Abstract
Background:
Chronic immunity to hepatitis A (HA) is largely influenced by environmental factors, such as socioeconomic indicators, public health conditions, and access to safe water. In the past two decades, Iran has witnessed improvements in socioeconomic status, increased urbanization, enhanced health education, improved access to safe drinking water sources, and better public health conditions. However, these changes have not been uniform across all regions of Iran, and varying epidemiological situations are expected.Objectives:
This study aimed to delineate the pattern of HA chronic immunity across different regions of Iran using geographical information system (GIS) mapping.Methods:
The study included a total of 3255 individuals who tested positive for anti-hepatitis A virus (anti-HAV) immunoglobulin G (IgG). This study analyzed factors such as place of residence, marital status, age, and gender to explore possible relationships. Univariate and multivariable analyses were conducted to identify independently associated factors for HA. A locally weighted scatterplot smoothing (LOWESS) multivariate model was developed using a backward stepwise approach. Geographical variations in the prevalence of HA chronic immunity in the general population of Iran were assessed to understand spatial effects and risk factors. A Bayesian spatial model was employed to identify the spatial pattern of HA chronic immunity prevalence, using OpenBUGS version 3.2.3.Results:
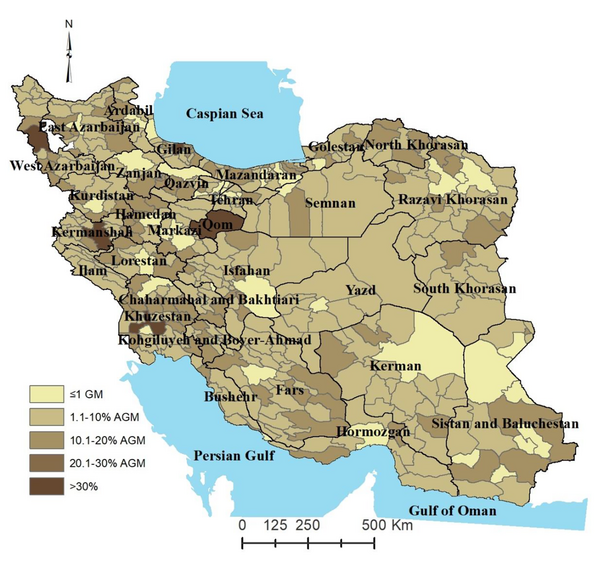
The prevalence of HAV immunity was higher in regions with mild semi-dry climates (aPR = 2.37, 95% confidence interval [CI] = 2.30 - 3.33, P< 0.001), medium semi-dry climates (aPR = 1.37, 95% CI = 1.14 - 1.63, P< 0.001), dry climates (aPR = 1.13, 95% CI = 0.9 - 1.4), and ultra-dry climates (aPR = 1.79, 95% CI = 1.05 - 2.98, P = 0.033), compared to semi-humid climates. Other variables did not exhibit a significant relationship with HA chronic immunity. The GIS analysis map revealed that immunity to HA was generally lower in the capital cities of Iran’s provinces. However, most central regions of Iran exhibit medium endemicity; nevertheless, higher immunity to HA was observed in border areas and coastal regions, particularly in the northern part of the country.Conclusions:
Different regions of Iran display distinct patterns of HAV endemicity, influenced by the country’s climatic diversity.Keywords
Drought Geographic Information Systems Endemicity Climate Hepatitis A Socioeconomic Status
1. Background
Hepatitis A (HA) is recognized as the most common type of acute viral hepatitis worldwide (1). It is estimated that tens of millions of individuals worldwide are infected with the hepatitis A virus (HAV) each year, with only one and a half million becoming symptomatic (2). Due to the fecal-oral nature of the virus, it is primarily transmitted through the ingestion of contaminated food and beverages, followed by direct contact with an infected person, and, to a lesser extent, through blood (3). The severity of the disease largely depends on the age of the individual. Although young children often develop the infection asymptomatically, the severity of symptoms, hospitalization, and mortality increase with age in older children and adults (4). In addition to age, HA is significantly influenced by environmental factors, such as socioeconomic indicators, public health status, and access to safe water (5, 6). Considering the effect of temperature and humidity on the persistence of the A virus in the environment (7, 8) and the impact of climate on virus exposure to the host (9), climate can be one of the environmental factors affecting HA immunity.
Over the past two decades, Iran has experienced improvements in socioeconomic status, increased urbanization, the development of health education, improved access to safe drinking water resources, and enhanced public health conditions in society. Therefore, it was expected that these changes would affect the prevalence of HA (10). The declining trend in HA endemicity in studies conducted during this period supports this hypothesis (11). In the latest assessment recently conducted by our team, the endemicity of this disease in Iran was estimated to be low (12). However, this downward trend is not uniform across all parts of Iran, leading to varying epidemiological situations.
2. Objectives
Consequently, this study aimed to illustrate the pattern of HA chronic immunity in various regions of the country and potential environmental factors related to this pattern, including climate. To the best of our knowledge, no similar study has been published on HA immunity based on geographical information system (GIS) mapping. The findings of this GIS-based study will provide insights into the necessary policies for HA vaccination and the prediction of HA outbreaks in different areas of Iran.
3. Methods
3.1. Study Design
This is a secondary and supplementary report on a nationwide population-based study of HA chronic immunity seroprevalence conducted across Iran in 2019 (12).
3.2. Data Collection
In the primary study (12), a multi-stage cluster random sampling method was employed based on the socio-demographic status of the population in both urban and rural areas. A total sample size of 5 655 participants was calculated based on certain assumptions and was proportionally collected from 42 cities across the country. The selection of these cities was determined using a simple random sampling technique within seven socio-demographic strata, established through K-means clustering. These strata were defined based on factors such as population, mortality of children under 5 years of age, gender ratio, family size, and rural/urban population ratio. These cities were located in 12 provinces, including Razavi Khorasan (n = 927, 17.1%), Tehran (n = 773, 14.3%), Khuzestan (n = 602, 11.1%), Sistan and Baluchestan (n = 493, 9.1%), Fars (n = 468, 8.6%), Lorestan (n = 405, 7.5%), Kurdistan (n = 341, 6.3%), Hormozgan (n = 328, 6%), Ardebil (n = 265, 4.9%), Kerman (n = 279, 5.1%), Mazandaran (n = 274, 5%), and Isfahan (n = 264, 4.9%). The questionnaire gathered information on age, gender, marital status, place of residence, socioeconomic status, and laboratory findings from all age groups over 1 year and both genders. A total of 5 673 individuals participated in the study, with 254 individuals excluded due to lost or low-quality blood samples and incomplete checklist information. Consequently, blood samples from 5 419 participants were used for testing. Further details on sampling, data acquisition, and laboratory procedures have been previously published (12).
Climate data for various regions of Iran and related maps were compiled using information from the Meteorological Organization and the Organization of Forests and Pastures of Iran. These data were used to categorize different regions of the country into 6 climate zones: semi-humid, mild semi-dry, medium semi-dry, severe semi-dry, dry, and ultra-dry climates (13).
3.3. Statistical and Mapping Analyses
Data entry was double-checked by a biostatistics expert for accuracy. The data were cleaned to identify internal and external inconsistencies, outliers, and extreme values. Data preparation was conducted based on the study protocol. Data extraction, recoding, and both descriptive and analytical analyses were carried out using STATA software (version 16). Continuous variables were presented as mean and standard deviation (SD), while categorical variables were reported as frequencies and percentages. Univariate and multivariable analyses were performed to identify independently associated factors of HA. A locally weighted scatterplot smoothing (LOWESS) multivariate model was developed using a backward stepwise method, including all variables with a P-value < 0.2 in the univariate analysis. Furthermore, prevalence ratios and their 95% confidence intervals (CIs) were estimated using a Poisson regression (PR) model with robust error variance to quantify the strength of statistical associations. In all cases, a P-value less than 0.05 was considered statistically significant.
Moreover, in this study, geographical variations in the prevalence of HA chronic immunity in the general population of Iran across 428 counties were analyzed. The data were assessed to understand spatial effects and the risk factors associated with the prevalence of HA chronic immunity. A Bayesian spatial model was employed to identify the spatial pattern of HA chronic immunity prevalence. The spatial model was coded using OpenBUGS version 3.2.3 (14, 15). ArcGIS software 10.1 (16) was also used to display the results on maps. We ran two chains with 1000 samples as a burn-in and 10,000 samples as iterations. Convergence for the chains was confirmed through auto-correlations, trace plots, and density plots (17).
4. Results
Out of 3 603 cases that tested seropositive for anti-hepatitis A virus (anti-HAV) immunoglobulin G (IgG), 348 were excluded from the study due to incomplete climatic information in their regions. Finally, 3 255 individuals were included in the final analysis, with a mean age of 34.2 (SD = 10.2) years. The study sample consisted of 1,687 (51.8%) females and 1,568 (49.2%) males. Most of the subjects (72%) were married, and 69.2% of them lived in cities (12).
Among the 42 cities, 4 (9.5%), 9 (21.4%), 2 (4.8%), 10 (23.8%), 11 (26.2%), and 6 (14.3%) cities had semi-humid, mild semi-dry, medium semi-dry, severe semi-dry, dry, and ultra-dry climates, respectively (Table 1). Since there were six different climates, it was observed that the highest serological positivity rate belonged to the mild semi-dry climate (37.1%); nevertheless, the lowest was related to the medium semi-dry climate (4.2%). Additionally, the highest and lowest mean ages were associated with mild semi-dry climates (41.03 ± 18.6 years) and ultra-dry climates (27.76 ± 2.9 years), respectively. The highest percentage of women and men was related to ultra-dry (59.3%) and mild semi-dry (52.2%) climates, respectively. Ultra-dry and mild semi-dry climates had the highest and lowest percentages of singles (50.8%) and married individuals (94.1%), respectively. Moreover, the highest and lowest percentages of individuals living in cities and villages were in dry (76.8%) and severe semi-dry (44.9%) climates, respectively. The frequency of positive anti-HAV IgG in different climates was as follows:
Mild semi-dry (37.1%), dry (27.1%), severe semi-dry (14.9%), ultra-dry (9.7%), semi-humid (5.3%), and medium Semi-dry (4.2%), respectively, based on univariate analysis (Table 2)
Status of Chronic Immunity to Hepatitis A and Climate in Sampled Cities and Provinces
| City | Province | Hepatitis A Seroprevalence (% GM) | Climate | |
|---|---|---|---|---|
| 1 | Juybar | Mazandaran | 1.1 - 10 | Semi-humid |
| 2 | Fereidunkenar | Mazandaran | 1.1 - 10 | Semi-humid |
| 3 | Babolsar | Mazandaran | 10.1 - 20 | Semi-humid |
| 4 | Sanandaj | Kordestan | <1 | Medium semi-dry |
| 5 | Nur | Mazandaran | 10.1 - 20 | Semi-humid |
| 6 | Bastak | Hormozgan | 1.1 - 10 | Ultra-dry |
| 7 | Boroujerd | Lorestan | 1.1 - 10 | Medium semi-dry |
| 8 | Abadeh | Fars | 1.1 - 10 | Mild semi-dry |
| 9 | Ardabil | Ardabil | < 1 | Mild semi-dry |
| 10 | Tehran | Tehran | < 1 | Severe semi-dry |
| 11 | Baghmalek | Khuzestan | 10.1 - 20 | Severe semi-dry |
| 12 | Shiraz | Fars | <1 | Severe semi-dry |
| 13 | Marivan | Kordestan | 10.1 - 20 | Mild semi-dry |
| 14 | Kazerun | Fars | 1.1 -10 | Severe semi-dry |
| 15 | Khamir | Hormozgan | 1.1 - 10 | Ultra-dry |
| 16 | Eghlid | Fars | 1.1 - 10 | Mild semi-dry |
| 17 | Azna | Lorestan | 1.1 - 10 | Mild semi-dry |
| 18 | Firouzababd | Fars | 1.1 - 10 | Severe semi-dry |
| 19 | Fariman | Razavi Khorasan | 1.1 - 10 | Severe semi-dry |
| 20 | Bileh Savar | Ardabil | 1.1 - 10 | Mild semi-dry |
| 21 | Zabol | Sistan and Baluchestan | 10.1 - 20 | Ultra-dry |
| 22 | Hoveizeh | Khuzestan | 1.1 - 10 | Ultra-dry |
| 23 | Rafsanjan | Kerman | 1.1 - 10 | Dry |
| 24 | Kouhdasht | Lorestan | 10.1 - 20 | Mild semi-dry |
| 25 | Khomeinishahr | Isfahan | 1.1 - 10 | Dry |
| 26 | Rostam | Fars | 1.1 - 10 | Severe semi-dry |
| 27 | Khalkhal | Ardabil | 1.1 - 10 | Mild semi-dry |
| 28 | Ravar | Kerman | 1.1 - 10 | Dry |
| 29 | Fasa | Fars | 10.1 - 20 | Severe semi-dry |
| 30 | Naein | Isfahan | 1.1 - 10 | Dry |
| 31 | Larestan | Fars | 10.1 - 20 | Dry |
| 32 | Lamerd | Fars | 1.1 - 10 | Dry |
| 33 | Zarand | Kerman | 10.1 - 20 | Dry |
| 34 | Bandar Abbas | Hormozgan | < 1 | Dry |
| 35 | Mashhad | Razavi Khorasan | < 1 | Severe semi-dry |
| 36 | Bandar Lengeh | Hormozgan | 10.1 - 20 | Dry |
| 37 | Torbat Jam | Razavi Khorasan | 1.1 - 10 | Dry |
| 38 | Ahvaz | Khuzestan | 20.1 - 30 | Dry |
| 39 | Darab | Fars | 10.1 - 20 | Severe semi-dry |
| 40 | Chabahar | Sistan and Baluchestan | 1.1 -10 | Ultra-dry |
| 41 | Aligudarz | Lorestan | 10.1 - 20 | Mild semi-dry |
| 42 | Saravan | Sistan and Baluchestan | 10.1 - 20 | Ultra-dry |
Demographic Characteristics of Anti-hepatitis A Virus (Anti-HAV) Immunoglobulin G (IgG) Seropositivity in the Iranian Population
| Variables | Total | Semi-humid | Mild Semi-dry | Medium Semi-dry | Severe Semi-dry | Dry | Ultra-dry | P-Value |
|---|---|---|---|---|---|---|---|---|
| Frequency (%) | 3255 (100) | 172 (5.3) | 1207 (37.1) | 138 (4.2) | 485 (14.9) | 882 (27.1) | 317 (9.7) | < 0.001 |
| Age, mean ± SD (y) | 34.23 ± 10.21 | 32.39 ± 4.12 | 41.03 ± 18.60 | 35.16 ± 2.79 | 36.02 ± 5.49 | 31.09 ± 4.12 | 27.76 ± 2.94 | 0.99 |
| Gender | 0.973 | |||||||
| Female | 1687 (51.8) | 99 (57.6) | 637 (52.8) | 66 (47.8) | 247 (50.9) | 450 (51) | 188 (59.3) | |
| Male | 1568 (49.2) | 73 (42.4) | 570 (47.2) | 72 (52.2) | 238 (49.1) | 432 (49) | 183 (40.7) | |
| Marital status | 0.532 | |||||||
| Single | 910 (28) | 48 (27.9) | 292 (24.2) | 22 (15.9) | 124 (25.6) | 263 (29.8) | 161 (50.8) | |
| Married | 2345 (72) | 124 (72.1) | 915 (75.8) | 116 (94.1) | 361 (74.4) | 619 (70.2) | 210 (49.2) | |
| Residency place | < 0.001 | |||||||
| Urban | 2254 (69.2) | 107 (62.2) | 912 (75.6) | 83 (60.1) | 276 (55.1) | 677 (76.8) | 199 (62.8) | |
| Village | 1001 (30.8) | 65 (37.8) | 295 (24.4) | 55 (39.9) | 209 (44.9) | 205 (23.2) | 172 (37.2) |
In the multivariable model, the prevalence of HAV immunity in mild semi-dry climates (aPR = 2.37, 95% CI = 2.30-3.33, P < 0.001), medium semi-dry (aPR = 1.37, 95% CI = 1.14-1.63, P < 0.001), dry (aPR = 1.13, 95% CI = 0.91-1.43), and ultra-dry (aPR = 1.79, 95% CI = 1.05-2.98, P = 0.033) was significantly higher than in semi-humid climates. Other variables did not show any significant relationship with HA chronic immunity.
Figure 1 displays the distribution of HA chronic immunity prevalence in cities and provinces across Iran. Immunity to HA varies greatly in different regions of the country, influenced by socioeconomic conditions, climatic factors, population density, and proximity to borders and seas. Different regions were categorized into five groups based on their endemicity level compared to the global mean (GM). The majority fell within the range of 1.1 to 10% above the GM, with the fewest regions exceeding 30% above the GM.
Prevalence of hepatitis A chronic immunity in the general population of Iran
5. Discussion
In this study, we discovered significant regional diversity in HA chronic immunity across different regions of Iran. Although socioeconomic and geographical factors played a role, it became evident that climate was related to HAV chronic immunity. However, age, marital status, and gender did not show significant associations. Many of Iran’s capital cities exhibited a lower prevalence of HAV chronic immunity, in contrast to border areas.
A review study reported a wide range of HAV IgG seroprevalence rates in different parts of Iran, ranging from 8 to 99% (18). Hepatitis A virus infection and consequently immunity to it are influenced by various individual and environmental factors. Social determinants of health (SDH) are among the primary factors affecting HA endemicity. The income and social protection available to residents, influenced by the economic and industrial development of the region, have been identified as factors impacting the prevalence of HA (19). This condition can also affect the local education system. The literacy level of individuals and their parents, in addition to the knowledge of individuals about disease care and transmission, have been suggested as risk factors for HA in various studies (20). These factors, combined with a lower unemployment rate, higher food security, and better access to healthcare services, could explain the lower prevalence of HA infection and, consequently, lower immunity in many of Iran’s provincial capital cities, as indicated in the resulting map of this study.
Conversely, most central regions of Iran exhibit medium endemicity, and many areas with high HA immunity are situated in the eastern, southern, and southwestern parts of the country, adjacent to the three countries of Afghanistan, Pakistan, and Iraq. Pakistan is considered endemic due to issues related to drinking water quality and poor hygiene (21). Although few epidemiological studies have been conducted on HA in Afghanistan and Iraq, available results suggest very high endemicity, likely influenced by prolonged conflicts and instability in these countries (22). Iraq receives the highest number of Iranian foreign travelers, and most of the travel to all three countries occurs via land borders and neighboring provinces. Therefore, it is justified that border areas adjacent to these countries share a similar epidemiological situation in terms of contracting communicable diseases, including HA. The relationship between HA prevalence and residence in border areas was previously established in an earlier study (8) and has been further demonstrated in this mapping study.
In border areas, especially in the coastal provinces, particularly in the northern part of the country, higher immunity to HA was observed. The probable cause of the high HA seroprevalence in coastal areas could be attributed to international relations. Additionally, concerning the coastal areas along the northern seas of the country, another factor influencing HA immunity is the high population density, which has been proven to be a determining factor for HA prevalence (23). In this region, due to the fertility and suitability of the agricultural fields, the population usually exceeds the environment’s capacity. This leads to denser cities and villages with shorter distances between them.
Since Iran is a vast country with diverse weather conditions, it was hypothesized that one of the possible environmental factors is climate. This study concluded that chronic immunity to HAV is significantly associated with climate. According to the results, the highest prevalence of HAV-IgG seropositivity was observed in regions with a mild semi-dry climate; however, the lowest was related to semi-humid climates.
One of the reasons for the variation in the prevalence of HA immunity in different climatic regions might be related to the characteristics of the disease pathogen. Hepatitis A virus is a member of the Picornaviridae family, Hepatovirus genus, with aquatic environments serving as its reservoir (13). Previous studies have shown that the stability and longevity of the virus in different environments, including water, soil, and various foods, are related to ambient temperature (8). The ideal temperature range for HAV is 25 to 42 degrees Celsius, and as the temperature increases, the virus’s inactivation time decreases (7, 8). This is one of the reasons why hepatitis outbreaks are more common in late fall and early winter (24). There is a similarly significant relationship between ambient humidity and HAV, with the highest durability of the virus observed within the range of moderate relative humidity (50 - 85%) (7, 25).
The characteristics of HAV mentioned above are not consistent with the results of the present study. However, it should be noted that when examining factors affecting an infectious disease, one must consider not only its pathogen but also two other important components of the disease: the host and the disease transmission environment (9). Climatic conditions can indirectly alter exposure to individual and environmental risk factors for the disease, leading to variability in the prevalence of HA. One such factor is the source of drinking water supply, which can affect the prevalence of HA through various mechanisms (26). In areas with heavy rainfall, flooding can lead to soil leaching and an increased chance of contaminating drinking water sources, thereby raising the incidence of HA (27). However, in arid and low-rainfall areas, due to the lack of sustainable natural water sources, some populations might turn to unhealthy water sources (26). In certain central and southern regions of Iran, the dispersion of residential areas has made it impossible to pipe drinking water to the entire population. Consequently, in some areas, the source of water supply for various uses is surface water from rainwater storage, which is sometimes even shared with animals.
On the other hand, it has been shown that in some infectious diseases, an increase in ambient temperature can lead to increased activities and social interactions, thereby increasing the incidence by elevating the chance of person-to-person transmission (28). Direct interpersonal transmission of HA has been previously established, especially in areas with low levels of health (29).
One of the key findings related to climate is a better understanding of potential changes in the prevalence of HA due to climate change over time. Climate change has become one of the major global challenges in recent decades, with an estimated 5 billion individuals expected to experience water scarcity and its consequences by 2025 (30). The consequences of these changes will affect various aspects of human life, including human health. Several studies have examined changes in the epidemiological trends of infectious diseases caused by climate change, including a study in China that also mentioned HA as one of these diseases (31).
Comparing the present study’s findings to the findings of previous studies, the relationship between age and hepatitis immunity has been a topic of interest. Some studies have reported a positive correlation between age and hepatitis immunity, suggesting that older individuals are more likely to be immune due to previous exposure to the virus (32). However, other studies have presented conflicting results, reporting no significant association between age and hepatitis immunity (33). The current study aligns with the latter group, supporting the notion that the relationship between age and hepatitis immunity might not be as straightforward as previously assumed. One possible explanation for the lack of a significant relationship between age and hepatitis immunity could be the widespread implementation of HA vaccination programs. Vaccination campaigns targeting specific age groups, such as children or adolescents, have been successful in reducing the incidence of HA in many countries (34). However, in Iran, HA vaccination has not been implemented as part of the routine immunization program. Therefore, this finding might be attributed to factors other than vaccination, including the downward trend of hepatitis endemicity during the last few decades. This factor can lead to varying levels of exposure to the virus in childhood among different current age groups, resulting in differences in immunity.
Regarding marital status, previous research exploring its association with hepatitis immunity is limited. Although some studies have suggested a potential link between marital status and HA seroprevalence, with higher rates observed in married individuals (35), the present study’s findings do not support such an association. It is important to note that marital status might not directly influence HA immunity but could be a proxy for other factors, such as lifestyle behaviors, which were not assessed in the present study. Future research should explore these potential confounders to gain a more comprehensive understanding of the relationship between marital status and hepatitis immunity.
One of the main limitations of epidemiological studies in reporting the spread of a disease in a wide area is the lack of information related to some parts of that area, which also applies to the epidemiological reports of HA (36). Based on the current knowledge, only a few studies have evaluated and compared the endemicity of HA in different provinces of Iran using a single methodology. One of them is a previous study that achieved this goal in 12 provinces of the country (12). Another study by Mostafavi et al. has succeeded in reporting the information on HA immunity only in the 10-to-18-year-old population in 16 out of 31 provinces of Iran (37).
Different methods have been used to address this limitation, including dividing and evaluating the studied area into larger geographical units as much as possible. For example, a study by Hoseini et al. divided Iran into four central, western, northern, and southern regions and compared the endemicity of HA in Iran at the level of these four regions (rather than provinces) (38). Another solution is to leave the areas without information blank on the map (39). However, both of these approaches leave the mission of using the map, which is to determine the pattern and show the differences in disease endemicity in the smaller areas of the same region and reduce its efficiency (40).
In such a situation, it might be possible to get help from the capabilities of GIS. The geographical information system is an electronic information system that collects and processes geographic information and can display the information of geographic locations and all the phenomena that are somehow related to these geographic locations in the form of maps, charts, and tables, providing this information for different applications (41). In health studies, this system is mostly used to show the prevalence of diseases and to investigate possible associations between non-geographical phenomena (e.g., diseases) and environmental and geographic factors (42). One of the applications of GIS is interpolation techniques, which are used to generalize the information obtained from the samples of study units in a population to the entire target population. These techniques can also use the available data to estimate the required information from areas where complete data were not obtained during sampling (43). Therefore, such a capability is very valuable in epidemiological studies, especially in infectious diseases. As far as we know, none of the previous studies related to HA has used this method to overcome the limitation of incomplete data.
However, a limitation of this study can be pointed out: collecting data from more cities and villages in the original survey (12) could have provided more accurate results about immunity to HA in this study.
As a strength of this study, the researchers tried to consider social determinants of health, such as residency place (rural/urban), marital status, age, and gender, which have been identified as effective factors in HA endemicity in previous studies. Additionally, to the best of our knowledge, this is the first study to investigate the relationship between climate and immunity to HA. This GIS mapping study is based on a homogeneous approach, which might be more representative of the true condition of HAV chronic immunity in different regions of Iran than previous heterogeneous studies with various methods.
5.1. Conclusions
Hepatitis A virus chronic immunity exhibits different patterns in different parts of Iran, as depicted in this study. Meanwhile, climate shows a significant relationship with this immunity; nevertheless, age, marital status, and gender were not significantly related. Therefore, a surveillance system for HAV should be established, taking into account various factors, such as the increasing trend of drought in Iran, to monitor and respond to any potential outbreaks in the country.
References
-
1.
Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol. 2012;4(3):74-80. [PubMed ID: 22489259]. [PubMed Central ID: PMC3321493]. https://doi.org/10.4254/wjh.v4.i3.74.
-
2.
Wasley A, Fiore A, Bell BP. Hepatitis A in the era of vaccination. Epidemiol Rev. 2006;28:101-11. [PubMed ID: 16775039]. https://doi.org/10.1093/epirev/mxj012.
-
3.
Jacobsen KH, Koopman JS. The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int J Epidemiol. 2005;34(3):600-9. [PubMed ID: 15831565]. https://doi.org/10.1093/ije/dyi062.
-
4.
Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26(49):6266-73. [PubMed ID: 18848855]. https://doi.org/10.1016/j.vaccine.2008.09.056.
-
5.
Van Herck K, Van Damme P. Prevention of hepatitis A by Havrix: a review. Expert Rev Vaccines. 2005;4(4):459-71. [PubMed ID: 16117704]. https://doi.org/10.1586/14760584.4.4.459.
-
6.
Saberifiroozi M. Hepatitis A virus infection: Is it an important hazard to public health?: hazards of HAV for public health. Hepat Mon. 2011;11(4):235-7. [PubMed ID: 22087149]. [PubMed Central ID: PMC3206698].
-
7.
Nelson NP, Murphy TV. Hepatitis A: The Changing Epidemiology of Hepatitis A. Clin Liver Dis (Hoboken). 2013;2(6):227-30. [PubMed ID: 26566433]. [PubMed Central ID: PMC4641046]. https://doi.org/10.1002/cld.230.
-
8.
Yeargin T, Buckley D, Fraser A, Jiang X. The survival and inactivation of enteric viruses on soft surfaces: A systematic review of the literature. Am J Infect Control. 2016;44(11):1365-73. [PubMed ID: 27160982]. https://doi.org/10.1016/j.ajic.2016.03.018.
-
9.
Wu X, Lu Y, Zhou S, Chen L, Xu B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environ Int. 2016;86:14-23. [PubMed ID: 26479830]. https://doi.org/10.1016/j.envint.2015.09.007.
-
10.
Movahedi M, Haghdoost AA, Pournik O, Hajarizadeh B, Fallah MS. Temporal variations of health indicators in Iran comparing with other Eastern Mediterranean Region countries in the last two decades. J Public Health (Oxf). 2008;30(4):499-504. [PubMed ID: 18772146]. https://doi.org/10.1093/pubmed/fdn071.
-
11.
Merat S, Rezvan H, Nouraie M, Abolghasemi H, Jamali R, Amini-Kafiabad S, et al. Seroprevalence and risk factors of hepatitis A virus infection in Iran: a population based study. Arch Iran Med. 2010;13(2):99-104. [PubMed ID: 20187662].
-
12.
Lankarani KB, Honarvar B, Molavi Vardanjani H, Kharmandar A, Gouya MM, Zahraei SM, et al. Immunity to Hepatitis-A virus: A nationwide population-based seroprevalence study from Iran. Vaccine. 2020;38(45):7100-7. [PubMed ID: 32917416]. https://doi.org/10.1016/j.vaccine.2020.08.071.
-
13.
Fatemi M. Comprehensive weather and climatology of Iran. 2023. Available from: https://climatology.ir/?p=4758.
-
14.
Lawson AB, Browne WJ, Vidal Rodeiro CL. Disease Mapping with WinBUGS and MLwiN. John Wiley & Sons, Ltd; 2003.
-
15.
Spiegelhalter D, Thomas A, Best N, Lunn D. OpenBUGS Version 3.2. 3 user manual. Cambridge, England: Medical Research Council Biostatistics Unit, University of Cambridge; 2014.
-
16.
Esri R. ArcGIS Desktop: Release 10. Redlands, USA: Environmental Systems Research Institute; 2011.
-
17.
Asmarian N, Sharafi Z, Mousavi A, Jacques R, Tamayo I, Bind MA, et al. Multiple sclerosis incidence rate in southern Iran: a Bayesian epidemiological study. BMC Neurol. 2021;21(1):309. [PubMed ID: 34376167]. [PubMed Central ID: PMC8353854]. https://doi.org/10.1186/s12883-021-02342-1.
-
18.
Saffar MJ, Abedian O, Ajami A, Abedian F, Mirabi AM, Khalilian AR, et al. Age-specific seroprevalence of anti-hepatitis a antibody among 1-30 years old population of savadkuh, mazandaran, iran with literature review. Hepat Mon. 2012;12(5):326-32. [PubMed ID: 22783344]. [PubMed Central ID: PMC3389358]. https://doi.org/10.5812/hepatmon.6035.
-
19.
Koroglu M, Jacobsen KH, Demiray T, Ozbek A, Erkorkmaz U, Altindis M. Socioeconomic indicators are strong predictors of hepatitis A seroprevalence rates in the Middle East and North Africa. J Infect Public Health. 2017;10(5):513-7. [PubMed ID: 28162965]. https://doi.org/10.1016/j.jiph.2016.09.020.
-
20.
Guenifi W, Laouamri S, Lacheheb A. Changes in prevalence of hepatitis A and associated factors in Setif-Algeria. Rev Epidemiol Sante Publique. 2017;65(6):437-42. [PubMed ID: 29050813]. https://doi.org/10.1016/j.respe.2017.05.009.
-
21.
Daud MK, Nafees M, Ali S, Rizwan M, Bajwa RA, Shakoor MB, et al. Drinking Water Quality Status and Contamination in Pakistan. Biomed Res Int. 2017;2017:7908183. [PubMed ID: 28884130]. [PubMed Central ID: PMC5573092]. https://doi.org/10.1155/2017/7908183.
-
22.
Mirminachi B, Mohammadi Z, Merat S, Neishabouri A, Sharifi AH, Alavian SH, et al. Update on the Prevalence of Hepatitis C Virus Infection Among Iranian General Population: A Systematic Review and Meta-Analysis. Hepat Mon. 2017;17(2). e42291. https://doi.org/10.5812/hepatmon.42291.
-
23.
Enoch A, Hardie DR, Hussey GD, Kagina BM. Hepatitis A seroprevalence in Western Cape Province, South Africa: Are we in epidemiological transition? S Afr Med J. 2019;109(5):314-8. [PubMed ID: 31131797]. https://doi.org/10.7196/SAMJ.2019.v109i5.13410.
-
24.
Yilmaz A. Hepatitis A seroprevalence in Erzurum, Turkey. Ann Agric Environ Med. 2020;27(3):481-4. [PubMed ID: 32955233]. https://doi.org/10.26444/aaem/125394.
-
25.
Kim SJ, Si J, Lee JE, Ko G. Temperature and humidity influences on inactivation kinetics of enteric viruses on surfaces. Environ Sci Technol. 2012;46(24):13303-10. [PubMed ID: 23152976]. https://doi.org/10.1021/es3032105.
-
26.
Ahmed J, Wong LP, Chua YP, Yasmin A, Channa N, VanDerslice JA. Estimation of Hepatitis A Virus Infection Prevalence Through Drinking Water Supply of Primary Schools of Sindh, Pakistan. Hepat Mon. 2020;20(5). e98412. https://doi.org/10.5812/hepatmon.98412.
-
27.
Lloyd SJ, Kovats RS, Armstrong BG. Global diarrhoea morbidity, weather and climate. Climate Research. 2007;34:119-27. https://doi.org/10.3354/cr034119.
-
28.
Hubalek Z. North Atlantic weather oscillation and human infectious diseases in the Czech Republic, 1951-2003. Eur J Epidemiol. 2005;20(3):263-70. [PubMed ID: 15921044]. https://doi.org/10.1007/s10654-004-6518-3.
-
29.
Patterson J, Abdullahi L, Hussey GD, Muloiwa R, Kagina BM. A systematic review of the epidemiology of hepatitis A in Africa. BMC Infect Dis. 2019;19(1):651. [PubMed ID: 31331281]. [PubMed Central ID: PMC6647100]. https://doi.org/10.1186/s12879-019-4235-5.
-
30.
McCarthy JJ, Canziani OF, Leary NA, Dokken DJ, White KS. Climate change 2001: impacts, adaptation, and vulnerability: contribution of Working Group II to the third assessment report of the Intergovernmental Panel on Climate Change. 2. Cambridge University Press; 2001.
-
31.
Wang Y, Rao Y, Wu X, Zhao H, Chen J. A method for screening climate change-sensitive infectious diseases. Int J Environ Res Public Health. 2015;12(1):767-83. [PubMed ID: 25594780]. [PubMed Central ID: PMC4306891]. https://doi.org/10.3390/ijerph120100767.
-
32.
Franco E, Meleleo C, Serino L, Sorbara D, Zaratti L. Hepatitis A: Epidemiology and prevention in developing countries. World J Hepatol. 2012;4(3):68-73. [PubMed ID: 22489258]. [PubMed Central ID: PMC3321492]. https://doi.org/10.4254/wjh.v4.i3.68.
-
33.
Iorio N, John S. Hepatitis A. StatPearls [Internet]. StatPearls Publishing; 2023.
-
34.
Herzog C, Van Herck K, Van Damme P. Hepatitis A vaccination and its immunological and epidemiological long-term effects - a review of the evidence. Hum Vaccin Immunother. 2021;17(5):1496-519. [PubMed ID: 33325760]. [PubMed Central ID: PMC8078665]. https://doi.org/10.1080/21645515.2020.1819742.
-
35.
Yin S, Barker L, Ly KN, Kilmer G, Foster MA, Drobeniuc J, et al. Susceptibility to Hepatitis A Virus Infection in the United States, 2007-2016. Clin Infect Dis. 2020;71(10):e571-9. [PubMed ID: 32193542]. https://doi.org/10.1093/cid/ciaa298.
-
36.
Mohd Hanafiah K, Jacobsen KH, Wiersma ST. Challenges to mapping the health risk of hepatitis A virus infection. Int J Health Geogr. 2011;10:57. [PubMed ID: 22008459]. [PubMed Central ID: PMC3210090]. https://doi.org/10.1186/1476-072X-10-57.
-
37.
Mostafavi N, Kelishadi R, Kazemi E, Ataei B, Yaran M, Motlagh ME, et al. Comparison of the Prevalence and Risk Factors of Hepatitis A in 10 to 18-Year-Old Adolescents of Sixteen Iranian Provinces: The CASPIAN-III Study. Hepat Mon. 2016;16(9). e36437. [PubMed ID: 27822259]. [PubMed Central ID: PMC5091029]. https://doi.org/10.5812/hepatmon.36437.
-
38.
Hoseini SG, Kelishadi R, Ataei B, Yaran M, Motlagh ME, Ardalan G, et al. Seroprevalence of hepatitis A in Iranian adolescents: is it time to introduce a vaccine? Epidemiol Infect. 2016;144(2):291-6. [PubMed ID: 26083105]. https://doi.org/10.1017/S0950268815001302.
-
39.
Lewandowsky S, Behrens JT, Pickle LW, Herrmann DJ, White AA. Perception of clusters in mortality maps: representing magnitude and statistical reliability. Cognitive aspects of statistical mapping. 1995. p. 107-32.
-
40.
MacEachren AM, Brewer CA, Pickle LW. Visualizing georeferenced data: representing reliability of health statistics. Environ Plan A. 1998;30(9):1547-61. https://doi.org/10.1068/a301547.
-
41.
Rezaeian M. An introduction to the practical methods for mapping the geographical morbidity and mortality rates. Tollo-e-Behdasht. 2004;2(4):41-51. https://doi.org/10.1515/pm-2004-410108.
-
42.
Musa GJ, Chiang PH, Sylk T, Bavley R, Keating W, Lakew B, et al. Use of GIS Mapping as a Public Health Tool-From Cholera to Cancer. Health Serv Insights. 2013;6:111-6. [PubMed ID: 25114567]. [PubMed Central ID: PMC4089751]. https://doi.org/10.4137/HSI.S10471.
-
43.
Mugglin AS, Carlin BP. Hierarchical modeling in geographic information systems: Population interpolation over incompatible zones. J Agric Biol Environ Stat. 1998:111-30. https://doi.org/10.2307/1400646.
