Abstract
Background:
Various micronutrients have been used to manage patients affected by coronavirus disease 2019 (COVID-19). However, data on the potential benefits of selenium for COVID-19 patients are scarce.Objectives:
This study was conducted to monitor the efficacy and safety of selenium supplementation in COVID-19 patients hospitalized at Shahid Mostafa Khomeini Hospital in Ilam, Iran, in 2021.Methods:
In this randomized controlled trial, 100 COVID-19 cases were included and divided into two groups: The selenium group (n = 50) was prescribed the hospital treatment protocol as well as selenium supplementation in a daily dose of 200 µg, and the control group (n = 50) that received only the hospital treatment protocol. Laboratory tests, including platelet, white blood count (WBC), hemoglobin, ferritin, c-reactive protein (CRP), and erythrocyte sedimentation rate (ESR), were performed before the trial and on the 14th day. Two groups were compared based on laboratory findings, duration of hospitalization, and dependency on mechanical ventilation.Results:
In the selenium group, platelets, WBC, and hemoglobin rose remarkably compared to their pre-treatment concentrations (P < 0.05), while a considerable drop was detected in levels of ESR, CRP, and ferritin (P < 0.05). The mean length of stay in the selenium and control groups was 9.27 ± 13.50 and 11.45 ± 14.33 days, respectively (P = 0.401). The mean mechanical ventilation dependency days in the selenium and control groups were 9.65 ± 11.71 and 7.45 ± 4.93, respectively, implying no statistically significant difference (P = 0.307).Conclusions:
Selenium supplement may reduce levels of inflammatory markers in COVID-19 patients. Nevertheless, further trials are required to monitor its clinical effectiveness.Keywords
1. Background
The coronavirus disease 2019 (COVID-19) pandemic appeared as a result of an expeditious spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), leading to abysmal effects on the lives of people worldwide (1). The clinical course of COVID-19 varies from a symptomless infection to multisystem organ damage and death (2). The COVID-19 pandemic led to a drastic death toll, reducing overall life expectancy by 1.5 years in 2020 (3). Despite the production of several vaccines and medications, the mortality rate in patients affected by COVID-19 infection remains high, indicating the need to develop more effective therapeutic strategies (4).
Reports worldwide suggest that certain supplements could be potential candidates for adjuvant therapy. However, the efficacy of these supplements has not been thoroughly evaluated (5). Since lower levels of circulating trace elements were detected in COVID-19 patients, questions were raised about the possible advantages of these minerals in treating COVID-19 infection. Trace elements contribute to various processes, which modulate immune responses and also perform as cofactors for several antioxidant molecules. Deficient concentrations of these elements can influence innate and adaptive immune functions, predisposing individuals to severe infectious diseases (6). In critically ill patients, mineral deficiencies are accompanied by an elevation in free radical production and an attenuation in the systemic inflammatory processes, thereby directly participating in cell death and a higher mortality risk (7).
Previous studies have investigated the role of different trace elements in treating COVID-19 infection. However, a systematic review reported that up to 2022, no trial had assessed selenium's prophylactic or therapeutic effects in COVID-19 patients (8). As an antioxidant and anti-inflammatory agent, selenium is influentially involved in the glutathione peroxidase system (9). Before the current pandemic, several clinical trials had studied the impact of this element on the clinical outcomes of individuals suffering from critical conditions. However, the results appeared to be controversial. In a meta-analysis conducted by Allingstrup and Afshari (10) in 2015, selenium supplements appeared to reduce mortality in critically ill patients. Nevertheless, a year later, Manzanares et al. (11) observed that selenium supplementation neither lowered the mortality rate nor improved the clinical outcomes of critically ill patients.
2. Objectives
Given the known antioxidant and anti-inflammatory properties of selenium and the lack of clinical trials on the effects of selenium supplementation on the course of COVID-19, we conducted this study to monitor the efficacy and safety of selenium supplementation in COVID-19 patients. We hypothesized that selenium supplementation could improve the clinical outcomes of individuals affected by COVID-19.
3. Methods
3.1. Study Design and Participants
This open-label randomized controlled trial was conducted at Shahid Mostafa Khomeini Hospital in Ilam, Iran, in 2021. The inclusion criteria were as follows: Hospitalized patients aged 18 to 65 years who were diagnosed with COVID-19 infection based on a positive RT-PCR test, clinical and para-clinical evidence, including a respiratory rate of ≥ 30 per minute, arterial oxygen saturation lower than 90%, and arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) ratio < 300 mmHg (12). The exclusion criteria were as follows: Acute renal failure, chronic kidney disease, pregnancy or breastfeeding, history of allergic reaction to selenium, pneumonia due to viral, bacterial, and fungi microorganisms or noninfectious causes, cirrhosis, a prior history of active or latent forms of tuberculosis, and acquired immunodeficiency syndrome. The sample size was calculated based on the formula below, considering the confidence interval of 95%, a type 1 error of 5%, a type 2 error of 20%, and a power of 80%. As this research needed follow-up, we considered the possibility of dropping samples; hence, there were five additional participants in each group, and the total sample size was 100 (13).
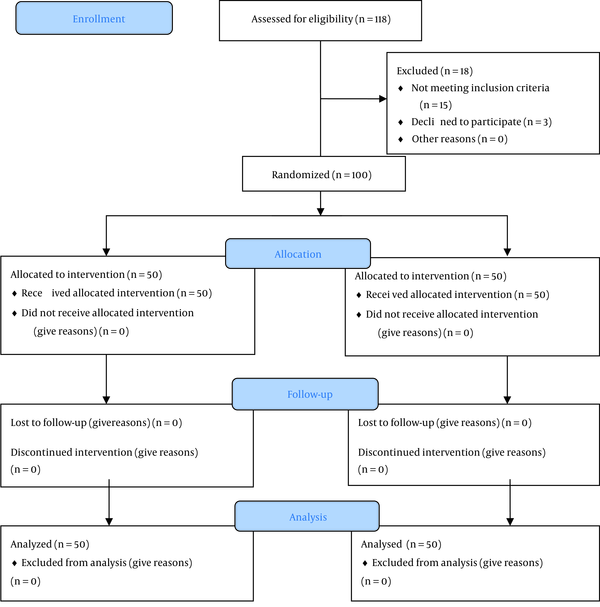
Figure 1 illustrates the studied population's CONSORT 2010 flow diagram (14). A total of 100 subjects who met the inclusion criteria were recruited. Participants were randomized and divided at a 1:1 ratio into two groups utilizing a simple permuted block randomization method. Participants in the control group were treated according to the hospital’s protocol for the treatment of COVID-19, which consisted of 400 mg of hydroxychloroquine twice daily on day one, thenceforth 200 mg twice daily. The intervention group received a selenium capsule in a daily dose of 200 micrograms in addition to 400 mg of hydroxychloroquine twice daily on day one, thenceforth 200 mg twice daily.
CONSORT 2010 flow diagram (14) for the studied population
3.2. Data Collection
Data related to age, gender, duration of hospitalization, period of mechanical ventilation dependency, and laboratory tests, including white blood count (WBC), hemoglobin, platelet, ferritin, erythrocyte sedimentation rate (ESR), and c-reactive protein (CRP) values of patients diagnosed with COVID-19 were collected and recorded in the follow-up form on the 1st and 14th day. Laboratory tests and length of stay were considered the study outcomes.
3.3. Statistical Analysis
Quantitative variables were described as mean and standard deviation (SD). The independent samples t-test and the repeated measures ANOVA were used to compare the mean of quantitative variables in different groups. The statistical analyses were conducted in STATA software version 12 at a 0.05 significance level.
3.4. Ethical Considerations
The study was approved by the Research Ethics Committee of Ilam University of Medical Sciences with the ethical code IR.MEDILAM.REC.1399.010. Written, informed, and voluntary consent was obtained from all participants in the study. The patients were assured that non-participation in the survey would not prevent them from receiving routine treatments. Hence, the principles of the Declaration of Helsinki were observed.
3.5. Study Protocol Registration
This trial was registered at the Iranian Registry of Clinical Trials with the identifier IRCT20190418043307N1.
4. Results
4.1. Demographic Data
Data related to gender, age, and BMI are listed in Table 1. The mean age in the selenium and control groups were 56.54 ± 17.91 and 57.14 ± 18.31 years, respectively.
Characteristics of the Patients Hospitalized for COVID-19 in the Selenium and Control Groups
| Characteristics | Control Group | Selenium Group |
|---|---|---|
| Age (y) | 57.14 ± 18.31 | 56.54 ± 17.91 |
| Weight (kg) | 66.00 ± 13.3 | 69.2 ± 14.4 |
| BMI (kg/m2) | 27.1 ± 5.0 | 28.3 ± 5.7 |
4.2. Laboratory Findings
On the 14th day after intervention, platelets, WBC, and hemoglobin increased significantly in the selenium group compared to their pre-treatment levels (P < 0.05). At the same time, a considerable drop was detected in ESR, CRP, and ferritin (P < 0.05). Please refer to Table 2 for additional data.
Distribution of Pre- and Post-intervention Laboratory Values in the Selenium and Control Groups
| Groups | Before Intervention | 14th Day | P-Value |
|---|---|---|---|
| WBC | 0.05 | ||
| Selenium | 9.49 ± 4.62 | 12.26 ± 2.33 | |
| Control | 8.80 ± 4.81 | 9.34 ± 3.11 | |
| Platelet count (1000/µL) | 0.04 | ||
| Selenium | 228 ± 71 | 243 ± 75 | |
| Control | 230 ± 83 | 239 ± 68 | |
| ESR (mm/hour) | 0.02 | ||
| Selenium | 71.6 ± 28.8 | 39.4 ± 36.5 | |
| Control | 68.9 ± 27.3 | 35.5 ± 34.2 | |
| CRP (mg/dL) | < 0.001 | ||
| Selenium | 4.6 ± 1.3 | 0.98 ± 0.5 | |
| Control | 3.9 ± 0.2 | 2.2 ± 0.1 | |
| Ferritin (mg/dL) | < 0.001 | ||
| Selenium | 275.4 ± 11.1 | 259 ± 9.6 | |
| Control | 279.0 ± 12.3 | 273 ± 10.9 | |
| Hemoglobin (g/dL) | 0.04 | ||
| Selenium | 12.9 ± 1.9 | 15.2 ± 1.2 | |
| Control | 12.1 ± 0.1 | 13.9 ± 0.5 |
4.3. Clinical Outcomes
The mean hospital stay duration in the selenium and control groups were 9.27 ± 13.50 and 11.45 ± 14.33 days, respectively (P = 0.401). The mean mechanical ventilation dependency days in the selenium and control groups were 9.65 ± 11.71 and 7.45 ± 4.93, respectively, which showed no statistically significant difference (P = 0.307). Table 3 tabulates further information.
Comparison of Clinical Outcomes of Patients in Control and Intervention Groups
| Characteristics | Selenium Group | Control Group | P-Value |
|---|---|---|---|
| Hospitalization time (days) | 9.27 ± 13.50 | 11.45 ± 14.33 | 0.401 |
| Mechanical ventilation dependency (days) | 7.45 ± 4.93 | 9.65 ± 11.71 | 0.307 |
5. Discussion
This study investigated the effects of selenium supplementation on clinical and paraclinical outcomes in COVID-19 patients. Selenium supplementation led to an increase in platelets, WBC, and hemoglobin and a reduction in ESR, CRP, and ferritin levels.
Direct indications that selenium could affect the COVID-19 course and the outcome of patients are limited and from observational studies. Nevertheless, based on prior experience with selenium adjuvant therapy for SARS and other viral infections, we highlight evidence suggesting dietary supplements' roles in promoting host resistance against viral infections. Selenium supplementation seems to be a beneficial add-on treatment for critically ill patients; however, there are some discrepancies in previous findings. Several studies have shown that this trace element can boost the proliferation of T lymphocytes and natural killer (NK) cells and the activities of the innate immune system (15, 16). The results of a previous work demonstrated that selenium supplementation in a dose of 200 µg/d boosted the cytotoxicity of CD8+ T cells, the lytic activity of NK cells, and the number of lymphocyte populations in human peripheral blood (16). Besides, Sakr et al. (17) reported that plasma selenium concentrations reversely correlated with inflammatory markers, including interleukin 6, CRP, and leucocyte count.
Concerning COVID-19 infection, the data on selenium supplementation are mostly restricted to observational findings. Selenium has been proposed as a micronutrient influential in the immune and inflammatory systems, which can aid in recognizing patients at a higher risk (18). In this regard, Im et al. (19) showed that selenium shortage in COVID-19 patients attenuated the immune responses and resulted in accelerated disease progression. A study conducted by Majeed et al. (20) in the Indian population revealed that plasma selenium concentrations were lower in COVID-19 patients than in the healthy control group.
In a prospective analysis performed by Razeghi Jahromi et al., although a drop in CRP levels accompanied elevated selenium levels, no remarkable association was found between disease severity and selenium levels (21). As reported in a retrospective study, arginine combined with other micronutrients, including selenium, shortened the length of stay and duration of ventilation in COVID-19 cases (22). On the contrary, the findings of Ramezaninejad et al. demonstrated that regarding the survival rate and need for ventilation, recipients of vitamin C, D, and selenium were not fundamentally different from patients who did not receive these supplements (23). It should be noted that in their study, only eight subjects received selenium, which might be insufficient to provide conclusive evidence.
The available data on the implication of selenium in COVID-19 are primarily observational, while randomized controlled trials are the only reliable methodology to verify the efficacy and safety of such interventions. A review article recommended that clinical studies with large sample sizes be conducted to confirm the potential benefits of selenium for reducing the incidence or severity of COVID-19 (24). Although our findings indicated that selenium supplementation reduced some inflammatory markers, this evidence may be insufficient to justify routine selenium supplementation to treat COVID-19 patients.
Overall, the results of the present work provided evidence that may benefit future research. However, due to possible limitations that might affect the generalizability of the findings, the results of this study should be treated with caution.
5.1. Limitations
This was an open-label trial, and the control group did not receive a placebo. Due to the study's novelty, we couldn't analyze some variables, such as lung involvement extent, recovery rates, and impact of other treatments.
5.2. Conclusions
Fulfilling the body's nutritional requirements is crucial for optimal immune system performance, improving COVID-19 patient survival rates. In this study, we focused on the importance of selenium in strengthening the immune system. The present study showed that after the intervention, the inflammatory markers decreased in the majority of patients. Since COVID-19 patients have reportedly low levels of several trace elements, it would be feasible to assume that nutritional deficiencies can influence the onset and progression of COVID-19. Hence, repeated measurements of blood parameters could help determine the prognosis of COVID-19 patients and monitor the efficacy of treatment.
References
-
1.
Joulaei H, Fatemi M, Hooshyar D, Karimi Rouzbahani A, Joulaei R, Foroozanfar Z. Analyzing delay in referral of pregnant women and children under five years old during the COVID-19 pandemic: Fars Province, Iran. Health Care Women Int. 2023;44(7-8):807-23. [PubMed ID: 35917555]. https://doi.org/10.1080/07399332.2022.2105845.
-
2.
Nilav A, Karimi Rouzbahani A, Mahmoudvand G, Zavari T. Evaluation of the Effect of Underlying Diseases on Mortality of COVID-19 Patients: A Study of 19,985 Cases. Jundishapur J Microbiol. 2022;15(11):e133603. https://doi.org/10.5812/jjm-133603.
-
3.
Lundberg DJ, Cho A, Raquib R, Nsoesie EO, Wrigley-Field E, Stokes AC. Geographic and Temporal Patterns in Covid-19 Mortality by Race and Ethnicity in the United States from March 2020 to February 2022. Preprint. medRxiv. Posted online July 21, 2022. https://doi.org/10.1101/2022.07.20.22277872.
-
4.
Bemtgen X, Kruger K, Supady A, Duerschmied D, Schibilsky D, Bamberg F, et al. First Successful Treatment of Coronavirus Disease 2019 Induced Refractory Cardiogenic Plus Vasoplegic Shock by Combination of Percutaneous Ventricular Assist Device and Extracorporeal Membrane Oxygenation: A Case Report. ASAIO J. 2020;66(6):607-9. [PubMed ID: 32472827]. [PubMed Central ID: PMC7217127]. https://doi.org/10.1097/MAT.0000000000001178.
-
5.
Damiescu R, Lee DYW, Efferth T. Can Essential Oils Provide an Alternative Adjuvant Therapy for COVID-19 Infections and Pain Management at the Same Time? Pharmaceuticals (Basel). 2022;15(11):1387. [PubMed ID: 36355558]. [PubMed Central ID: PMC9693097]. https://doi.org/10.3390/ph15111387.
-
6.
Li Y, Luo W, Liang B. Circulating trace elements status in COVID-19 disease: A meta-analysis. Front Nutr. 2022;9:982032. [PubMed ID: 36034929]. [PubMed Central ID: PMC9411985]. https://doi.org/10.3389/fnut.2022.982032.
-
7.
Heyland DK, Dhaliwal R, Day AG, Muscedere J, Drover J, Suchner U, et al. Reducing Deaths due to OXidative Stress (The REDOXS Study): Rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically-ill patients. Proc Nutr Soc. 2006;65(3):250-63. [PubMed ID: 16923310]. https://doi.org/10.1079/pns2006505.
-
8.
Balboni E, Zagnoli F, Filippini T, Fairweather-Tait SJ, Vinceti M. Zinc and selenium supplementation in COVID-19 prevention and treatment: a systematic review of the experimental studies. J Trace Elem Med Biol. 2022;71:126956. [PubMed ID: 35217499]. [PubMed Central ID: PMC8853960]. https://doi.org/10.1016/j.jtemb.2022.126956.
-
9.
Karimi M, Haghpanah S, Azarkeivan A, Zahedi Z, Zarei T, Akhavan Tavakoli M, et al. Prevalence and Mortality Due to Outbreak of Novel Coronavirus Disease (COVID-19) in β-Thalassemias: The Nationwide Iranian Experience. Preprint. SSRN Electronic Journal. Posted online July 2, 2020. https://doi.org/10.2139/ssrn.3605175.
-
10.
Allingstrup M, Afshari A. Selenium supplementation for critically ill adults. Cochrane Database Syst Rev. 2015;2015(7):CD003703. [PubMed ID: 26214143]. [PubMed Central ID: PMC6517228]. https://doi.org/10.1002/14651858.CD003703.pub3.
-
11.
Manzanares W, Lemieux M, Elke G, Langlois PL, Bloos F, Heyland DK. High-dose intravenous selenium does not improve clinical outcomes in the critically ill: a systematic review and meta-analysis. Crit Care. 2016;20(1):356. [PubMed ID: 27788688]. [PubMed Central ID: PMC5084353]. https://doi.org/10.1186/s13054-016-1529-5.
-
12.
Satici MO, Islam MM, Satici C, Uygun CN, Ademoglu E, Altunok I, et al. The role of a noninvasive index 'Spo2/ Fio2' in predicting mortality among patients with COVID-19 pneumonia. Am J Emerg Med. 2022;57:54-9. [PubMed ID: 35525158]. [PubMed Central ID: PMC9044731]. https://doi.org/10.1016/j.ajem.2022.04.036.
-
13.
Jamal A, Seifati SM, Dehghani Ashkezari M, Soleimani A. [The effect of selenium supplementation on the expression of some of the genes associated with inflammatory markers in diabetic hemodialysis patients]. Feyz. 2021;25(4):1055-63. Persian. https://doi.org/10.48307/fmsj.2021.25.4.1055.
-
14.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1(2):100-7. [PubMed ID: 21350618]. [PubMed Central ID: PMC3043330]. https://doi.org/10.4103/0976-500X.72352.
-
15.
Huang H, Jiao X, Xu Y, Han Q, Jiao W, Liu Y, et al. Dietary selenium supplementation alleviates immune toxicity in the hearts of chickens with lead-added drinking water. Avian Pathol. 2019;48(3):230-7. [PubMed ID: 30663336]. https://doi.org/10.1080/03079457.2019.1572102.
-
16.
Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium and human immune cell functions. II. Effect on cytotoxic lymphocytes and natural killer cells. Biol Trace Elem Res. 1994;41(1-2):115-27. [PubMed ID: 7946899]. https://doi.org/10.1007/BF02917222.
-
17.
Sakr Y, Reinhart K, Bloos F, Marx G, Russwurm S, Bauer M, et al. Time course and relationship between plasma selenium concentrations, systemic inflammatory response, sepsis, and multiorgan failure. Br J Anaesth. 2007;98(6):775-84. [PubMed ID: 17478454]. https://doi.org/10.1093/bja/aem091.
-
18.
Eden T, McAuliffe S, Crocombe D, Neville J, Ray S. Nutritional parameters and outcomes in patients admitted to intensive care with COVID-19: a retrospective single-centre service evaluation. BMJ Nutr Prev Health. 2021;4(2):416-24. [PubMed ID: 35024546]. [PubMed Central ID: PMC8350973]. https://doi.org/10.1136/bmjnph-2021-000270.
-
19.
Im JH, Je YS, Baek J, Chung MH, Kwon HY, Lee JS. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390-3. [PubMed ID: 32795605]. [PubMed Central ID: PMC7418699]. https://doi.org/10.1016/j.ijid.2020.08.018.
-
20.
Majeed M, Nagabhushanam K, Gowda S, Mundkur L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition. 2021;82:111053. [PubMed ID: 33321395]. [PubMed Central ID: PMC7657009]. https://doi.org/10.1016/j.nut.2020.111053.
-
21.
Razeghi Jahromi S, Moradi Tabriz H, Togha M, Ariyanfar S, Ghorbani Z, Naeeni S, et al. The correlation between serum selenium, zinc, and COVID-19 severity: an observational study. BMC Infect Dis. 2021;21(1):899. [PubMed ID: 34479494]. [PubMed Central ID: PMC8414458]. https://doi.org/10.1186/s12879-021-06617-3.
-
22.
Bologna C, Pone E. Clinical Study on the Efficacy and Safety of Arginine Administered Orally in Association with Other Active Ingredients for the Prevention and Treatment of Sarcopenia in Patients with COVID-19-Related Pneumonia, Hospitalized in a Sub-Intensive Care Unit. Healthcare (Basel). 2022;10(1):162. [PubMed ID: 35052325]. [PubMed Central ID: PMC8776134]. https://doi.org/10.3390/healthcare10010162.
-
23.
Ramezaninejad S, Sohrabi M, Alikhani A, Davoudi Badabi A, Abbaspour Kasgari H. [Relationship between Vitamin D, Vitamin C, and Selenium Intake and Disease Severity and Outcomes in Patients Hospitalized with COVID-19: A Retrospective Study]. J Mazandaran Univ Med Sci. 2022;32(213):73-81. Persian.
-
24.
Ambra R, Melloni S, Venneria E. Could Selenium Supplementation Prevent COVID-19? A Comprehensive Review of Available Studies. Molecules. 2023;28(10):4130. [PubMed ID: 37241870]. [PubMed Central ID: PMC10222736]. https://doi.org/10.3390/molecules28104130.