Therapeutic Targeting of the Regulators of Cancer Epigenomes
DOI:
https://doi.org/10.54133/ajms.v5i.128Keywords:
Epigenetics and cancer, Epigenomic and cancer, Cancer epigenome and epidrugsAbstract
Aim: To assess the value of targeting the various molecules that regulate the epigenome in the management of cancer. Method: Peer-reviewed articles were examined in PubMed, Google Scholar, and ResearchGate search tools using keywords given in the manuscript. Main points: Three major epigenomic modifications, namely DNA methylation, histone methylation, and histone acetylation, attracted the most research interest and led to a few globally approved drugs for the treatment of various malignancies. The DNA methylation profiles of cancer have been successfully employed in many aspects of the management of this disease. Conclusion: Epigenomic profiling of cancer specimens has already been successfully employed in the management of cancer in a handful of specialized clinics, and this application could be extended further following more in-depth investigations in this field.
Downloads
References
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-74. doi: 10.1016/j.cell.2011.02.013. DOI: https://doi.org/10.1016/j.cell.2011.02.013
Locke WJ, Guanzon D, Ma C, Liew YJ, Duesing KR, Fung KYC, et al. DNA Methylation Cancer Biomarkers: Translation to the Clinic. Front Genet. 2019;10:1150. doi: 10.3389/fgene.2019.01150. DOI: https://doi.org/10.3389/fgene.2019.01150
Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12(1):31-46. doi: 10.1158/2159-8290.CD-21-1059. DOI: https://doi.org/10.1158/2159-8290.CD-21-1059
Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11(10):726-34. doi: 10.1038/nrc3130. DOI: https://doi.org/10.1038/nrc3130
Berdasco M, Esteller M. Clinical epigenetics: seizing opportunities for translation. Nat Rev Genet. 2019;20(2):109-127. doi: 10.1038/s41576-018-0074-2. DOI: https://doi.org/10.1038/s41576-018-0074-2
Davalos V, Esteller M. Cancer epigenetics in clinical practice. CA Cancer J Clin. 2022. doi: 10.3322/caac.21765. DOI: https://doi.org/10.3322/caac.21765
Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23(7):781-783. doi: 10.1101/gad.1787609. DOI: https://doi.org/10.1101/gad.1787609
Waddington CH. The epigenotype. 1942. Int J Epidemiol. 2012;41(1):10-13. doi: 10.1093/ije/dyr184. DOI: https://doi.org/10.1093/ije/dyr184
Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148-1159. doi: 10.1056/NEJMra072067. DOI: https://doi.org/10.1056/NEJMra072067
Ramachandran S, Vogel L, Strahl BD, Dokholyan NV. Thermodynamic stability of histone H3 is a necessary but not sufficient driving force for its evolutionary conservation. PLoS Comput Biol. 2011;7(1):e1001042. doi: 10.1371/journal.pcbi.1001042. DOI: https://doi.org/10.1371/journal.pcbi.1001042
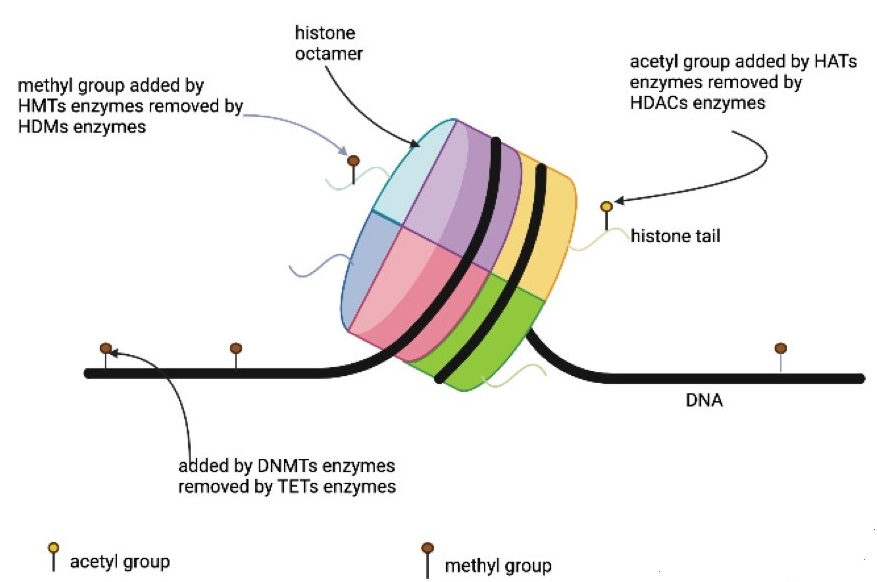
Annunziato A. DNA Packaging: Nucleosomes and chromatin. Nature Edu. 2008;1(1):26.
Zhou BR, Feng H, Kale S, Fox T, Khant H, de Val N, et al. Distinct structures and dynamics of chromatosomes with different human linker histone isoforms. Mol Cell. 2021;81(1):166-182.e6. doi: 10.1016/j.molcel.2020.10.038. DOI: https://doi.org/10.1016/j.molcel.2020.10.038
Biswas S, Rao CM. Epigenetic tools (The Writers, The Readers and The Erasers) and their implications in cancer therapy. Eur J Pharmacol. 2018;837:8-24. doi: 10.1016/j.ejphar.2018.08.021. DOI: https://doi.org/10.1016/j.ejphar.2018.08.021
Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4(2):143-153. doi: 10.1038/nrc1279. DOI: https://doi.org/10.1038/nrc1279
Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12-27. doi: 10.1016/j.cell.2012.06.013. DOI: https://doi.org/10.1016/j.cell.2012.06.013
You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22(1):9-20. doi: 10.1016/j.ccr.2012.06.008. DOI: https://doi.org/10.1016/j.ccr.2012.06.008
Shen H, Laird PW. Interplay between the cancer genome and epigenome. Cell. 2013;153(1):38-55. doi: 10.1016/j.cell.2013.03.008. DOI: https://doi.org/10.1016/j.cell.2013.03.008
Roy DM, Walsh LA, Chan TA. Driver mutations of cancer epigenomes. Protein Cell. 2014;5(4):265-296. doi: 10.1007/s13238-014-0031-6. DOI: https://doi.org/10.1007/s13238-014-0031-6
Lu Y, Chan YT, Tan HY, Li S, Wang N, Feng Y. Epigenetic regulation in human cancer: the potential role of epi-drug in cancer therapy. Mol Cancer. 2020;19(1):79. doi: 10.1186/s12943-020-01197-3. DOI: https://doi.org/10.1186/s12943-020-01197-3
Feng S, De Carvalho DD. Clinical advances in targeting epigenetics for cancer therapy. FEBS J. 2022;289(5):1214-1239. doi: 10.1111/febs.15750. DOI: https://doi.org/10.1111/febs.15750
Cheng Y, He C, Wang M, Ma X, Mo F, Yang S, et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther. 2019;4:62. doi: 10.1038/s41392-019-0095-0. DOI: https://doi.org/10.1038/s41392-019-0095-0
Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101-108. doi: 10.1038/nature11233. DOI: https://doi.org/10.1038/nature11233
Wei JW, Huang K, Yang C, Kang CS. Non-coding RNAs as regulators in epigenetics (Review). Oncol Rep. 2017;37(1):3-9. doi: 10.3892/or.2016.5236. DOI: https://doi.org/10.3892/or.2016.5236
Wu L, Zhou H, Zhang Q, Zhang J, Ni F, Liu C, et al. DNA methylation mediated by a microRNA pathway. Mol Cell. 2010;38(3):465-475. doi: 10.1016/j.molcel.2010.03.008. DOI: https://doi.org/10.1016/j.molcel.2010.03.008
Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301(5895):89-92. doi: 10.1038/301089a0. DOI: https://doi.org/10.1038/301089a0
Jin B, Li Y, Robertson KD. DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer. 2011;2(6):607-617. doi: 10.1177/1947601910393957. DOI: https://doi.org/10.1177/1947601910393957
Mattei AL, Bailly N, Meissner A. DNA methylation: a historical perspective. Trends Genet. 2022;38(7):676-707. doi: 10.1016/j.tig.2022.03.010. DOI: https://doi.org/10.1016/j.tig.2022.03.010
Baylin SB. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol. 2005;2(Suppl 1):S4-11. doi: 10.1038/ncponc0354. DOI: https://doi.org/10.1038/ncponc0354
Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8(4):286-298. doi: 10.1038/nrg2005. DOI: https://doi.org/10.1038/nrg2005
Aran D, Toperoff G, Rosenberg M, Hellman A. Replication timing-related and gene body-specific methylation of active human genes. Hum Mol Genet. 2011;20(4):670-680. doi: 10.1093/hmg/ddq513. DOI: https://doi.org/10.1093/hmg/ddq513
Clark SJ, Harrison J, Paul CL, Frommer M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 1994;22(15):2990-2997. doi: 10.1093/nar/22.15.2990. DOI: https://doi.org/10.1093/nar/22.15.2990
Gowher H, Liebert K, Hermann A, Xu G, Jeltsch A. Mechanism of stimulation of catalytic activity of Dnmt3A and Dnmt3B DNA-(cytosine-C5)-methyltransferases by Dnmt3L. J Biol Chem. 2005;280(14):13341-13348. doi: 10.1074/jbc.M413412200. DOI: https://doi.org/10.1074/jbc.M413412200
FDA approves azacitadine for newly diagnosed juvenile myelomonocytic leukaemia. FDA website, https://www.fda.gov. Last accessed March 2023.
Issa JJ, Roboz G, Rizzieri D, Jabbour E, Stock W, O'Connell C, et al. Safety and tolerability of guadecitabine (SGI-110) in patients with myelodysplastic syndrome and acute myeloid leukaemia: a multicenter, randomized, dose-escalation phase 1 study. Lancet Oncol. 2015;16(9):1099-1110. doi: 10.1016/S1470-2045(15)00038-8. DOI: https://doi.org/10.1016/S1470-2045(15)00038-8
Kantarjian HM, Roboz GJ, Kropf PL, Yee KWL, O'Connell CL, Tibes R, et al. Guadecitabine (SGI-110) in treatment-naive patients with acute myeloid leukaemia: phase 2 results from a multicenter, randomized, phase 1/2 trial. Lancet Oncol. 2017;18(10):1317-1326. doi: 10.1016/S1470-2045(17)30576-4. DOI: https://doi.org/10.1016/S1470-2045(17)30576-4
Daher-Reyes GS, Merchan BM, Yee KWL. Guadecitabine (SGI-110): an investigational drug for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Expert Opin Investig Drugs. 2019;28(10):835-849. doi: 10.1080/13543784.2019.1667331. DOI: https://doi.org/10.1080/13543784.2019.1667331
Roboz GJ, Döhner H, Gobbi M, Kropf PL, Mayer J, Krauter J, et al. Results from a global randomized phase 3 study of guadecitabine (G) vs. treatment choice (TC) in 815 patients with treatment naïve (TN) AML unfit for intensive chemotherapy (IC) ASTRAL-1 Study: Analysis by number of cycles. Blood 2019;134(Suppl. 1):2591. doi: doi: 10.1182/blood-2019-127253. DOI: https://doi.org/10.1182/blood-2019-127253
Brueckner B, Garcia Boy R, Siedlecki P, Musch T, Kliem HC, Zielenkiewicz, P et al. Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res. 2005;65(14):6305-6311. doi: 10.1158/0008-5472.CAN-04-2957. DOI: https://doi.org/10.1158/0008-5472.CAN-04-2957
Chen S, Wang Y, Zhou W, Li S, Peng J, Shi Z, et al. Identifying novel selective non-nucleoside DNA methyltransferase 1 inhibitors through docking-based virtual screening. J Med Chem. 2014;57(21):9028-9041. doi: 10.1021/jm501134e. DOI: https://doi.org/10.1021/jm501134e
Gilmartin AG, Groy A, Gore ER, Atkins C, Long ER, Montoute MN, et al. In vitro and in vivo induction of fetal hemoglobin with a reversible and selective DNMT1 inhibitor. Haematologica. 2021;106(7):1979-1987. doi: 10.3324/haematol.2020.248658. DOI: https://doi.org/10.3324/haematol.2020.248658
Robert MF, Morin S, Beaulieu N, Gauthier F, Chute IC, Barsalou A, et al. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat Genet. 2003;33(1):61-65. doi: 10.1038/ng1068. DOI: https://doi.org/10.1038/ng1068
Arce C, Segura-Pacheco B, Perez-Cardenas E, Taja-Chayeb L, Candelaria M, Dueñnas-Gonzalez A. Hydralazine target: from blood vessels to the epigenome. J Transl Med. 2006;4:10. doi: 10.1186/1479-5876-4-10. DOI: https://doi.org/10.1186/1479-5876-4-10
Zambrano P, Segura-Pacheco B, Perez-Cardenas E, Cetina L, Revilla-Vazquez A, Taja-Chayeb L, et al. A phase I study of hydralazine to demethylate and reactivate the expression of tumor suppressor genes. BMC Cancer. 2005;5:44. doi: 10.1186/1471-2407-5-44. DOI: https://doi.org/10.1186/1471-2407-5-44
Isakovic L, Saavedra OM, Llewellyn DB, Claridge S, Zhan L, Bernstein N, et al. Constrained (l-)-S-adenosyl-l-homocysteine (SAH) analogues as DNA methyltransferase inhibitors. Bioorg Med Chem Lett. 2009;19(10):2742-2746. doi: 10.1016/j.bmcl.2009.03.132. DOI: https://doi.org/10.1016/j.bmcl.2009.03.132
Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11(19 Pt 1):7033-7041. doi: 10.1158/1078-0432.CCR-05-0406. DOI: https://doi.org/10.1158/1078-0432.CCR-05-0406
Medina-Franco JL, López-Vallejo F, Kuck D, Lyko F. Natural products as DNA methyltransferase inhibitors: a computer-aided discovery approach. Mol Divers. 2011;15(2):293-304. doi: 10.1007/s11030-010-9262-5. DOI: https://doi.org/10.1007/s11030-010-9262-5
Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324(5929):930-5. doi: 10.1126/science.1170116. DOI: https://doi.org/10.1126/science.1170116
Rawłuszko-Wieczorek AA, Siera A, Jagodziński PP. TET proteins in cancer: Current 'state of the art'. Crit Rev Oncol Hematol. 2015;96(3):425-436. doi: 10.1016/j.critrevonc.2015.07.008. DOI: https://doi.org/10.1016/j.critrevonc.2015.07.008
Tefferi A, Pardanani A, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia. 2009;23(5):905-911. doi: 10.1038/leu.2009.47. DOI: https://doi.org/10.1038/leu.2009.47
Huang H, Jiang X, Li Z, Li Y, Song CX, He C, et al. TET1 plays an essential oncogenic role in MLL-rearranged leukemia. Proc Natl Acad Sci U S A. 2013;110(29):11994-11999. doi: 10.1073/pnas.1310656110. DOI: https://doi.org/10.1073/pnas.1310656110
Hsu CH, Peng KL, Kang ML, Chen YR, Yang YC, Tsai CH, et al. TET1 suppresses cancer invasion by activating the tissue inhibitors of metalloproteinases. Cell Rep. 2012;2(3):568-579. doi: 10.1016/j.celrep.2012.08.030. DOI: https://doi.org/10.1016/j.celrep.2012.08.030
Müller T, Gessi M, Waha A, Isselstein LJ, Luxen D, Freihoff D, et al. Nuclear exclusion of TET1 is associated with loss of 5-hydroxymethylcytosine in IDH1 wild-type gliomas. Am J Pathol. 2012;181(2):675-683. doi: 10.1016/j.ajpath.2012.04.017. DOI: https://doi.org/10.1016/j.ajpath.2012.04.017
Yang H, Liu Y, Bai F, Zhang JY, Ma SH, Liu J, et al. Tumor development is associated with decrease of TET gene expression and 5-methylcytosine hydroxylation. Oncogene. 2013;32(5):663-669. doi: 10.1038/onc.2012.67. DOI: https://doi.org/10.1038/onc.2012.67
Mahmood N, Rabbani SA. DNA methylation readers and cancer: Mechanistic and therapeutic applications. Front Oncol. 2019;9:489. doi: 10.3389/fonc.2019.00489. DOI: https://doi.org/10.3389/fonc.2019.00489
Ateeq B, Unterberger A, Szyf M, Rabbani SA. Pharmacological inhibition of DNA methylation induces proinvasive and prometastatic genes in vitro and in vivo. Neoplasia. 2008;10(3):266-278. doi: 10.1593/neo.07947. DOI: https://doi.org/10.1593/neo.07947
Pandey M, Shukla S, Gupta S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int J Cancer. 2010;126(11):2520-2533. doi: 10.1002/ijc.24988.
Mirza S, Sharma G, Parshad R, Gupta SD, Pandya P, Ralhan R. Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J Breast Cancer. 2013;16(1):23-31. doi: 10.4048/jbc.2013.16.1.23. DOI: https://doi.org/10.4048/jbc.2013.16.1.23
Campbell PM, Bovenzi V, Szyf M. Methylated DNA-binding protein 2 antisense inhibitors suppress tumourigenesis of human cancer cell lines in vitro and in vivo. Carcinogenesis. 2004;25(4):499-507. doi: 10.1093/carcin/bgh045. DOI: https://doi.org/10.1093/carcin/bgh045
Herz HM, Garruss A, Shilatifard A. SET for life: biochemical activities and biological functions of SET domain-containing proteins. Trends Biochem Sci. 2013;38(12):621-639. doi: 10.1016/j.tibs.2013.09.004. DOI: https://doi.org/10.1016/j.tibs.2013.09.004
Hatzimichael E, Crook T. Cancer epigenetics: new therapies and new challenges. J Drug Deliv. 2013;2013:529312. doi: 10.1155/2013/529312. DOI: https://doi.org/10.1155/2013/529312
Zhao Z, Shilatifard A. Epigenetic modifications of histones in cancer. Genome Biol. 2019;20(1):245. doi: 10.1186/s13059-019-1870-5. DOI: https://doi.org/10.1186/s13059-019-1870-5
Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14(11):1008-1016. doi: 10.1038/nsmb1337. DOI: https://doi.org/10.1038/nsmb1337
Copeland RA, Solomon ME, Richon VM. Protein methyltransferases as a target class for drug discovery. Nat Rev Drug Discov. 2009;8(9):724-732. doi: 10.1038/nrd2974. DOI: https://doi.org/10.1038/nrd2974
Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419(6907):624-629. doi: 10.1038/nature01075. DOI: https://doi.org/10.1038/nature01075
Knutson SK, Wigle TJ, Warholic NM, Sneeringer CJ, Allain CJ, Klaus CR, et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol. 2012;8(11):890-896. doi: 10.1038/nchembio.1084. DOI: https://doi.org/10.1038/nchembio.1084
McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492(7427):108-112. doi: 10.1038/nature11606. DOI: https://doi.org/10.1038/nature11606
Qi W, Chan H, Teng L, Li L, Chuai S, Zhang R, et al. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci U S A. 2012;109(52):21360-21365. doi: 10.1073/pnas.1210371110. DOI: https://doi.org/10.1073/pnas.1210371110
Verma SK, Tian X, LaFrance LV, Duquenne C, Suarez DP, Newlander KA, et al. Identification of Potent, Selective, Cell-Active Inhibitors of the Histone Lysine Methyltransferase EZH2. ACS Med Chem Lett. 2012;3(12):1091-6. doi: 10.1021/ml3003346. DOI: https://doi.org/10.1021/ml3003346
Garapaty-Rao S, Nasveschuk C, Gagnon A, Chan EY, Sandy P, Busby J, et al. Identification of EZH2 and EZH1 small molecule inhibitors with selective impact on diffuse large B cell lymphoma cell growth. Chem Biol. 2013;20(11):1329-1339. doi: 10.1016/j.chembiol.2013.09.013. DOI: https://doi.org/10.1016/j.chembiol.2013.09.013
Chan-Penebre E, Armstrong K, Drew A, Grassian AR, Feldman I, Knutson SK, et al. Selective killing of SMARCA2- and SMARCA4-deficient small cell carcinoma of the ovary, hypercalcemic type cells by inhibition of EZH2: In vitro and in vivo preclinical models. Mol Cancer Ther. 2017;16(5):850-860. doi: 10.1158/1535-7163.MCT-16-0678. DOI: https://doi.org/10.1158/1535-7163.MCT-16-0678
Ler LD, Ghosh S, Chai X, Thike AA, Heng HL, Siew EY, et al. Loss of tumor suppressor KDM6A amplifies PRC2-regulated transcriptional repression in bladder cancer and can be targeted through inhibition of EZH2. Sci Transl Med. 2017;9(378):eaai8312. doi: 10.1126/scitranslmed.aai8312. DOI: https://doi.org/10.1126/scitranslmed.aai8312
Wang Y, Chen SY, Karnezis AN, Colborne S, Santos ND, Lang JD, et al. The histone methyltransferase EZH2 is a therapeutic target in small cell carcinoma of the ovary, hypercalcaemic type. J Pathol. 2017;242(3):371-383. doi: 10.1002/path.4912. DOI: https://doi.org/10.1002/path.4912
Hess JL. MLL: a histone methyltransferase disrupted in leukemia. Trends Mol Med. 2004;10(10):500-507. doi: 10.1016/j.molmed.2004.08.005. DOI: https://doi.org/10.1016/j.molmed.2004.08.005
Wang X, Chen CW, Armstrong SA. The role of DOT1L in the maintenance of leukemia gene expression. Curr Opin Genet Dev. 2016;36:68-72. doi: 10.1016/j.gde.2016.03.015. DOI: https://doi.org/10.1016/j.gde.2016.03.015
Kuntimaddi A, Achille NJ, Thorpe J, Lokken AA, Singh R, Hemenway CS, et al. Degree of recruitment of DOT1L to MLL-AF9 defines level of H3K79 Di- and tri-methylation on target genes and transformation potential. Cell Rep. 2015;11(5):808-820. doi: 10.1016/j.celrep.2015.04.004. DOI: https://doi.org/10.1016/j.celrep.2015.04.004
Krivtsov AV, Feng Z, Lemieux ME, Faber J, Vempati S, Sinha AU, et al. H3K79 methylation profiles define murine and human MLL-AF4 leukemias. Cancer Cell. 2008;14(5):355-368. doi: 10.1016/j.ccr.2008.10.001. DOI: https://doi.org/10.1016/j.ccr.2008.10.001
Brown PJ, Müller S. Open access chemical probes for epigenetic targets. Future Med Chem. 2015;7(14):1901-1917. doi: 10.4155/fmc.15.127. DOI: https://doi.org/10.4155/fmc.15.127
Blanc RS, Richard S. Arginine methylation: The coming of age. Mol Cell. 2017;65(1):8-24. doi: 10.1016/j.molcel.2016.11.003. DOI: https://doi.org/10.1016/j.molcel.2016.11.003
Wysocka J, Allis CD, Coonrod S. Histone arginine methylation and its dynamic regulation. Front Biosci. 2006;11:344-355. doi: 10.2741/1802. DOI: https://doi.org/10.2741/1802
Zhao Q, Rank G, Tan YT, Li H, Moritz RL, Simpson RJ, et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol. 2009;16(3):304-311. doi: 10.1038/nsmb.1568. DOI: https://doi.org/10.1038/nsmb.1568
Eram MS, Shen Y, Szewczyk M, Wu H, Senisterra G, Li F, et al. A Potent, selective, and cell-active inhibitor of human type I protein arginine methyltransferases. ACS Chem Biol. 2016;11(3):772-781. doi: 10.1021/acschembio.5b00839. DOI: https://doi.org/10.1021/acschembio.5b00839
Dhar S, Vemulapalli V, Patananan AN, Huang GL, Di Lorenzo A, Richard S, et al. Loss of the major type I arginine methyltransferase PRMT1 causes substrate scavenging by other PRMTs. Sci Rep. 2013;3:1311. doi: 10.1038/srep01311. DOI: https://doi.org/10.1038/srep01311
Chan-Penebre E, Kuplast KG, Majer CR, Boriack-Sjodin PA, Wigle TJ, Johnston LD, et al. A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat Chem Biol. 2015;11(6):432-437. doi: 10.1038/nchembio.1810. DOI: https://doi.org/10.1038/nchembio.1810
Duncan KW, Rioux N, Boriack-Sjodin PA, Munchhof MJ, Reiter LA, Majer CR, et al. Structure and property guided design in the identification of PRMT5 tool compound EPZ015666. ACS Med Chem Lett. 2015;7(2):162-166. doi: 10.1021/acsmedchemlett.5b00380. DOI: https://doi.org/10.1021/acsmedchemlett.5b00380
NCT02783300 an open-label dose escalation study @https://clinicaltrials.gov. Last accessed March 2023.
Punnia-Moorthy G, Hersey P, Emran AA, Tiffen J. Lysine demethylases: Promising drug targets in melanoma and other cancers. Front Genet. 2021;12:680633. doi: 10.3389/fgene.2021.680633. DOI: https://doi.org/10.3389/fgene.2021.680633
Walport LJ, Hopkinson RJ, Schofield CJ. Mechanisms of human histone and nucleic acid demethylases. Curr Opin Chem Biol. 2012;16(5-6):525-534. doi: 10.1016/j.cbpa.2012.09.015. DOI: https://doi.org/10.1016/j.cbpa.2012.09.015
Narayanan SP, Singh S, Gupta A, Yadav S, Singh SR, Shukla S. Integrated genomic analyses identify KDM1A's role in cell proliferation via modulating E2F signaling activity and associate with poor clinical outcome in oral cancer. Cancer Lett. 2015;367(2):162-72. doi: 10.1016/j.canlet.2015.07.022. DOI: https://doi.org/10.1016/j.canlet.2015.07.022
Ciccone DN, Su H, Hevi S, Gay F, Lei H, Bajko J, et al. KDM1B is a histone H3K4 demethylase required to establish maternal genomic imprints. Nature. 2009;461(7262):415-418. doi: 10.1038/nature08315. DOI: https://doi.org/10.1038/nature08315
Chang B, Chen Y, Zhao Y, Bruick RK. JMJD6 is a histone arginine demethylase. Science. 2007;318(5849):444-447. doi: 10.1126/science.1145801. DOI: https://doi.org/10.1126/science.1145801
Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25(11):1106-1118. doi: 10.1002/bies.10357. DOI: https://doi.org/10.1002/bies.10357
Musselman CA, Khorasanizadeh S, Kutateladze TG. Towards understanding methyllysine readout. Biochim Biophys Acta. 2014;1839(8):686-693. doi: 10.1016/j.bbagrm.2014.04.001. DOI: https://doi.org/10.1016/j.bbagrm.2014.04.001
Gayatri S, Bedford MT. Readers of histone methylarginine marks. Biochim Biophys Acta. 2014;1839(8):702-710. doi: 10.1016/j.bbagrm.2014.02.015. DOI: https://doi.org/10.1016/j.bbagrm.2014.02.015
Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693-705. doi: 10.1016/j.cell.2007.02.005. DOI: https://doi.org/10.1016/j.cell.2007.02.005
McBrian MA, Behbahan IS, Ferrari R, Su T, Huang TW, Li K, et al. Histone acetylation regulates intracellular pH. Mol Cell. 2013;49(2):310-321. doi: 10.1016/j.molcel.2012.10.025. DOI: https://doi.org/10.1016/j.molcel.2012.10.025
Audia JE, Campbell RM. Histone modifications and cancer. Cold Spring Harb Perspect Biol. 2016;8(4):a019521. doi: 10.1101/cshperspect.a019521. DOI: https://doi.org/10.1101/cshperspect.a019521
Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37(4):391-400. doi: 10.1038/ng1531. DOI: https://doi.org/10.1038/ng1531
Seligson DB, Horvath S, McBrian MA, Mah V, Yu H, Tze S, et al. Global levels of histone modifications predict prognosis in different cancers. Am J Pathol. 2009;174(5):1619-1628. doi: 10.2353/ajpath.2009.080874. DOI: https://doi.org/10.2353/ajpath.2009.080874
Baylin SB, Jones PA. Epigenetic determinants of cancer. Cold Spring Harb Perspect Biol. 2016;8(9):a019505. doi: 10.1101/cshperspect.a019505. DOI: https://doi.org/10.1101/cshperspect.a019505
Hou X, Li Y, Luo RZ, Fu JH, He JH, Zhang LJ, et al. High expression of the transcriptional co-activator p300 predicts poor survival in resectable non-small cell lung cancers. Eur J Surg Oncol. 2012;38(6):523-530. doi: 10.1016/j.ejso.2012.02.180. DOI: https://doi.org/10.1016/j.ejso.2012.02.180
Di Cerbo V, Schneider R. Cancers with wrong HATs: the impact of acetylation. Brief Funct Genomics. 2013;12(3):231-243. doi: 10.1093/bfgp/els065. DOI: https://doi.org/10.1093/bfgp/els065
Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6(4):a018713. doi: 10.1101/cshperspect.a018713. DOI: https://doi.org/10.1101/cshperspect.a018713
Dell'Aversana C, Lepore I, Altucci L. HDAC modulation and cell death in the clinic. Exp Cell Res. 2012;318(11):1229-1244. doi: 10.1016/j.yexcr.2012.01.025. DOI: https://doi.org/10.1016/j.yexcr.2012.01.025
Grabowska W, Sikora E, Bielak-Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology. 2017;18(4):447-476. doi: 10.1007/s10522-017-9685-9. DOI: https://doi.org/10.1007/s10522-017-9685-9
Qi J. Bromodomain and extraterminal domain inhibitors (BETi) for cancer therapy: chemical modulation of chromatin structure. Cold Spring Harb Perspect Biol. 2014;6(12):a018663. doi: 10.1101/cshperspect.a018663. DOI: https://doi.org/10.1101/cshperspect.a018663
Schaefer U. Pharmacological inhibition of bromodomain-containing proteins in inflammation. Cold Spring Harb Perspect Biol. 2014;6(6):a018671. doi: 10.1101/cshperspect.a018671. DOI: https://doi.org/10.1101/cshperspect.a018671
Derissen EJ, Beijnen JH, Schellens JH. Concise drug review: azacitidine and decitabine. Oncologist. 2013;18(5):619-624. doi: 10.1634/theoncologist.2012-0465. DOI: https://doi.org/10.1634/theoncologist.2012-0465
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomized, open-label, phase III study. Lancet Oncol. 2009;10(3):223-232. doi: 10.1016/S1470-2045(09)70003-8. DOI: https://doi.org/10.1016/S1470-2045(09)70003-8
Garcia-Manero G, Roboz G, Walsh K, Kantarjian H, Ritchie E, Kropf P, et al. Guadecitabine (SGI-110) in patients with intermediate or high-risk myelodysplastic syndromes: phase 2 results from a multicenter, open-label, randomized, phase 1/2 trial. Lancet Haematol. 2019;6(6):e317-e327. doi: 10.1016/S2352-3026(19)30029-8. DOI: https://doi.org/10.1016/S2352-3026(19)30029-8
Ou Y, Zhang Q, Tang Y, Lu Z, Lu X, Zhou X, et al. DNA methylation enzyme inhibitor RG108 suppresses the radioresistance of esophageal cancer. Oncol Rep. 2018;39(3):993-1002. doi: 10.3892/or.2018.6210. DOI: https://doi.org/10.3892/or.2018.6210
Amato RJ, Stephenson J, Hotte S, Nemunaitis J, Bélanger K, Reid G, et al. MG98, a second-generation DNMT1 inhibitor, in the treatment of advanced renal cell carcinoma. Cancer Invest. 2012;30(5):415-421. doi: 10.3109/07357907.2012.675381. DOI: https://doi.org/10.3109/07357907.2012.675381
Deng C, Lu Q, Zhang Z, Rao T, Attwood J, Yung R, et al. Hydralazine may induce autoimmunity by inhibiting extracellular signal-regulated kinase pathway signaling. Arthritis Rheum. 2003;48(3):746-756. doi: 10.1002/art.10833. DOI: https://doi.org/10.1002/art.10833
Segura-Pacheco B, Trejo-Becerril C, Perez-Cardenas E, Taja-Chayeb L, Mariscal I, Chavez A, et al. Reactivation of tumor suppressor genes by the cardiovascular drugs hydralazine and procainamide and their potential use in cancer therapy. Clin Cancer Res. 2003;9(5):1596-1603.
Song Y, Zhang C. Hydralazine inhibits human cervical cancer cell growth in vitro in association with APC demethylation and re-expression. Cancer Chemother Pharmacol. 2009;63(4):605-613. doi: 10.1007/s00280-008-0773-z. DOI: https://doi.org/10.1007/s00280-008-0773-z
Graça I, Sousa EJ, Costa-Pinheiro P, Vieira FQ, Torres-Ferreira J, Martins MG, et al. Anti-neoplastic properties of hydralazine in prostate cancer. Oncotarget. 2014;5(15):5950-5964. doi: 10.18632/oncotarget.1909. DOI: https://doi.org/10.18632/oncotarget.1909
Villar-Garea A, Fraga MF, Espada J, Esteller M. Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells. Cancer Res. 2003;63(16):4984-4989. PMID: 12941824.
Tada M, Imazeki F, Fukai K, Sakamoto A, Arai M, Mikata R, et al. Procaine inhibits the proliferation and DNA methylation in human hepatoma cells. Hepatol Int. 2007;1(3):355-364. doi: 10.1007/s12072-007-9014-5. DOI: https://doi.org/10.1007/s12072-007-9014-5
Gao Z, Xu Z, Hung MS, Lin YC, Wang T, Gong M, et al. Procaine and procainamide inhibit the Wnt canonical pathway by promoter demethylation of WIF-1 in lung cancer cells. Oncol Rep. 2009;22(6):1479-1484. doi: 10.3892/or_00000590. DOI: https://doi.org/10.3892/or_00000590
Pandey M, Shukla S, Gupta S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int J Cancer. 2010;126(11):2520-2533. doi: 10.1002/ijc.24988. DOI: https://doi.org/10.1002/ijc.24988
Straining R, Eighmy W. Tazemetostat: EZH2 inhibitor. J Adv Pract Oncol. 2022;13(2):158-163. doi: 10.6004/jadpro.2022.13.2.7. DOI: https://doi.org/10.6004/jadpro.2022.13.2.7
He Y, Xu W, Xiao YT, Huang H, Gu D, Ren S. Targeting signaling pathways in prostate cancer: mechanisms and clinical trials. Signal Transduct Target Ther. 2022;7(1):198. doi: 10.1038/s41392-022-01042-7. DOI: https://doi.org/10.1038/s41392-022-01042-7
EZHARMIA® approved in Japan as first dual EZH1 and EZH2 inhibitor therapy for patients with adult T-cell leukaemia/lymphoma, press release dated 26th September 2022 from Daiichi-Sankyo. https://www.daiichisankyo.com. Last accessed March 2023.
Stein EM, Garcia-Manero G, Rizzieri DA, Tibes R, Berdeja JG, Savona MR, et al. The DOT1L inhibitor pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood. 2018;131(24):2661-2669. doi: 10.1182/blood-2017-12-818948. DOI: https://doi.org/10.1182/blood-2017-12-818948
Menghrajani K, Cai SF, Devlin SM, Armstrong SA, Piekarz R, Rudek M, et al. A phase Ib/II study of the histone methyltransferase inhibitor pinometostat in combination with azacitidine in patients with 11q23-rearranged acute myeloid leukemia. Blood. 2019;134(Suppl. 1): 2655. doi: 10.1182/blood-2019-121926. DOI: https://doi.org/10.1182/blood-2019-121926
Siu LL, Rasco DW. Postel Vinay S. 3410–METEOR-1: A phase I study of GSK3326595, a first-in-class protein arginine methyltransferase 5 (PRMT5) inhibitor, in advanced solid tumors. Ann Oncol. 2019;30(Suppl. 5):v159-v193. DOI: https://doi.org/10.1093/annonc/mdz244
Blaheta RA, Cinatl J. Anti-tumor mechanisms of valproate: a novel role for an old drug. Med Res Rev. 2002;22(5):492-511. doi: 10.1002/med.10017. DOI: https://doi.org/10.1002/med.10017
Yoon S, Eom GH. HDAC and HDAC inhibitor: From cancer to cardiovascular diseases. Chonnam Med J. 2016;52(1):1-11. doi: 10.4068/cmj.2016.52.1.1. DOI: https://doi.org/10.4068/cmj.2016.52.1.1
Foss F, Advani R, Duvic M, Hymes KB, Intragumtornchai T, Lekhakula A, et al. A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015;168(6):811-819. doi: 10.1111/bjh.13222. DOI: https://doi.org/10.1111/bjh.13222
Prince HM, Bishton MJ, Johnstone RW. Panobinostat (LBH589): a potent pan-deacetylase inhibitor with promising activity against hematologic and solid tumors. Future Oncol. 2009;5(5):601-612. doi: 10.2217/fon.09.36. DOI: https://doi.org/10.2217/fon.09.36
Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280(2):233-241. doi: 10.1016/j.canlet.2009.02.019. DOI: https://doi.org/10.1016/j.canlet.2009.02.019
Connolly RM, Rudek MA, Piekarz R. Entinostat: a promising treatment option for patients with advanced breast cancer. Future Oncol. 2017;13(13):1137-1148. doi: 10.2217/fon-2016-0526. DOI: https://doi.org/10.2217/fon-2016-0526
VanderMolen KM, McCulloch W, Pearce CJ, Oberlies NH. Romidepsin (Istodax, NSC 630176, FR901228, FK228, depsipeptide): a natural product recently approved for cutaneous T-cell lymphoma. J Antibiot (Tokyo). 2011;64(8):525-531. doi: 10.1038/ja.2011.35. DOI: https://doi.org/10.1038/ja.2011.35
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 1969 Al-Rafidain Journal of Medical Sciences ( ISSN: 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











