Abstract
Context:
There is a link between proton pump inhibitors (PPIs) use and the occurrence of spontaneous bacterial peritonitis (SBP) in cirrhotic patients in some studies; however, in other studies, such a link does not exist.Objectives:
The aim of the current systematic review and meta-analysis was to evaluate the association between PPI and the occurrence of SBP or hepatic encephalopathy (HE) in cirrhotic patients.Data Sources:
A systematic search of sources was conducted in order to evaluate for any relationship between PPI and the risk of SBP in patients with liver diseases. Medline, Scopus, Ovid, ProQuest, Google Scholar, and Web of Science were searched to find any evidence in this regard from 1980 to November of 2021.Study Selection:
The articles were evaluated by two independent reviewers according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses). After deleting the duplicates, first, the titles of the studies were evaluated, and then the full texts were evaluated. Any disagreement between the two researchers was solved by discussion or a third reviewer.Data Extraction:
Appropriate Critical Appraisal Checklists of Joanna Briggs Institute (JBI) were used for the quality assessment of eligible studies. Statistical analysis was performed by CMA software (version 2.0), and a P-value of less than 0.05 was considered a significant level.Results:
In the systematic search of sources, 3705 articles were identified. Finally, 33 studies were included in this meta-analysis study. A total of 6370 PPI users and 8037 patients in the control group experienced at least one of the complications of liver cirrhosis, including SBP or HE. According to meta-analysis, the risk of SBP or HE in the intervention group was 1.95 times higher than in the control group (RR = 1.95; 95% confidence interval [CI]: 1.53 - 2.48, P < 0.001).Conclusions:
The use of PPIs is associated with a higher risk of SBP and HE in cirrhotic patients. However, the quality of included studies in the current systematic review and meta-analysis was moderate, and high-quality studies with a larger sample size are required.Keywords
Liver Diseases Proton Pump Inhibitors (PPI) Spontaneous Bacterial Peritonitis (SBP) Hepatic Encephalopathy (HE)
1. Context
In patients with cirrhosis, spontaneous bacterial peritonitis (SBP) is a frequent and serious consequence that can be fatal (1). Bacterial translocation from the intestinal flora to the mesenteric lymph nodes is the first stage in the pathophysiology of SBP (2). In liver cirrhosis, there is a clear increase in gut permeability and small intestinal bacterial overgrowth (SIBO), both of which can promote bacterial translocation (2, 3). Through a variety of causes, including weakened immunity brought on by a reduction in the reticuloendothelial system’s phagocytic activity, a lack of complement, and neutrophil dysfunction, cirrhotic individuals are more vulnerable to infections (4). Reduced gastric acidity leads to an increase in bacterial growth in the stomach and small intestine. Gastric acidity is a defense mechanism against ingested germs. Enteric infections become more likely as a result (5).
Strong stomach acid inhibitors known as proton pump inhibitors (PPIs) have been linked to a higher risk of developing enteric infections brought on by different enteropathogens, such as Salmonella, Campylobacter, and Clostridium difficile (6, 7). Additionally, some results raise doubts about the relationship between PPI usage and the emergence of SBP in cirrhotic patients with ascites (8-10). The higher prevalence of enteric infections linked to PPI medication has been explained by a variety of reasons. In vitro, reduction of neutrophil function, altered microbial flora, increased small intestine overgrowth, and delayed stomach emptying are a few of the aforementioned results (6).
Proton pump inhibitor metabolism (except for rabeprazole) might be greatly hindered in advanced cirrhosis, which might have an impact on the infection risk associated with PPI use. As a result, higher exposure to PPIs might occur (11). Proton pump inhibitors work well and are well tolerated. In several acid-related illnesses, they are widely used and possibly misused (12-14). There is evidence of PPI usage in cirrhotic individuals in the literature (15, 16). The term “hepatic encephalopathy” (HE) refers to a variety of neuropsychiatric symptoms connected to both acute and long-term liver impairment (17, 18). Proton pump inhibitor abuse is widespread among cirrhotic patients. Proton pump inhibitor misuse, however, can potentially result in uncommon but severe negative effects.
Proton pump inhibitor use has been linked to an increased risk of HE in patients with liver impairment, according to previous studies (19). The most thoroughly researched adverse effect is the link between PPI use and the occurrence of SBP or HE in cirrhotic patients. Some studies found the link; however, there was no such link in other studies.
2. Objectives
The aim of the current systematic review and meta-analysis was to evaluate the association between PPI administration and the occurrence of SBP or HE in cirrhotic patients.
3. Data Sources
A systematic search of sources was conducted in order to evaluate any relationship between PPI and the risk of SBP or HE. Medline, ScienceDirect, Scopus, Ovid, ProQuest, Google Scholar, and Web of Science were searched to find any evidence in this regard from 1980 to November 2021. Iranian information databases included IranMedex, Barakat Knowledge Network System, Magiran, Scientific Information Database (SID), and Iranian Research Institute for Information Science (IranDoc). Grey literature and conference articles were also searched (Appendix I).
Search keywords were liver diseases, liver cirrhosis, hepatic cirrhosis, proton pump inhibitors (PPIs), spontaneous bacterial peritonitis (SBP), hepatic encephalopathy (HE).
PICO Definition of the Study:
P: Patients with liver diseases, including liver cirrhosis
I: Proton pump inhibitors
C: Placebo or no treatment
O: Outcome: Primary outcome was the occurrence of SBP and HE. The secondary outcomes were the occurrence of gastrointestinal bleeding, hepatorenal syndrome, mortality, and SIBO.
4. Study Selection
4.1. Inclusion Criteria
All studies that evaluated the association between PPIs and the occurrence of SBP or HE, or other complications in cirrhotic patients.
4.2. Exclusion Criteria
Animal studies, non-English language studies, and studies that did not have the required quality.
4.3. Screening Selection and Quality Assessment
The articles were evaluated by two individual researchers according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses). After deleting the duplicates, first, the titles of the studies were evaluated, and then the whole content of the articles was studied. Any disagreement between the two researchers was solved by discussion or a third reviewer. This process was managed through EndNote 8 software.
The evaluation of the quality of the articles was carried out by one researcher, and the second researcher evaluated the articles accidentally. Appropriate Critical Appraisal Checklists of Joanna Briggs Institute (JBI) were used for the critical appraisal of the eligible studies.
5. Data Extraction
Data extraction was performed through the modified JBI data extraction table. The extracted data included the year of issue of the article, authors, country, period of study, type of study, number of cases, mean age of patients, male-to-female ratio, outcome, type of drug, and side effects.
5.1. Data Synthesis and Analysis
Statistical analysis was performed by CMA software (version 2.0), and a P-value of less than 0.05 was considered a significant level. For this purpose, the heterogeneity of the studies was evaluated by Cochrane (Q) and I2, measuring the percentage of differences. If the amount of I2 was less than 50% constant, the Mantel-Haenszel method was used, and if it was more than 50% or P-value < 0.05, a random effect model was used.
6. Results
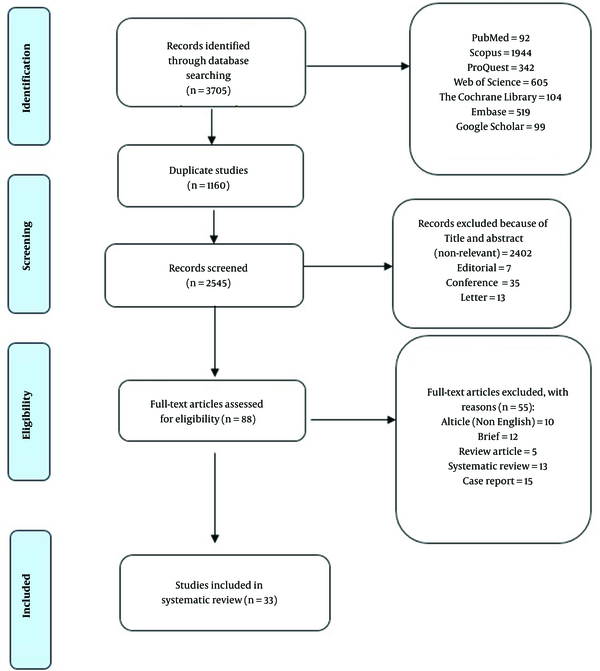
A total number of 3705 articles were obtained through a systematic electronic databases search. Of these papers, 1160 articles were omitted due to duplication, and 2457 articles were excluded after screening the titles and abstracts. Moreover, 55 articles were excluded after reading the full text. Finally, 33 studies entered the systematic review and meta-analysis (Figure 1). The characteristics of the included studies are summarized in Table 1. A total of 94.93% of the studies reported SBP as the outcome.
Flow diagram of trials for inclusion in the systematic review and meta-analysis.
Prevalence of Complications in Studied Articles
| Complications of Liver Cirrhosis | Number of Studies Reported, No. (%) |
|---|---|
| SBP | 31 (93.94) |
| HE | 14 (42.42) |
| Infection | 11 (33.33) |
| Variceal bleeding | 9 (27.27) |
| Ascites | 9 (27.27) |
| Mortality | 9 (27.27) |
| GIB | 3 (9.09) |
| Cirrhosis | 2 (6.06) |
| Hepatorenal syndrome | 1 (3.03) |
| SIBO | 1 (3.03) |
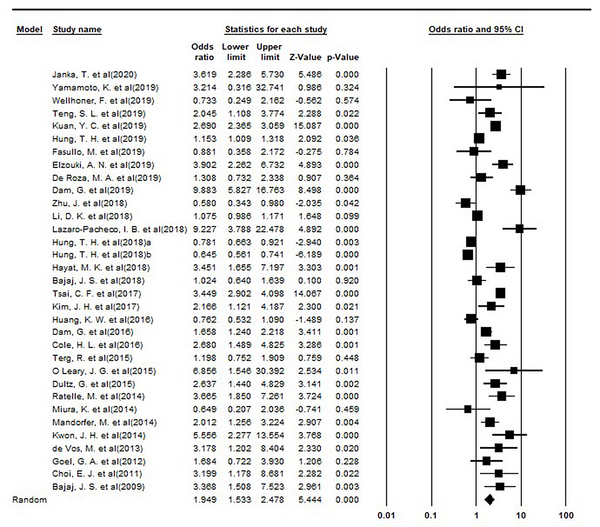
In the eligible articles for this systematic review and meta-analysis, 28778 patients used PPI, and 34644 cases were non-users. Of these cases, 6370 and 8037 patients in the intervention and control groups experienced at least one of the complications of liver cirrhosis, respectively. According to the meta-analysis, the risk of complications in the intervention group was 1.95 times higher than in the control group (RR = 1.95; 95% confidence interval [CI]: 1.53 - 2.48, P < 0.001). The forest plot of the meta-analysis is illustrated in Figure 2.
Forest Plot of Meta-analysis.
Since there was heterogeneity among studies, a subgroup analysis was performed based on the date of publishing the papers, and the results showed that there was no significant difference between the studies based on the years of publication (Figure 3). The odds ratio (OR) value for studies by the years of publication of the articles is shown in Table 2.
Forest Plot of Subgroup Analysis.
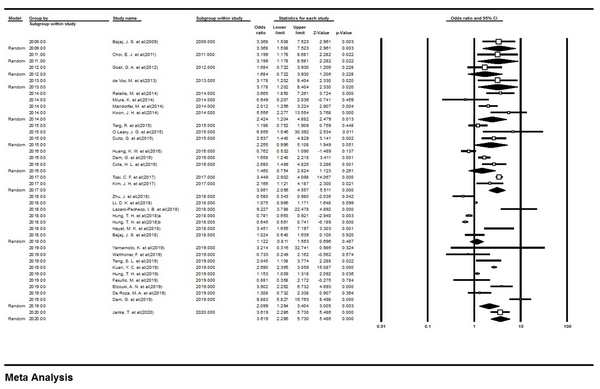
Results of Subgroup Analysis Based on the Year of Publication
| Group | Number of Studies | Point Estimate | Lower Limit | Upper Limit | Z-Value | P-Value | Q-Value | df (Q) | P-Value | I-Squared |
|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 1.00 | 3.37 | 1.51 | 7.52 | 2.96 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| 2011 | 1.00 | 3.20 | 1.18 | 8.68 | 2.28 | 0.02 | 0.00 | 0.00 | 1.00 | 0.00 |
| 2012 | 1.00 | 1.68 | 0.72 | 3.93 | 1.21 | 0.23 | 0.00 | 0.00 | 1.00 | 0.00 |
| 2013 | 1.00 | 3.18 | 1.20 | 8.40 | 2.33 | 0.02 | 0.00 | 0.00 | 1.00 | 0.00 |
| 2014 | 4.00 | 2.42 | 1.20 | 4.88 | 2.48 | 0.01 | 10.43 | 3.00 | 0.02 | 71.24 |
| 2015 | 3.00 | 2.26 | 1.00 | 5.11 | 1.95 | 0.05 | 7.54 | 2.00 | 0.02 | 73.46 |
| 2016 | 3.00 | 1.46 | 0.75 | 2.82 | 1.12 | 0.26 | 16.94 | 2.00 | 0.00 | 88.19 |
| 2017 | 2.00 | 3.06 | 2.06 | 4.56 | 5.51 | 0.00 | 1.79 | 1.00 | 0.18 | 44.13 |
| 2018 | 7.00 | 1.12 | 0.81 | 1.55 | 0.70 | 0.49 | 82.85 | 6.00 | 0.00 | 92.76 |
| 2019 | 9.00 | 2.10 | 1.29 | 3.40 | 3.01 | 0.00 | 133.51 | 8.00 | 0.00 | 94.01 |
| 2020 | 1.00 | 3.62 | 2.29 | 5.73 | 5.49 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
6.1. Publication Bias
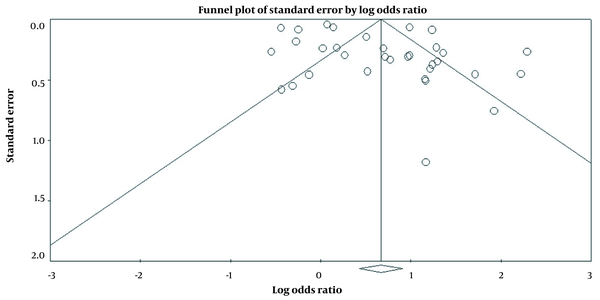
According to the results of the funnel plot, there was no publication bias in the included studies (t-value = 1.94, df = 31, P = 0.06) (Figure 4).
Funnel Plot of Standard Error by Log Odds Ratio.
The Critical Appraisal Checklists of the JBI (Available at https://jbi.global/critical-appraisal-tools) were used to evaluate the methodological quality, and according to the results, the studies had moderate quality (Table 3).
Methodology Quality According to the Joanna Briggs Institute (JBI) Critical Appraisal Checklist
| Author | Ref | Q1 a | Q2 b | Q3 c | Q4 d | Q5 e | Q6 f | Q7 g | Q8 h | Q9 i | Q10 j | Q11 k |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janka et al., 2019 | (20) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Yamamoto et al., 2019 | (21) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Wellhöner, 2019 | (22) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Teng et al., 2019 | (23) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Kuan et al., 2019 | (24) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Huang et al., 2019 | (25) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Fasullo et al., 2019 | (26) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Elzouki et al., 2019 | (27) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| De Roza et al., 2019 | (28) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Dam et al., 2019 | (29) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Zhu et al., 2018 | (30) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||
| Li et al., 2017 | (31) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Lazaro-Pacheco et al., 2017 | (32) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Hung et al., 2018 | (33) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Hung et al., 2018 | (34) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Hayat et al., 2018 | (35) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Bajaj et al., 2009 | (10) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Tsai et al., 2017 | (36) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Kim et al., 2017 | (37) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Huang et al., 2016 | (38) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Dam et al., 2016 | (39) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Cole et al., 2016 | (40) | N/A | N/A | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Terg et al., 2015 | (41) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| O’Leary et al., 2015 | (42) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Dultz et al., 2015 | (43) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ratelle et al., 2014 | (44) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Miura et al., 2013 | (45) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Mandorfer et al., 2014 | (46) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Kwon et al., 2014 | (47) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| De Vos et al., 2013 | (48) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Goel et al., 2012 | (8) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Choi et al., 2011 | (9) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bajaj et al., 2008 | (49) | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
7. Discussion
This systematic review and meta-analysis showed that there was an association between PPI use and the occurrence of SBP or HE, and cirrhotic PPI users are at a two-fold risk of SBP or HE than non-users. Gastrointestinal bleeding, such as esophageal variceal bleeding, peptic ulcer bleeding, portal hypertensive gastropathy, and stomach vascular ectasia syndrome, are frequent in cirrhotic patients. Proton pump inhibitors are applied to these patients to treat or stop bleeding following endoscopic hemostasis (50, 51). Recent research, however, suggested that PPIs might make SBP more common in cirrhotic patients (39).
Proton pump inhibitors are frequently utilized in therapeutic settings for a variety of patient indications. The treatment of peptic ulcer disease, gastroesophageal reflux disease, Zollinger-Ellison syndrome, non-steroidal anti-inflammatory drug (NSAID)-associated ulcers, and elimination of Helicobacter pylori are among the indications for PPIs (52, 53). In order to avoid peptic problems in patients receiving multidrug therapy for variceal or hypertensive gastropathy hemorrhage, PPIs are frequently used in patients with cirrhosis, sometimes even in the absence of a specific acid-related condition. The use of this class of medications appears to be more habitual than evidence-based ones, ultimately jeopardizing patient safety and raising healthcare expenditures (51).
Healthcare professionals managing patients with cirrhosis should be aware that the majority of these individuals do not require the usage of PPIs and should take all reasonable steps to actively review and reassess the current PPI therapy. Prescribers should use PPIs sparingly and only when there is clear evidence of their benefits. Although a small number of the studies included in the present systematic review contradicted this relationship, most of the included studies demonstrated a risk between the use of PPIs and the emergence of SBP. The patients with considerable liver damage in the included studies might be responsible for the disparity. Additionally, drug dosage or types might have an impact on the outcome of treatment. The use of PPIs and its relationship with the prevalence of SBP in cirrhotic patients is debatable, which is likely due to the heterogeneity of the individuals included between studies and other methodological problems, such as retrospective design and inadequate follow-up. Additionally, the negative effects of PPIs might only be experienced by certain subgroups, such as patients with decompensated cirrhosis, particularly in the case of ascites presence.
Using PPI can interfere with the immune response to pathogens that enter through the mouth, decrease the motility of the digestion system, delay the evacuation of gastric, and decrease gastric mucous viscosity. All of the aforementioned issues can affect intestine flora which might cause SIBO. In addition to acid suppression, PPIs interfere with neutrophil action and innate immunity. The appropriate action of neutrophils is required for the release of active oxygen and the production of interleukin 8 (IL-8). These mechanisms shed light on the role of PPIs in the spread of pneumonia and other serious healthcare-associated and hospital-acquired infections (15, 20). With a 4-fold increase, infection is the primary factor in cirrhotic patients’ mortality (16, 54). The presence of infection, even after it has resolved, is suggestive of the next prognostic stage; therefore, it must be taken into account when grading cirrhosis, according to a recent study by Arvaniti et al. (16, 55).
Spontaneous bacterial peritonitis is the illness that cirrhotic patients encounter the most commonly and is always linked to a poor prognosis (56). The prevalence changes throughout time and depending on the population. However, over the past two decades, the prevalence has ranged from 10% to 30% (55, 57, 58), with the most recent systematic study indicating a pooled frequency of 17.12% (59). Spontaneous bacterial peritonitis has a 40% (60) survival rate after the initial episode and a 70% (61) recurrence rate, accounting for 4% (59) of emergency department visits in the cirrhotic population. About 33 - 50% of patients with SBP have been observed to have acute renal damage (62).
Prophylactic and therapeutic antibiotic use, intravenous albumin infusion, and risk factor modification are all examples of management options for SBP. Old age, female gender, HE, coagulopathy, and variceal hemorrhage are previously recognized risk factors for SBP based on research (63). In addition, the use of PPI but not H2-blocker is an independent risk factor for the development of SBP in cirrhotic patients with ascites. The use of β-blockers is protective against the development of SBP, as has already been highlighted in the literature (64, 65). The infection with Clostridium difficile and nosocomial and community-acquired pneumonia have all been linked to long-term PPI use (66, 67).
A substantial body of evidence has emerged recently linking the usage of PPIs with the emergence of SBP in cirrhotic individuals. Regarding immunological dysfunction, PPIs and liver dysfunction share a similar mechanism, going back to the physiopathology of cirrhosis and SBP. Proton pump inhibitor use has been linked to increased risks of bacterial infections, such as SBP, by inhibiting phagocytosis, reducing oxidative burst, and promoting bacterial translocation (68-71). Clinical results on this subject, however, are still debatable. This issue makes it difficult to provide specific recommendations and emphasizes the need for larger, population-based research to support this association.
Some earlier studies identified no link between PPI use and the onset of SBP (37, 41, 46, 72, 73). The aforementioned studies, however, used smaller samples, had inconsistent definitions of exposures and outcomes, or failed to take suitable confounders into account. However, a growing number of studies have found a positive correlation (41, 42, 68, 74), and the majority of these studies have stronger levels of evidence (67, 75, 76). Variceal bleeding is much more common among PPI users, as demonstrated by Mandorfer et al. (46). A previous history of SBP episodes is a substantial risk factor for the development of a second episode; nevertheless, the database used does not provide any information regarding those episodes.
In terms of the association of PPIs and HE, short-term PPI consumption is associated with a significant risk of HE in patients with decompensated cirrhosis at varied periods of time, regardless of age, gender, and recent comorbidities. The highest risk is for the 28-day window, and the lowest risk is for the 7-day window. Esomeprazole, rabeprazole, and lansoprazole (more potent to suppress the effect of gastric acid) were associated with the risk of HE, but not omeprazole and pantoprazole (24). The use of PPIs might affect cirrhotic patients by changing the pH of the stomach and leading to the proliferation of the intestinal microbiome; therefore, it increases ammonia production and bacterial transport. Proton pump inhibitor use is associated with worse degrees of HE (26). The use of PPIs in patients who suffer from decompensated liver cirrhosis is associated with a higher mortality rate and major liver impairment complications that require hospitalization (28). The role of PPIs in cirrhotic patients with HE should focus on Helicobacter pylori eradication, not gastric acid suppression (33).
The current review is more recent and thorough, although recent systematic reviews have sought to investigate this association (77-80). With a larger patient group, this review included 33 published papers in this analysis, which is more than performed in earlier assessments. Although liver failure can occur due to various reasons, further prospective studies that stratify the population at risk of developing SBP according to the severity of their liver illness would be interesting, given that the risk of SBP is higher in individuals with severe liver disease (81-84).
7.1. Conclusions
Proton pump inhibitors increase the risk of SBP and HE and are associated with an increased risk of mortality in hospitalized or outpatient cirrhotic patients. In addition, subgroup analysis is suggested to evaluate which type and dosage of PPIs have a higher association with SBP or HE.
Acknowledgements
References
-
1.
Pinzello G, Simonetti RG, Craxi A, Di Piazza S, Spano C, Pagliaro L. Spontaneous bacterial peritonitis: A prospective investigation in predominantly nonalcoholic cirrhotic patients. Hepatology. 1983;3(4):545-9. [PubMed ID: 6862365]. https://doi.org/10.1002/hep.1840030411.
-
2.
Gines P, Arroyo V, Rodes J. Pathophysiology, complications, and treatment of ascites. Clin Liver Dis. 1997;1(1):129-55. [PubMed ID: 15562674]. https://doi.org/10.1016/s1089-3261(05)70261-0.
-
3.
Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, et al. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105(2):323-7. [PubMed ID: 19844200]. https://doi.org/10.1038/ajg.2009.558.
-
4.
Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000;182(2):526-33. [PubMed ID: 10915084]. https://doi.org/10.1086/315742.
-
5.
Martinsen TC, Bergh K, Waldum HL. Gastric juice: A barrier against infectious diseases. Basic Clin Pharmacol Toxicol. 2005;96(2):94-102. [PubMed ID: 15679471]. https://doi.org/10.1111/j.1742-7843.2005.pto960202.x.
-
6.
Bavishi C, Dupont HL. Systematic review: The use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34(11-12):1269-81. [PubMed ID: 21999643]. https://doi.org/10.1111/j.1365-2036.2011.04874.x.
-
7.
Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102(9):2047-56. quiz 2057. [PubMed ID: 17509031]. https://doi.org/10.1111/j.1572-0241.2007.01275.x.
-
8.
Goel GA, Deshpande A, Lopez R, Hall GS, van Duin D, Carey WD. Increased rate of spontaneous bacterial peritonitis among cirrhotic patients receiving pharmacologic acid suppression. Clin Gastroenterol Hepatol. 2012;10(4):422-7. [PubMed ID: 22155557]. https://doi.org/10.1016/j.cgh.2011.11.019.
-
9.
Choi EJ, Lee HJ, Kim KO, Lee SH, Eun JR, Jang BI, et al. Association between acid suppressive therapy and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Scand J Gastroenterol. 2011;46(5):616-20. [PubMed ID: 21275825]. https://doi.org/10.3109/00365521.2011.551891.
-
10.
Bajaj JS, Zadvornova Y, Heuman DM, Hafeezullah M, Hoffmann RG, Sanyal AJ, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol. 2009;104(5):1130-4. [PubMed ID: 19337238]. https://doi.org/10.1038/ajg.2009.80.
-
11.
Robinson M, Horn J. Clinical pharmacology of proton pump inhibitors: What the practising physician needs to know. Drugs. 2003;63(24):2739-54. [PubMed ID: 14664653]. https://doi.org/10.2165/00003495-200363240-00004.
-
12.
Naunton M, Peterson GM, Bleasel MD. Overuse of proton pump inhibitors. J Clin Pharm Ther. 2000;25(5):333-40. [PubMed ID: 11123484]. https://doi.org/10.1046/j.1365-2710.2000.00312.x.
-
13.
Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: A review of cost-effectiveness and risk [corrected]. Am J Gastroenterol. 2009;104 Suppl 2:S27-32. [PubMed ID: 19262544]. https://doi.org/10.1038/ajg.2009.49.
-
14.
Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: What the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-32. [PubMed ID: 22778788]. [PubMed Central ID: PMC3388523]. https://doi.org/10.1177/1756283X12437358.
-
15.
Fohl AL, Regal RE. Proton pump inhibitor-associated pneumonia: Not a breath of fresh air after all? World J Gastrointest Pharmacol Ther. 2011;2(3):17-26. [PubMed ID: 21731913]. [PubMed Central ID: PMC3124633]. https://doi.org/10.4292/wjgpt.v2.i3.17.
-
16.
Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139(4):1246-56. [PubMed ID: 20558165]. https://doi.org/10.1053/j.gastro.2010.06.019.
-
17.
Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34(5):768-73. [PubMed ID: 11434627]. https://doi.org/10.1016/s0168-8278(01)00026-5.
-
18.
Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375(17):1660-70. [PubMed ID: 27783916]. https://doi.org/10.1056/NEJMra1600561.
-
19.
Bian J, Wang A, Lin J, Wu L, Huang H, Wang S, et al. Association between proton pump inhibitors and hepatic encephalopathy: A meta-analysis. Medicine (Baltimore). 2017;96(17). e6723. [PubMed ID: 28445288]. [PubMed Central ID: PMC5413253]. https://doi.org/10.1097/MD.0000000000006723.
-
20.
Janka T, Tornai T, Borbely B, Tornai D, Altorjay I, Papp M, et al. Deleterious effect of proton pump inhibitors on the disease course of cirrhosis. Eur J Gastroenterol Hepatol. 2020;32(2):257-64. [PubMed ID: 31464790]. https://doi.org/10.1097/MEG.0000000000001499.
-
21.
Yamamoto K, Ishigami M, Honda T, Takeyama T, Ito T, Ishizu Y, et al. Influence of proton pump inhibitors on microbiota in chronic liver disease patients. Hepatol Int. 2019;13(2):234-44. [PubMed ID: 30737678]. https://doi.org/10.1007/s12072-019-09932-9.
-
22.
Wellhoner F, Doscher N, Tergast TL, Vital M, Plumeier I, Kahl S, et al. The impact of proton pump inhibitors on the intestinal microbiota in chronic hepatitis C patients. Scand J Gastroenterol. 2019;54(8):1033-41. [PubMed ID: 31361979]. https://doi.org/10.1080/00365521.2019.1647280.
-
23.
Teng SL, Hsu CH, Hsu CC, Shin JS, Huang JC. Association of gastric acid suppressants with the development of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Adv Dig Med. 2019;7:139-46. https://doi.org/10.1002/aid2.13164.
-
24.
Kuan YC, Huang KW, Lin CL, Luo JC, Kao CH. Short-term proton pump inhibitor use and hepatic encephalopathy risk in patients with decompensated cirrhosis. J Clin Med. 2019;8(8). [PubMed ID: 31349746]. [PubMed Central ID: PMC6723586]. https://doi.org/10.3390/jcm8081108.
-
25.
Huang ST, Tseng LY, Chen LK, Peng LN, Hsiao FY. Does Long-Term Proton Pump Inhibitor Use Increase Risk of Dementia? Not Really! Results of the Group-Based Trajectory Analysis. Clin Pharmacol Ther. 2019;106(3):616-22. [PubMed ID: 30861103]. https://doi.org/10.1002/cpt.1430.
-
26.
Fasullo M, Rau P, Liu DQ, Holzwanger E, Mathew JP, Guilarte-Walker Y, et al. Proton pump inhibitors increase the severity of hepatic encephalopathy in cirrhotic patients. World J Hepatol. 2019;11(6):522-30. [PubMed ID: 31293720]. [PubMed Central ID: PMC6603505]. https://doi.org/10.4254/wjh.v11.i6.522.
-
27.
Elzouki AN, Neffati N, Rasoul FA, Abdallah A, Othman M, Waness A. Increased risk of spontaneous bacterial peritonitis in cirrhotic patients using proton pump inhibitors. GE Port J Gastroenterol. 2019;26(2):83-9. [PubMed ID: 30976612]. [PubMed Central ID: PMC6454390]. https://doi.org/10.1159/000487963.
-
28.
De Roza MA, Kai L, Kam JW, Chan YH, Kwek A, Ang TL, et al. Proton pump inhibitor use increases mortality and hepatic decompensation in liver cirrhosis. World J Gastroenterol. 2019;25(33):4933-44. [PubMed ID: 31543684]. [PubMed Central ID: PMC6737311]. https://doi.org/10.3748/wjg.v25.i33.4933.
-
29.
Dam G, Vilstrup H, Andersen PK, Bossen L, Watson H, Jepsen P. Effect of proton pump inhibitors on the risk and prognosis of infections in patients with cirrhosis and ascites. Liver Int. 2019;39(3):514-21. [PubMed ID: 30472808]. https://doi.org/10.1111/liv.14012.
-
30.
Zhu J, Qi X, Yu H, Yoshida EM, Mendez-Sanchez N, Zhang X, et al. Association of proton pump inhibitors with the risk of hepatic encephalopathy during hospitalization for liver cirrhosis. United European Gastroenterol J. 2018;6(8):1179-87. [PubMed ID: 30288280]. [PubMed Central ID: PMC6169047]. https://doi.org/10.1177/2050640618773564.
-
31.
Li DK, Yan P, Abou-Samra AB, Chung RT, Butt AA. Proton pump inhibitors are associated with accelerated development of cirrhosis, hepatic decompensation and hepatocellular carcinoma in noncirrhotic patients with chronic hepatitis C infection: results from ERCHIVES. Aliment Pharmacol Ther. 2018;47(2):246-58. [PubMed ID: 29105111]. https://doi.org/10.1111/apt.14391.
-
32.
Lazaro-Pacheco IB, Servin-Caamano AI, Perez-Hernandez JL, Rojas-Loureiro G, Servin-Abad L, Tijera FH. Proton pump inhibitors increase the overall risk of developing bacterial infections in patients with cirrhosis. Arq Gastroenterol. 2018;55(1):28-32. [PubMed ID: 29561973]. https://doi.org/10.1590/S0004-2803.201800000-09.
-
33.
Hung TH, Lee HF, Tseng CW, Tsai CC, Tsai CC. Effect of proton pump inhibitors in hospitalization on mortality of patients with hepatic encephalopathy and cirrhosis but no active gastrointestinal bleeding. Clin Res Hepatol Gastroenterol. 2018;42(4):353-9. [PubMed ID: 29551615]. https://doi.org/10.1016/j.clinre.2017.11.011.
-
34.
Hung SC, Liao KF, Hung HC, Lin CL, Lai SW, Lee PC, et al. Using proton pump inhibitors correlates with an increased risk of chronic kidney disease: A nationwide database-derived case-controlled study. Family Practice. 2018;35(2):166-171. [PubMed ID: 29045621]. https://doi.org/10.1093/fampra/cmx102.
-
35.
Hayat MK, Shah ZH, Hayat MF, Imran MY, Qureshi I. Comparative study of spontaneous bacterial peritonitis in cirrhosis patients managed with and without proton pump inhibitors. Pak J Med Health Sci. 2018;12:598-601.
-
36.
Tsai CF, Chen MH, Wang YP, Chu CJ, Huang YH, Lin HC, et al. Proton Pump Inhibitors Increase Risk for Hepatic Encephalopathy in Patients With Cirrhosis in A Population Study. Gastroenterology. 2017;152(1):134-41. [PubMed ID: 27639806]. https://doi.org/10.1053/j.gastro.2016.09.007.
-
37.
Kim JH, Lim KS, Min YW, Lee H, Min BH, Rhee PL, et al. Proton pump inhibitors do not increase the risk for recurrent spontaneous bacterial peritonitis in patients with cirrhosis. J Gastroenterol Hepatol. 2017;32(5):1064-70. [PubMed ID: 28449345]. https://doi.org/10.1111/jgh.13637.
-
38.
Huang KW, Kuan YC, Luo JC, Lin CL, Liang JA, Kao CH. Impact of long-term gastric acid suppression on spontaneous bacterial peritonitis in patients with advanced decompensated liver cirrhosis. Eur J Intern Med. 2016;32:91-5. [PubMed ID: 27139916]. https://doi.org/10.1016/j.ejim.2016.04.016.
-
39.
Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64(4):1265-72. [PubMed ID: 27474889]. https://doi.org/10.1002/hep.28737.
-
40.
Cole HL, Pennycook S, Hayes PC. The impact of proton pump inhibitor therapy on patients with liver disease. Aliment Pharmacol Ther. 2016;44(11-12):1213-23. [PubMed ID: 27774677]. https://doi.org/10.1111/apt.13827.
-
41.
Terg R, Casciato P, Garbe C, Cartier M, Stieben T, Mendizabal M, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol. 2015;62(5):1056-60. [PubMed ID: 25481567]. https://doi.org/10.1016/j.jhep.2014.11.036.
-
42.
O'Leary JG, Reddy KR, Wong F, Kamath PS, Patton HM, Biggins SW, et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(4):753-9 e1-2. [PubMed ID: 25130937]. [PubMed Central ID: PMC4326601]. https://doi.org/10.1016/j.cgh.2014.07.060.
-
43.
Dultz G, Piiper A, Zeuzem S, Kronenberger B, Waidmann O. Proton pump inhibitor treatment is associated with the severity of liver disease and increased mortality in patients with cirrhosis. Aliment Pharmacol Ther. 2015;41(5):459-66. [PubMed ID: 25523381]. https://doi.org/10.1111/apt.13061.
-
44.
Ratelle M, Perreault S, Villeneuve JP, Tremblay L. Association between proton pump inhibitor use and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Can J Gastroenterol Hepatol. 2014;28(6):330-4. [PubMed ID: 24945188]. [PubMed Central ID: PMC4072237]. https://doi.org/10.1155/2014/751921.
-
45.
Miura K, Tanaka A, Yamamoto T, Adachi M, Takikawa H. Proton pump inhibitor use is associated with spontaneous bacterial peritonitis in patients with liver cirrhosis. Intern Med. 2014;53(10):1037-42. [PubMed ID: 24827481]. https://doi.org/10.2169/internalmedicine.53.2021.
-
46.
Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Summereder C, et al. Proton pump inhibitor intake neither predisposes to spontaneous bacterial peritonitis or other infections nor increases mortality in patients with cirrhosis and ascites. PLoS One. 2014;9(11). e110503. [PubMed ID: 25369194]. [PubMed Central ID: PMC4219684]. https://doi.org/10.1371/journal.pone.0110503.
-
47.
Kwon JH, Koh SJ, Kim W, Jung YJ, Kim JW, Kim BG, et al. Mortality associated with proton pump inhibitors in cirrhotic patients with spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2014;29(4):775-81. [PubMed ID: 24219827]. https://doi.org/10.1111/jgh.12426.
-
48.
De Vos M, De Vroey B, Garcia BG, Roy C, Kidd F, Henrion J, et al. Role of proton pump inhibitors in the occurrence and the prognosis of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Liver Int. 2013;33(9):1316-23. [PubMed ID: 23730823]. https://doi.org/10.1111/liv.12210.
-
49.
Bajaj JS, Acharya C, Fagan A, White MB, Gavis E, Heuman DM, et al. Proton Pump Inhibitor Initiation and Withdrawal affects Gut Microbiota and Readmission Risk in Cirrhosis. Am J Gastroenterol. 2018;113(8):1177-1186. [PubMed ID: 29872220]. https://doi.org/10.1038/s41395-018-0085-9.
-
50.
Shaheen NJ, Stuart E, Schmitz SM, Mitchell KL, Fried MW, Zacks S, et al. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology. 2005;41(3):588-94. [PubMed ID: 15726658]. https://doi.org/10.1002/hep.20593.
-
51.
Lodato F, Azzaroli F, Di Girolamo M, Feletti V, Cecinato P, Lisotti A, et al. Proton pump inhibitors in cirrhosis: Tradition or evidence based practice? World J Gastroenterol. 2008;14(19):2980-5. [PubMed ID: 18494046]. [PubMed Central ID: PMC2712162]. https://doi.org/10.3748/wjg.14.2980.
-
52.
Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): Need for a reappraisal. Eur J Intern Med. 2017;37:19-24. [PubMed ID: 27784575]. https://doi.org/10.1016/j.ejim.2016.10.007.
-
53.
Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (1996). 2000;40(1):52-62. https://doi.org/10.1016/s1086-5802(16)31036-1.
-
54.
Fernandez J, Navasa M, Gomez J, Colmenero J, Vila J, Arroyo V, et al. Bacterial infections in cirrhosis: Epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35(1):140-8. [PubMed ID: 11786970]. https://doi.org/10.1053/jhep.2002.30082.
-
55.
European Association for the Study of the L. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397-417. [PubMed ID: 20633946]. https://doi.org/10.1016/j.jhep.2010.05.004.
-
56.
Marciano S, Diaz JM, Dirchwolf M, Gadano A. Spontaneous bacterial peritonitis in patients with cirrhosis: incidence, outcomes, and treatment strategies. Hepat Med. 2019;11:13-22. [PubMed ID: 30666172]. [PubMed Central ID: PMC6336019]. https://doi.org/10.2147/HMER.S164250.
-
57.
Koulaouzidis A, Bhat S, Karagiannidis A, Tan WC, Linaker BD. Spontaneous bacterial peritonitis. Postgrad Med J. 2007;83(980):379-83. [PubMed ID: 17551068]. [PubMed Central ID: PMC2600063]. https://doi.org/10.1136/pgmj.2006.056168.
-
58.
Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology. 2001;120(3):726-48. [PubMed ID: 11179247]. https://doi.org/10.1053/gast.2001.22580.
-
59.
Tay PWL, Xiao J, Tan DJH, Ng C, Lye YN, Lim WH, et al. An epidemiological meta-analysis on the worldwide prevalence, resistance, and outcomes of spontaneous bacterial peritonitis in cirrhosis. Front Med (Lausanne). 2021;8:693652. [PubMed ID: 34422858]. [PubMed Central ID: PMC8375592]. https://doi.org/10.3389/fmed.2021.693652.
-
60.
D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217-31. [PubMed ID: 16298014]. https://doi.org/10.1016/j.jhep.2005.10.013.
-
61.
Oliveira AM, Branco JC, Barosa R, Rodrigues JA, Ramos L, Martins A, et al. Clinical and microbiological characteristics associated with mortality in spontaneous bacterial peritonitis: a multicenter cohort study. Eur J Gastroenterol Hepatol. 2016;28(10):1216-22. [PubMed ID: 27391170]. https://doi.org/10.1097/MEG.0000000000000700.
-
62.
Devani K, Charilaou P, Jaiswal P, Patil N, Radadiya D, Patel P, et al. Trends in hospitalization, acute kidney injury, and mortality in patients with spontaneous bacterial peritonitis. J Clin Gastroenterol. 2019;53(2):e68-74. [PubMed ID: 29252684]. https://doi.org/10.1097/MCG.0000000000000973.
-
63.
Niu B, Kim B, Limketkai BN, Sun J, Li Z, Woreta T, et al. Mortality from spontaneous bacterial peritonitis among hospitalized patients in the USA. Dig Dis Sci. 2018;63(5):1327-33. [PubMed ID: 29480417]. [PubMed Central ID: PMC5897146]. https://doi.org/10.1007/s10620-018-4990-y.
-
64.
Bang UC, Benfield T, Hyldstrup L, Jensen JE, Bendtsen F. Effect of propranolol on survival in patients with decompensated cirrhosis: a nationwide study based Danish patient registers. Liver Int. 2016;36(9):1304-12. [PubMed ID: 26992041]. https://doi.org/10.1111/liv.13119.
-
65.
Bossen L, Krag A, Vilstrup H, Watson H, Jepsen P. Nonselective beta-blockers do not affect mortality in cirrhosis patients with ascites: Post Hoc analysis of three randomized controlled trials with 1198 patients. Hepatology. 2016;63(6):1968-76. [PubMed ID: 26599983]. https://doi.org/10.1002/hep.28352.
-
66.
Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955-60. [PubMed ID: 15507580]. https://doi.org/10.1001/jama.292.16.1955.
-
67.
Deshpande A, Pant C, Pasupuleti V, Rolston DD, Jain A, Deshpande N, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(3):225-33. [PubMed ID: 22019794]. https://doi.org/10.1016/j.cgh.2011.09.030.
-
68.
van Vlerken LG, Huisman EJ, van Hoek B, Renooij W, de Rooij FW, Siersema PD, et al. Bacterial infections in cirrhosis: Role of proton pump inhibitors and intestinal permeability. Eur J Clin Invest. 2012;42(7):760-7. [PubMed ID: 22288900]. https://doi.org/10.1111/j.1365-2362.2011.02643.x.
-
69.
Agastya G, West BC, Callahan JM. Omeprazole inhibits phagocytosis and acidification of phagolysosomes of normal human neutrophils in vitro. Immunopharmacol Immunotoxicol. 2000;22(2):357-72. [PubMed ID: 10952036]. https://doi.org/10.3109/08923970009016425.
-
70.
Chang SS, Lai CC, Lee MG, Lee YC, Tsai YW, Hsu WT, et al. Risk of spontaneous bacterial peritonitis associated with gastric Acid suppression. Medicine (Baltimore). 2015;94(22). e944. [PubMed ID: 26039135]. [PubMed Central ID: PMC4616349]. https://doi.org/10.1097/MD.0000000000000944.
-
71.
Garcia-Martinez I, Frances R, Zapater P, Gimenez P, Gomez-Hurtado I, Moratalla A, et al. Use of proton pump inhibitors decrease cellular oxidative burst in patients with decompensated cirrhosis. J Gastroenterol Hepatol. 2015;30(1):147-54. [PubMed ID: 25039465]. https://doi.org/10.1111/jgh.12667.
-
72.
Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci. 2008;53(2):394-8. [PubMed ID: 17616817]. https://doi.org/10.1007/s10620-007-9899-9.
-
73.
Miozzo SAS, John JA, Appel-da-Silva MC, Dossin IA, Tovo CV, Mattos AA. Influence of proton pump inhibitors in the development of spontaneous bacterial peritonitis. World J Hepatol. 2017;9(35):1278-85. [PubMed ID: 29290909]. [PubMed Central ID: PMC5740091]. https://doi.org/10.4254/wjh.v9.i35.1278.
-
74.
Parkman HP, Urbain JL, Knight LC, Brown KL, Trate DM, Miller MA, et al. Effect of gastric acid suppressants on human gastric motility. Gut. 1998;42(2):243-50. [PubMed ID: 9536950]. [PubMed Central ID: PMC1726985]. https://doi.org/10.1136/gut.42.2.243.
-
75.
Xu HB, Wang HD, Li CH, Ye S, Dong MS, Xia QJ, et al. Proton pump inhibitor use and risk of spontaneous bacterial peritonitis in cirrhotic patients: a systematic review and meta-analysis. Genet Mol Res. 2015;14(3):7490-501. [PubMed ID: 26214428]. https://doi.org/10.4238/2015.July.3.25.
-
76.
Trikudanathan G, Israel J, Cappa J, O'Sullivan DM. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients - a systematic review and meta-analysis. Int J Clin Pract. 2011;65(6):674-8. [PubMed ID: 21564440]. https://doi.org/10.1111/j.1742-1241.2011.02650.x.
-
77.
Khan MA, Kamal S, Khan S, Lee WM, Howden CW. Systematic review and meta-analysis of the possible association between pharmacological gastric acid suppression and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2015;27(11):1327-36. [PubMed ID: 26313401]. https://doi.org/10.1097/MEG.0000000000000448.
-
78.
Yu T, Tang Y, Jiang L, Zheng Y, Xiong W, Lin L. Proton pump inhibitor therapy and its association with spontaneous bacterial peritonitis incidence and mortality: A meta-analysis. Dig Liver Dis. 2016;48(4):353-9. [PubMed ID: 26795544]. https://doi.org/10.1016/j.dld.2015.12.009.
-
79.
Dong H, Luo SQ, Dong Y, Feng W, Wei Y. The use of PPI is associated with spontaneous bacterial peritonitis in cirrhotic patients of different ethnic groups : A meta-analysis. Int J Clin Exp Med. 2016;9(2):1227-35.
-
80.
Alhumaid S, Al Mutair A, Al Alawi Z, Zaidi ARZ, Rabaan AA, Elhazmi A, et al. Proton pump inhibitors use and risk of developing spontaneous bacterial peritonitis in cirrhotic patients: A systematic review and meta-analysis. Gut Pathog. 2021;13(1):17. [PubMed ID: 33741033]. [PubMed Central ID: PMC7977161]. https://doi.org/10.1186/s13099-021-00414-8.
-
81.
Soleimanpour H, Safari S, Rahmani F, Ameli H, Alavian SM. The role of inhalational anesthetic drugs in patients with hepatic dysfunction: A review article. Anesth Pain Med. 2015;5(1). https://doi.org/10.5812/aapm.23409.
-
82.
Soleimanpour M, Imani F, Safari S, Sanaie S, Soleimanpour H, Ameli H, et al. The role of non-steroidal anti-inflammatory drugs (NSAIDS) in the treatment of patients with hepatic disease: A review article. Anesth Pain Med. 2016;6(4). https://doi.org/10.5812/aapm.37822.
-
83.
Soleimanpour H, Safari S, Shahsavari Nia K, Sanaie S, Alavian SM. Opioid drugs in patients with liver disease: A systematic review. Hepat Mon. 2016;16(4). e32636. [PubMed ID: 27257423]. [PubMed Central ID: PMC4887963]. https://doi.org/10.5812/hepatmon.32636.
-
84.
Soleimanpour H, Shahsavari Nia K, Sanaie S, Ghojazadeh M, Alavian SM. Use of dexmedetomidine in liver disease: A systematic review and meta-analysis. Hepat Mon. 2019;19(10). https://doi.org/10.5812/hepatmon.98530.