients with Hepatocellular Carcinoma Undergoing Surgical Resection a
| Characteristics | Values |
|---|---|
| Sex | |
| Male | 44 |
| Female | 10 |
| Age (y), No. (mean ± SD) | 54 (54.85 ± 11.14) |
| Tumor size (cm) | |
| ≤ 5 | 30 |
| > 5 | 24 |
| Number of tumors | |
| Single | 27 |
| Multiple | 27 |
| TNM stage | |
| I - II | 43 |
| III - IV | 11 |
| Differentiation | |
| Low | 11 |
| Mild | 25 |
| High | 18 |
| Metastasis | |
| Yes | 8 |
| No | 46 |
| PVTT | |
| Yes | 12 |
| No | 42 |
| Abdominal dropsy | |
| Yes | 11 |
| No | 43 |
All the included patients were followed up for the next five years. The survival data were collected during the hospitalization period or by telephone contact post-discharge. The Ethics Committee of the First Affiliated Hospital of Henan University of Traditional Chinese Medicine reviewed and approved this study (approval number: 2018HL-079-01).
3.2. Immunohistochemistry Assay
The CD28 expression in HCC tissues was detected by the streptavidin-peroxidase (SP) immunohistochemistry two-step method. Briefly, tissue slices (5 µm thickness) were deparaffinized, dehydrated, and blocked with 3% H2O2 for 10 min at room temperature. After being washed with PBS three times, sections were incubated with the primary rabbit anti-CD28 antibody (1:1000, Beijing Boaosen Biotechnology Co., Ltd., Beijing, China), followed by incubation with peroxidase-conjugated IgG1 secondary antibody (1:1000, Beijing Boaosen Biotechnology Co., Ltd., Beijing, China) at room temperature for 20 min. Then, the sections were stained by diaminobenzidine (DAB) solution (AR1026-5, Bosch Bioengineering Co., LTD, Wuhan, China) at room temperature for 5 min and re-stained with hematoxylin.
3.3. Microscopic Analysis
After the immunohistochemistry assay, sections stained with the CD28 antibody were analyzed in five random high-power microscope fields. The positive CD28 protein expression was reflected by brown staining in the cytoplasm. The positive staining was evaluated according to the staining density (integrated optical density, IOD) and percentage (area) by two independent pathologists in random order. Based on the IOD value of CD28 staining, sections were divided into the negative expression group (IOD < 1000), low expression group (IOD range: 10000 - 250000), and high expression group (IOD range: 250000 - 450000). The semi-quantitative analysis of CD28 staining was achieved using Image-Pro Plus software.
3.4. Flow Cytometry
A blood sample of 2 mL was collected from each patient in a vacuum tube containing an ethylenediaminetetraacetic acid anticoagulant. Blood samples were prepared for the flow cytometry (FCM) assay within 6 h post-collection, followed by incubation with FITC-conjugated antibodies against CD4 and CD8. After red blood cells and unlabeled cells were lysed by FACS lysing solution (Becton-Dickinson, Le Pont-De-Claix, France) (13), the percentages of CD4+ and CD8+ cells were analyzed by FC500 Flow Cytometer (Beckman Coulter, Miami, USA).
3.5. TCGA Data Acquisition
The mRNA expression datasets of HCC samples were retrieved from the Cancer Genome Atlas (TCGA) online database (https://www.cancer.gov). The samples without survival data were excluded. The CD28 expression profiles were extracted from 406 clinical samples. Based on the median CD28 expression level, tumor samples were divided into high and low expression groups for survival curve analysis.
3.6. Statistical Analysis
We applied SPSS 21.0 software for statistical analysis. Data were expressed as mean ± standard deviation (SD). The correlations of the CD28 expression level with clinicopathological characteristics and AFP (alpha-fetoprotein) level of HCC patients were tested by the chi-square test. The Kaplan-Meier method was used for survival analysis. The correlation of CD28 expression level with CD4+ and CD8+ T-cells was tested by Wilcoxon signed rank-sum test. The differences were statistically significant at P < 0.05.
4. Results
4.1. CD28 Expression Level in HCC Tissues
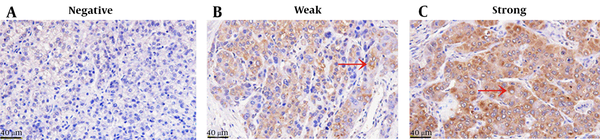
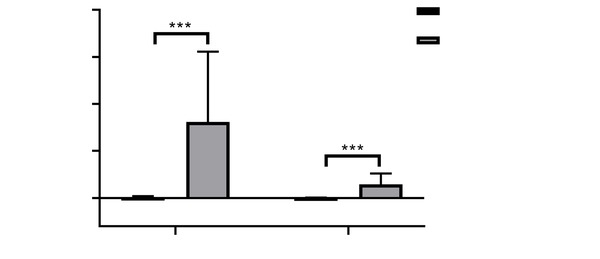
The positive CD28 expression showed diffuse cytoplasmic staining in HCC tissues, and the staining was brown or tan. As shown in Figure 1, CD28 expression was variable in HCC tissues, exhibiting negative expression in 16 cases but weak and strong expressions in 26 and 12 cases, respectively. In 54 HCC tissues, the positive expression rate of CD28 was 70.73% (38/54). The intensity and area of CD28 staining were remarkably higher in the positive expression group (area: 659174.9 ± 670060, IOD: 123348.3 ± 106348.6) than in the negative expression group (area: 8405.7 ± 9983.3, IOD: 1959.6 ± 2117.7) (P < 0.01, Figure 2).
Immunohistochemistry staining of CD28 in HCC tissues; A, Negative expression of CD28 in HCC tissues (SP × 400); B, Weak staining of CD28 in HCC tissues (SP × 400); C, Strong staining of CD28 in HCC tissues (SP × 400).
Quantitative analysis of CD28 expression in immunohistochemistry. Patients with weak and strong staining of CD28 were assigned to the positive expression group, while those with negative expression of CD28 were regarded as the negative expression group. The integrated optical density (IOD) value and area of CD28 staining were significantly higher in the positive expression group than in the negative expression group (*** P < 0.001).
4.2. Correlation Between CD28 Expression in HCC Tissues and Clinicopathological Characteristics
The correlation analysis between CD28 expression level and clinical variables was performed to understand the factors affecting CD28 expression. The results showed that CD28 expression was significantly correlated with the degree of tumor differentiation (P < 0.05). There was no correlation between CD28 and gender, age, tumor size, TNM stage, tumor thrombosis, metastasis, number of tumors, and AFP level (P > 0.05). The detailed information is shown in Table 2.
Relationship Between CD28 Expression Level and Clinicopathological Characteristics of Hepatocellular Carcinoma Patients
| Predictor | No. | CD28 Negative (N = 16) | CD28 Positive (N = 38) | χ2 | P |
|---|---|---|---|---|---|
| Gender | < 0.001 | 1.000 | |||
| Male | 44 | 13 | 31 | ||
| Female | 10 | 3 | 7 | ||
| Age (y) | 1.181 | 0.277 | |||
| < 60 | 33 | 8 | 25 | ||
| ≥ 60 | 21 | 8 | 13 | ||
| Tumor diameter (cm) | 0.004 | 0.947 | |||
| ≤ 5 | 30 | 9 | 21 | ||
| > 5 | 24 | 7 | 17 | ||
| Differentiation | 4.113 | 0.043 | |||
| Low | 11 | 6 | 5 | ||
| High | 43 | 10 | 33 | ||
| TNM staging | 0.316 | 0.574 | |||
| I - II | 43 | 14 | 29 | ||
| III - IV | 11 | 2 | 9 | ||
| Portal vein tumor thrombus | < 0.001 | 1.000 | |||
| Yes | 12 | 4 | 8 | ||
| No | 42 | 12 | 30 | ||
| Metastasis | < 0.001 | 1.000 | |||
| Yes | 8 | 2 | 6 | ||
| No | 46 | 14 | 32 | ||
| Number of tumors | 0.355 | 0.551 | |||
| Single | 27 | 9 | 18 | ||
| Multiple | 27 | 7 | 20 | ||
| AFP (ng/mL) | 0.031 | 0.860 | |||
| ≤ 20 | 28 | 8 | 20 | ||
| < 20 | 26 | 8 | 18 |
4.3. Relationship Between CD28 Expression Level in HCC Tissues and Number of CD4+ and CD8+ T-cells
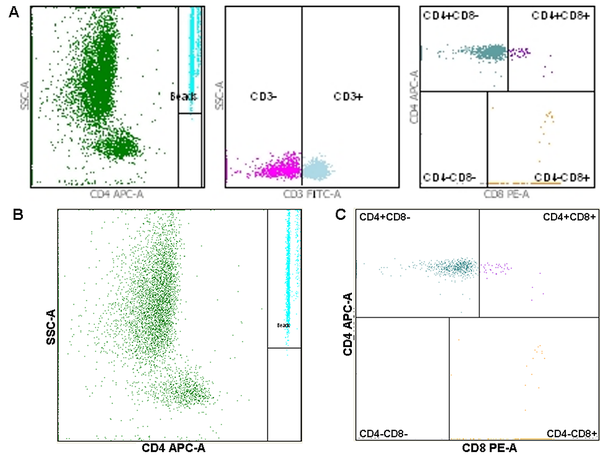
The numbers of CD4+ and CD8+ cells in blood samples of HCC patients were analyzed by flow cytometry (Figure 3). The results showed that the CD4+ and CD8+ cell counts were 526.13 ± 258.17 cells/µL and 383.93 ± 223.38 cells/µL in HCC patients. The Wilcoxon signed-rank test was used to analyze the correlation between the CD28 expression level in HCC tissues and the numbers of CD4+ and CD8+ T-cells in blood samples. The results showed that the CD28 expression level was correlated with the numbers of CD4+ T-cells (W = 327.000, P = 0.032) and CD8+ T-cells (W = 921.500, P = 0.019).
Flow cytometry for the number of CD4+ and CD8+ cells; A, General images of flow cytometry; B, Flow cytometry image for the number of CD4+ cells; C, Flow cytometry image for the number of CD8+ cells.
4.4. Prognostic Value of CD28 in HCC
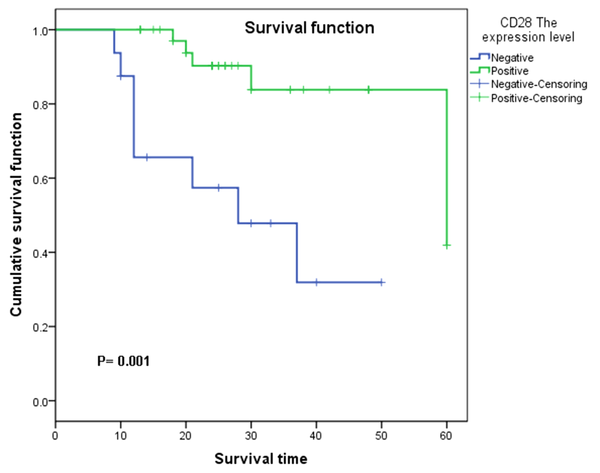
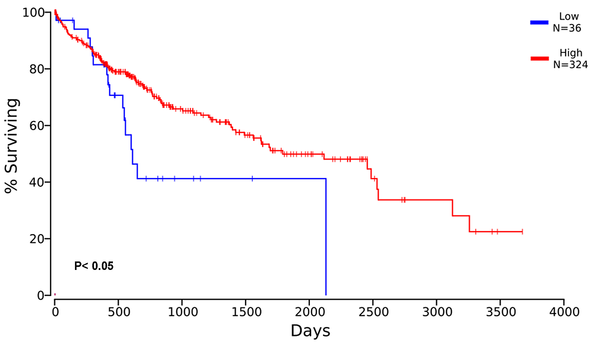
Survival analysis was performed to evaluate the relationship between CD28 expression and survival outcomes of HCC patients. The results showed that in the cohort of 54 HCC cases included in this study, the survival time was significantly longer in those with positive CD28 expression than those with negative CD28 expression (P = 0.001, Figure 4). To validate the predictive value of CD28 expression for survival outcomes, we performed Kaplan-Meier survival analysis in 406 HCC patients according to CD28 expression collected from the TCGA database. Based on the median of 68.95, HCC patients were divided into high and low CD28 expression groups. As illustrated in Figure 5, the survival time was significantly higher in patients with high CD28 expression (P < 0.05).
Kaplan-Meier curve analysis for 54 included HCC patients. Based on CD28 staining, patients were grouped into CD28 negative and positive expression groups. The survival time between the two groups was compared by the Kaplan-Meier method. The survival time was longer in patients with positive expression than those with negative expression.
Survival curve for HCC patients based on TCGA database. The expression profile of CD28 in 406 HCC patients was retrieved from the TCGA database. According to the median expression value of CD28, the patients were classified into high or low expression groups. The Kaplan-Meier analysis was performed on clinical data of the TCGA database. HCC patients with high CD28 expression showed a better prognosis than those with low CD28 expression.
5. Discussion
Hepatocellular carcinoma is a public health problem for its high recurrence rate and poor prognosis. The curative treatment for HCC remains challenging. Currently, immune-based therapy with checkpoint inhibitors has emerged as a promising candidate for cancer therapy (14). By targeting signaling pathways and immune checkpoint proteins, the molecule inhibitor sorafenib has been applied as the first-line systemic therapy for advanced-stage HCC; however, sorafenib systemic therapy for HCC is limited due to the modest benefits and serious side effects (1) Thus, a novel therapeutic approach to HCC treatment needs to be discovered.
CD28 is a founding member of a subfamily of costimulatory molecules characterized by extracellular variable immunoglobulin-like domains, which are constitutively expressed on T-cells (15, 16). CD28 is mainly expressed on CD4+ and CD8+ T-cells and provides unique signals to control a range of intracellular biochemical events, from post-translational protein modification (such as phosphorylation) to epigenetic changes, altering T-cell gene expression program (17). The central role of CD28 as the costimulatory signal in T-cell function makes it an attractive target for agents regulating the function of effector T-cells and Treg cells (18). In recent years, much evidence has suggested that CD28 plays a crucial role in anti-tumor immunity, preventing the occurrence and development of tumors so that its expression in lung cancer, gastric cancer, breast cancer, cervical cancer, and other tumor cells is closely related to patients’ prognosis (19-22). However, little is known about the expression level and role of CD28 in HCC patients.
This study included 54 HCC patients and analyzed CD28 expression in liver tumor tissues. Our data showed that the CD28 protein expression was correlated with the degree of tumor differentiation in HCC patients. The survival time of HCC patients was significantly higher in the positive CD28 expression group than in the negative expression group, verified by the survival analysis based on the TCGA dataset. The results indicated that HCC patients in the high CD28 expression group showed a better prognosis than those in the low expression group. Evidence shows that the impaired cellular immunity in HCC and hepatitis C virus (HCV) infection was accompanied by reduced CD28 expression (23). CD28 expression was decreased in advanced HCC patients coinfected with HBV infection (24) CD28 polymorphisms may increase the risk of HCC (2). Similarly, clinical significance analysis of CD28 in leukemia (ATL) revealed that patients with genetic abnormalities related to CD28 exhibited a worse prognosis than those without, indicating the beneficial effects of CD28 expression on the survival time of ATL patients (25). Controversially, the high expression of CD28 in lung cancer patients was associated with worse disease-free survival but a better overall survival (26). Thus, we speculated that CD28 might play a different role in the prognosis of different cancers.
Besides, our results showed that the CD28 expression was significantly correlated with the number of CD4+ and CD8+ T-cells. It is reported that CD8+ T-cells play a crucial role in inducing an adaptive immune response against the tumor. The CD4+ T-cells are necessary for antitumor immunity by eliminating tumor cells and modulating the tumor microenvironment (27, 28). The activation of T-cells in response to antigens is mediated by CD4-CD8-p56lck complexes and the costimulating molecule CD28 (24). CD28 generates positive signals conferring the T-cell response (29). Qiu et al. suggested that the percentages of CD8+ cells and CD8+CD28- cells were significantly increased, while the CD8+CD28+ cells were decreased in HCC patients compared to controls. There was a positive correlation between the numbers of CD8+CD28- cells and CD8+ cells (9). CD28 is necessary for the development and survival of CD4+ T-cells (30). The decreased expression of CD28 on CD4+ T-cells is correlated with the poor prognosis of idiopathic pulmonary fibrosis patients (31). Thus, CD28 may play a key role in cell immunity by interacting with CD4 and CD8. In this study, CD28 might have interacted with CD4+ and CD8+ T-cells to affect the HCC prognosis, but further studies are warranted.
Furthermore, AFP is a kind of glycoprotein secreted from endoderm-derived tumors. AFP is overexpressed in HCC and serves as a biomarker for HCC diagnosis and prognosis (32, 33). In this study, we performed correlation analysis between the AFP level and CD28 expression to evaluate the diagnostic and prognostic value of CD28. The results showed no association between AFP and CD28 expression, which might be attributed to the small sample size in our study.
There are some limitations to this study. First, the sample size in our study was relatively small. Second, this study failed to reflect the correlation between the CD28 expression level and other clinicopathological characteristics. Finally, healthy individuals were not examined since it is challenging to collect liver specimens from a healthy person. This study could not investigate the changes in CD28, CD4, and CD8 in HCC patients compared to controls. Thus, studies with a large sample size of HCC patients and healthy controls are needed to provide more data to support the role of CD28 as the immune checkpoint for HCC treatment.
In summary, CD28 may play a vital role in the occurrence and development of HCC, and its protein expression level is an independent factor affecting the HCC prognosis. CD28 could be suggested as an immune checkpoint target for HCC treatment.
References
-
1.
Cervello M, Emma MR, Augello G, Cusimano A, Giannitrapani L, Soresi M, et al. New landscapes and horizons in hepatocellular carcinoma therapy. Aging (Albany NY). 2020;12(3):3053-94. [PubMed ID: 32018226]. [PubMed Central ID: PMC7041742]. https://doi.org/10.18632/aging.102777.
-
2.
Yang J, Liu J, Chen Y, Tang W, Bo K, Sun Y, et al. Investigation of ICOS, CD28 and CD80 polymorphisms with the risk of hepatocellular carcinoma: a case-control study in eastern Chinese population. Biosci Rep. 2019;39(7). [PubMed ID: 31235485]. [PubMed Central ID: PMC6609557]. https://doi.org/10.1042/BSR20181824.
-
3.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589-604. [PubMed ID: 31439937]. [PubMed Central ID: PMC6813818]. https://doi.org/10.1038/s41575-019-0186-y.
-
4.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565-70. [PubMed ID: 21436444]. https://doi.org/10.1126/science.1203486.
-
5.
Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229(1):5-11. [PubMed ID: 19426211]. [PubMed Central ID: PMC2928676]. https://doi.org/10.1111/j.1600-065X.2009.00784.x.
-
6.
Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257(5071):789-92. [PubMed ID: 1323143]. https://doi.org/10.1126/science.1323143.
-
7.
Chen X, Li H, Qiao Y, Yu D, Guo H, Tan W, et al. Association of CD28 gene polymorphism with cervical cancer risk in a Chinese population. Int J Immunogenet. 2011;38(1):51-4. [PubMed ID: 20846283]. https://doi.org/10.1111/j.1744-313X.2010.00969.x.
-
8.
Chen S, Zhang Q, Shen L, Liu Y, Xu F, Li D, et al. Investigation of CD28 gene polymorphisms in patients with sporadic breast cancer in a Chinese Han population in Northeast China. PLoS One. 2012;7(10). e48031. [PubMed ID: 23133541]. [PubMed Central ID: PMC3485049]. https://doi.org/10.1371/journal.pone.0048031.
-
9.
Qiu YR, Yang CL, Chen LB, Wang Q. [Analysis of CD8(+) and CD8(+)CD28(-) cell subsets in patients with hepatocellular carcinoma]. Di Yi Jun Yi Da Xue Xue Bao. 2002;22(1):72-3. [PubMed ID: 12390853].
-
10.
Boleslawski E, Othman SB, Aoudjehane L, Chouzenoux S, Scatton O, Soubrane O, et al. CD28 expression by peripheral blood lymphocytes as a potential predictor of the development of de novo malignancies in long-term survivors after liver transplantation. Liver Transpl. 2011;17(3):299-305. [PubMed ID: 21384512]. https://doi.org/10.1002/lt.22232.
-
11.
Shen XH, Xu P, Yu X, Song HF, Chen H, Zhang XG, et al. Discrepant Clinical Significance of CD28(+)CD8(-) and CD4(+)CD25(high) Regulatory T Cells During the Progression of Hepatitis B Virus Infection. Viral Immunol. 2018;31(8):548-58. [PubMed ID: 30117787]. https://doi.org/10.1089/vim.2018.0035.
-
12.
Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44(4):723-31. [PubMed ID: 16488051]. https://doi.org/10.1016/j.jhep.2005.12.015.
-
13.
Vercruysse D, Dusa A, Stahl R, Vanmeerbeeck G, de Wijs K, Liu C, et al. Three-part differential of unlabeled leukocytes with a compact lens-free imaging flow cytometer. Lab Chip. 2015;15(4):1123-32. [PubMed ID: 25537881]. https://doi.org/10.1039/c4lc01131g.
-
14.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-65. [PubMed ID: 22658128]. [PubMed Central ID: PMC3563263]. https://doi.org/10.1056/NEJMoa1200694.
-
15.
Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227-42. [PubMed ID: 23470321]. [PubMed Central ID: PMC3786574]. https://doi.org/10.1038/nri3405.
-
16.
Topp MS, Riddell SR, Akatsuka Y, Jensen MC, Blattman JN, Greenberg PD. Restoration of CD28 expression in CD28- CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J Exp Med. 2003;198(6):947-55. [PubMed ID: 12963692]. [PubMed Central ID: PMC2194206]. https://doi.org/10.1084/jem.20021288.
-
17.
Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 Costimulation: From Mechanism to Therapy. Immunity. 2016;44(5):973-88. [PubMed ID: 27192564]. [PubMed Central ID: PMC4932896]. https://doi.org/10.1016/j.immuni.2016.04.020.
-
18.
Blanchet F, Schurter BT, Acuto O. Protein arginine methylation in lymphocyte signaling. Curr Opin Immunol. 2006;18(3):321-8. [PubMed ID: 16616479]. https://doi.org/10.1016/j.coi.2006.03.001.
-
19.
Liu C, Hu Q, Hu K, Su H, Shi F, Kong L, et al. Increased CD8+CD28+ T cells independently predict better early response to stereotactic ablative radiotherapy in patients with lung metastases from non-small cell lung cancer. J Transl Med. 2019;17(1):120. [PubMed ID: 30971280]. [PubMed Central ID: PMC6458628]. https://doi.org/10.1186/s12967-019-1872-9.
-
20.
Shen Y, Qu QX, Zhu YB, Zhang XG. Analysis of CD8+CD28- T-suppressor cells in gastric cancer patients. J Immunoassay Immunochem. 2012;33(2):149-55. [PubMed ID: 22471605]. https://doi.org/10.1080/15321819.2011.609575.
-
21.
Escarra-Senmarti M, Bueno-Topete MR, Jave-Suarez LF, Gomez-Banuelos E, Gutierrez-Franco J, Vega-Magana N, et al. Loss of CD28 within CD4(+) T cell subsets from cervical cancer patients is accompanied by the acquisition of intracellular perforin, and is further enhanced by NKG2D expression. Immunol Lett. 2017;182:30-8. [PubMed ID: 28087292]. https://doi.org/10.1016/j.imlet.2017.01.006.
-
22.
Zahran AM, Shaltout AS, Fakhry H, Khallaf SM, Abdel Fattah ON, Temerik DF, et al. Prognostic Significance of Circulating CD28 Negative Suppressor T Cells and Memory B Cells in Patients with Breast Cancer. Iran J Immunol. 2020;17(2):95-110. [PubMed ID: 32602464]. https://doi.org/10.22034/iji.2020.83420.1625.
-
23.
Maki A, Matsuda M, Asakawa M, Kono H, Fujii H, Matsumoto Y. Decreased expression of CD28 coincides with the down-modulation of CD3zeta and augmentation of caspase-3 activity in T cells from hepatocellular carcinoma-bearing patients and hepatitis C virus-infected patients. J Gastroenterol Hepatol. 2004;19(12):1348-56. [PubMed ID: 15610307]. https://doi.org/10.1111/j.1440-1746.2004.03455.x.
-
24.
Hsu PN, Yang TC, Kao JT, Cheng KS, Lee YJ, Wang YM, et al. Increased PD-1 and decreased CD28 expression in chronic hepatitis B patients with advanced hepatocellular carcinoma. Liver Int. 2010;30(9):1379-86. [PubMed ID: 20738778]. https://doi.org/10.1111/j.1478-3231.2010.02323.x.
-
25.
Sakamoto Y, Ishida T, Masaki A, Takeshita M, Iwasaki H, Yonekura K, et al. Clinical significance of CD28 gene-related activating alterations in adult T-cell leukaemia/lymphoma. Br J Haematol. 2021;192(2):281-91. [PubMed ID: 33205842]. [PubMed Central ID: PMC7894310]. https://doi.org/10.1111/bjh.17211.
-
26.
Sun D, Tian L, Bian T, Zhao H, Tao J, Feng L, et al. The role of CD28 in the prognosis of young lung adenocarcinoma patients. BMC Cancer. 2020;20(1):910. [PubMed ID: 32967633]. [PubMed Central ID: PMC7510131]. https://doi.org/10.1186/s12885-020-07412-0.
-
27.
Melssen M, Slingluff CL. Vaccines targeting helper T cells for cancer immunotherapy. Curr Opin Immunol. 2017;47:85-92. [PubMed ID: 28755541]. [PubMed Central ID: PMC5757837]. https://doi.org/10.1016/j.coi.2017.07.004.
-
28.
Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129-44. [PubMed ID: 18363998]. https://doi.org/10.1111/j.1600-065X.2008.00616.x.
-
29.
Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229(1):12-26. [PubMed ID: 19426212]. [PubMed Central ID: PMC4186963]. https://doi.org/10.1111/j.1600-065X.2009.00770.x.
-
30.
Guo F, Iclozan C, Suh WK, Anasetti C, Yu XZ. CD28 controls differentiation of regulatory T cells from naive CD4 T cells. J Immunol. 2008;181(4):2285-91. [PubMed ID: 18684917]. [PubMed Central ID: PMC2688779]. https://doi.org/10.4049/jimmunol.181.4.2285.
-
31.
Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, et al. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS One. 2010;5(1). e8959. [PubMed ID: 20126467]. [PubMed Central ID: PMC2813297]. https://doi.org/10.1371/journal.pone.0008959.
-
32.
Bei R, Mizejewski GJ. Alpha fetoprotein is more than a hepatocellular cancer biomarker: from spontaneous immune response in cancer patients to the development of an AFP-based cancer vaccine. Curr Mol Med. 2011;11(7):564-81. [PubMed ID: 21707514]. https://doi.org/10.2174/156652411800615162.
-
33.
Ma H, Sun X, Chen L, Cheng W, Han XX, Zhao B, et al. Multiplex Immunochips for High-Accuracy Detection of AFP-L3% Based on Surface-Enhanced Raman Scattering: Implications for Early Liver Cancer Diagnosis. Anal Chem. 2017;89(17):8877-83. [PubMed ID: 28770990]. https://doi.org/10.1021/acs.analchem.7b01349.