Choline chloride based eutectic solvent: a highly efficient reaction media for the synthesis of 3,4-dihydropyrimidin-2(1H)-thiones
DOI:
https://doi.org/10.52547/jcc.4.3.4Keywords:
Deep Eutectic Solvent, Green solvent, Dihydropyrimidine, Multicomponent, Biginelli condensationAbstract
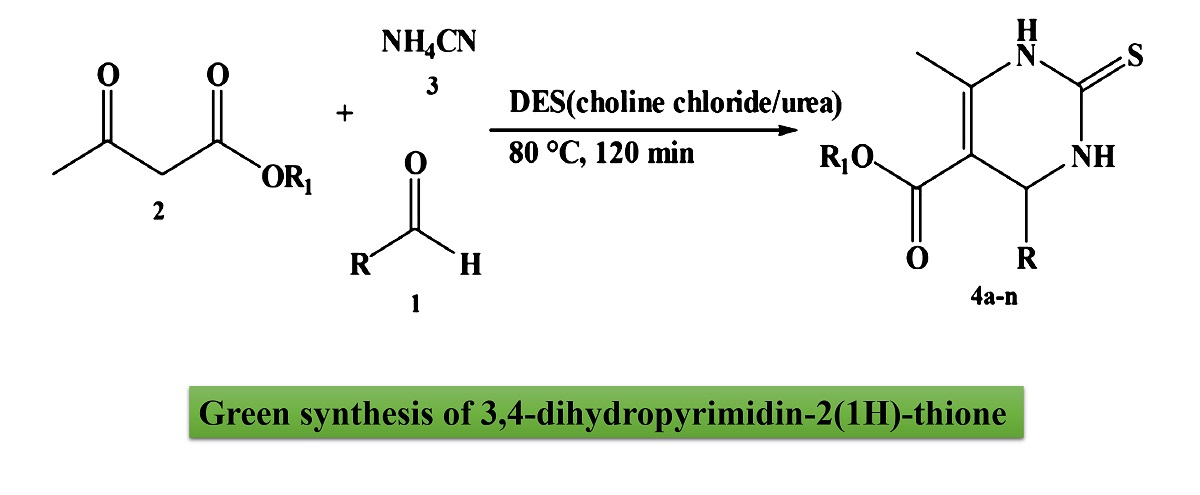
A mild and green protocol was developed to the 3,4-dihydropyrimidin-2(1H)-thione derivatives preparation in deep eutectic solvent without the use of a catalyst or any other additive. The procedure offers a number of benefits, including clean reaction profile, avoiding the use of typical toxic catalysts, an easy workup procedure, short reaction times, and low prices.
References
P. Liu, J.-W. Hao, L.-P. Mo, Z.-H. Zhang, Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions, RSC Advances 5(60) (2015) 48675-48704.
C. Ruß, B. König, Low melting mixtures in organic synthesis–an alternative to ionic liquids?, Green Chemistry 14(11) (2012) 2969-2982.
Q. Zhang, K.D.O. Vigier, S. Royer, F. Jerome, Deep eutectic solvents: syntheses, properties and applications, Chemical Society Reviews 41(21) (2012) 7108-7146.
Y. Yu, X. Lu, Q. Zhou, K. Dong, H. Yao, S. Zhang, Biodegradable naphthenic acid ionic liquids: synthesis, characterization, and quantitative structure–biodegradation relationship, Chemistry–A European Journal 14(35) (2008) 11174-11182.
K.D. Weaver, H.J. Kim, J. Sun, D.R. MacFarlane, G.D. Elliott, Cyto-toxicity and biocompatibility of a family of choline phosphate ionic liquids designed for pharmaceutical applications, Green Chemistry 12(3) (2010) 507-513.
S.K. Ghosh, R. Nagarajan, Deep eutectic solvent mediated synthesis of quinazolinones and dihydroquinazolinones: synthesis of natural products and drugs, RSC Advances 6(33) (2016) 27378-27387.
P.M. Pawar, K.J. Jarag, G.S. Shankarling, Environmentally benign and energy efficient methodology for condensation: an interesting facet to the classical Perkin reaction, Green Chemistry 13(8) (2011) 2130-2134.
G. Imperato, E. Eibler, J. Niedermaier, B. König, Low-melting sugar–urea–salt mixtures as solvents for Diels–Alder reactions, Chemical Communications (9) (2005) 1170-1172.
F. Ilgen, B. König, Organic reactions in low melting mixtures based on carbohydrates and L-carnitine—a comparison, Green Chemistry 11(6) (2009) 848-854.
G. Imperato, S. Höger, D. Lenoir, B. Koenig, Low melting sugar–urea–salt mixtures as solvents for organic reactions—estimation of polarity and use in catalysis, Green Chemistry 8(12) (2006) 1051-1055.
U.B. Patil, A.S. Singh, J.M. Nagarkar, Choline chloride based eutectic solvent: an efficient and reusable solvent system for the synthesis of primary amides from aldehydes and from nitriles, RSC Advances 4(3) (2014) 1102-1106.
A. Shaabani, S.E. Hooshmand, M.T. Nazeri, R. Afshari, S. Ghasemi, Deep eutectic solvent as a highly efficient reaction media for the one-pot synthesis of benzo-fused seven-membered heterocycles, Tetrahedron Letters 57(33) (2016) 3727-3730.
S. Gore, S. Baskaran, B. Koenig, Efficient synthesis of 3, 4-dihydropyrimidin-2-ones in low melting tartaric acid–urea mixtures, Green Chemistry 13(4) (2011) 1009-1013.
Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte, Y.H. Choi, Ionic liquids and deep eutectic solvents in natural products research: mixtures of solids as extraction solvents, Journal of natural products 76(11) (2013) 2162-2173.
A.P. Abbott, G. Capper, D.L. Davies, R.K. Rasheed, V. Tambyrajah, Novel solvent properties of choline chloride/urea mixtures, Chemical Communications (1) (2003) 70-71.
R. Gautam, N. Kumar, J.G. Lynam, Theoretical and experimental study of choline chloride-carboxylic acid deep eutectic solvents and their hydrogen bonds, Journal of Molecular Structure 1222 (2020) 128849.
P. Biginelli, Aldehyde-urea derivatives of aceto-and oxaloacetic acids, Gazz. chim. ital 23(1) (1893) 360-413.
M. Karimi, E. Sadeghi, S.K. Bigdeli, M. Zahedifar, Synthesis, feasibility study of production of singlet oxygen and hydroxyl radical and performance in antibacterial activity of ZnS: Eu QDs, Journal of Composites and Compounds 4(11) (2022) 77-82.
R. Kaur, S. Chaudhary, K. Kumar, M.K. Gupta, R.K. Rawal, Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review, European journal of medicinal chemistry 132 (2017) 108-134.
A.H. Saleh, D. Kumar, I. Sirakov, P. Shafiee, M. Arefian, Application of nano compounds for the prevention, diagnosis, and treatment of SARS-coronavirus: A review, Journal of Composites and Compounds 3(9) (2021) 230-246.
A. Bakhtiari, A. Cheshmi, M. Naeimi, S.M. Fathabad, M. Aliasghari, A.M. Chahardehi, S. Hassani, V. Elhami, Synthesis and characterization of the novel 80S bioactive glass: bioactivity, biocompatibility, cytotoxicity, Journal of Composites and Compounds 2(4) (2020) 110-114.
H. Murata, H. Ishitani, M. Iwamoto, Synthesis of Biginelli dihydropyrimidinone derivatives with various substituents on aluminium-planted mesoporous silica catalyst, Organic and biomolecular chemistry 8(5) (2010) 1202-1211.
T.U. Mayer, T.M. Kapoor, S.J. Haggarty, R.W. King, S.L. Schreiber, T.J. Mitchison, Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen, Science 286(5441) (1999) 971-974.
D. Russowsky, R.m.F. Canto, S.A. Sanches, M.G. D’Oca, Â. de Fátima, R.A. Pilli, L.K. Kohn, M.A. Antoˆnio, J.E. de Carvalho, Synthesis and differential antiproliferative activity of Biginelli compounds against cancer cell lines: monastrol, oxo-monastrol and oxygenated analogues, Bioorganic chemistry 34(4) (2006) 173-182.
R.S. Chang, T.-B. Chen, S.S. O'Malley, D.J. Pettibone, J. DiSalvo, B. Francis, M.G. Bock, R. Freidinger, D. Nagarathnam, S.W. Miao, In vitro studies on L-771,688 (SNAP 6383), a new potent and selective ?1A-adrenoceptor antagonist, European journal of pharmacology 409(3) (2000) 301-312.
K.S. Atwal, B.N. Swanson, S.E. Unger, D.M. Floyd, S. Moreland, A. Hedberg, B.C. O'Reilly, Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1, 2, 3, 4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents, Journal of medicinal chemistry 34(2) (1991) 806-811.
R.N. Azadani, M. Sabbagh, H. Salehi, A. Cheshmi, A. Raza, B. Kumari, G. Erabi, Sol-gel: Uncomplicated, routine and affordable synthesis procedure for utilization of composites in drug delivery, Journal of Composites and Compounds 3(6) (2021) 57-70.
P. Fazlali, A. Mahdian, M.S. Soheilifar, S.M. Amininasab, P. Shafiee, I.A. Wani, A.M.A.A. AL-Mokaram, Nanobiosensors for early detection of neurodegenerative disease, Journal of Composites and Compounds 4(10) (2022) 24-36.
P. Biginelli, Aldureides of ethylic acetoacetate and ethylic oxalacetate, Gazz. Chim. Ital 23 (1893) 360-416.
V.P.S. Sidhu, R. Borges, M. Yusuf, S. Mahmoudi, S.F. Ghorbani, M. Hosseinikia, P. Salahshour, F. Sadeghi, M. Arefian, A comprehensive review of bioactive glass: synthesis, ion substitution, application, challenges, and future perspectives, Journal of Composites and Compounds 3(9) (2021) 247-261.
S. Padervand, M. Amiri, Optimization of electrolyte concentration for surface modification of tantalum using plasma electrolytic nitridation, International Journal of Refractory Metals and Hard Materials 87 (2020) 105146.
S. Askari, M. Ghashang, G. Sohrabi, Synthesis and mechanical properties of Bi2O3-Al4Bi2O9 nanopowders, Journal of Composites and Compounds 2(5) (2020) 171-174.
P. Shafiee, M.R. Nafchi, S. Eskandarinezhad, S. Mahmoudi, E. Ahmadi, Sol-gel zinc oxide nanoparticles: advances in synthesis and applications, Synthesis and Sintering 1(4) (2021) 242-254.
N. Ahmed, Z.N. Siddiqui, Sulphated silica tungstic acid as a highly efficient and recyclable solid acid catalyst for the synthesis of tetrahydropyrimidines and dihydropyrimidines, Journal of Molecular Catalysis A: Chemical 387 (2014) 45-56.
S.D. Salim, K.G. Akamanchi, Sulfated tungstate: an alternative, eco-friendly catalyst for Biginelli reaction, Catalysis Communications 12(12) (2011) 1153-1156.
C.K. Khatri, D.S. Rekunge, G.U. Chaturbhuj, Sulfated polyborate: a new and eco-friendly catalyst for one-pot multi-component synthesis of 3, 4-dihydropyrimidin-2 (1 H)-ones/thiones via Biginelli reaction, New Journal of Chemistry 40(12) (2016) 10412-10417.
S. Khaksar, S.M. Vahdat, R.N. Moghaddamnejad, Pentafluorophenylammonium triflate: an efficient, practical, and cost-effective organocatalyst for the Biginelli reaction, Monatshefte für Chemie-Chemical Monthly 143(12) (2012) 1671-1674.
O.M. Singh, M.L. Singh, S.J. Singh, SNCL2-CATALYZED SYNTHESIS OF DIHYDROPYRIMIDINONES UNDER SOLVENT-FREE CONDITIONS, Heterocyclic Communications 13(5) (2007) 277-282.
J. Safari, S. Gandomi-Ravandi, S. Ashiri, Organosilane sulfonated graphene oxide in the Biginelli and Biginelli-like reactions, New Journal of Chemistry 40(1) (2016) 512-520.
S. Nagarajan, T.M. Shaikh, E. Kandasamy, Synthesis of 1-alkyl triazolium triflate room temperature ionic liquids and their catalytic studies in multi-component Biginelli reaction, Journal of Chemical Sciences 127(9) (2015) 1539-1545.
Q. Liu, N. Pan, J. Xu, W. Zhang, F. Kong, Microwave-assisted and iodine-catalyzed synthesis of dihydropyrimidin-2-thiones via biginelli reaction under solvent-free conditions, Synthetic Communications 43(1) (2013) 139-146.
D. Bhuyan, M. Saikia, L. Saikia, ZnO nanoparticles embedded in SBA-15 as an efficient heterogeneous catalyst for the synthesis of dihydropyrimidinones via Biginelli condensation reaction, Microporous and Mesoporous Materials 256 (2018) 39-48.
M. Moghaddas, A. Davoodnia, M.M. Heravi, N. Tavakoli-Hoseini, Sulfonated carbon catalyzed Biginelli reaction for one-pot synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones and-thiones, Chinese Journal of Catalysis 33(4-6) (2012) 706-710.
S. Padervand, S. Sarihi, S. Mousavi Khoei, N. Shakiba, Determining the Optimal Processing Time for Tantalum Surface Modification through Plasma Electrolytic Nitridation, Journal of Surface Investigation: X-ray, Synchrotron and Neutron Techniques 15(4) (2021) 877-884.
S. Padervand, S. Khoei, N. Shakiba, Access to Optimum Working Voltage of Plasma Electrolytic Nitridation of Tantalum Alloys, Surface Engineering and Applied Electrochemistry 56(6) (2020) 704-711.
N. Aboualigaledari, M. Rahmani, A review on the synthesis of the TiO2-based photocatalyst for the environmental purification, Journal of Composites and Compounds 3(6) (2021) 25-42.
A.J. Rad, Synthesis of copper oxide nanoparticles on activated carbon for pollutant removal in Tartrazine structure, Journal of Composites and Compounds 2(3) (2020) 99-104.
E.M. Abdelraheem, S. Khaksar, K. Kurpiewska, J. Kalinowska-T?u?cik, S. Shaabani, A. Dömling, Two Step Macrocycle Synthesis by Classical Ugi Reaction, The Journal of organic chemistry (2018).
S. Khaksar, M. Gholami, An eco-benign and highly efficient access to dihydro-1H-indeno [1, 2-b] pyridines in 2, 2, 2-trifluoroethanol, Journal of Molecular Liquids 196 (2014) 159-162.
S. Khaksar, H. Radpeyma, Pentafluorophenylammonium triflate: A highly efficient catalyst for the synthesis of quinoxaline derivatives in water, Comptes Rendus Chimie 17(10) (2014) 1023-1027.
S. Khaksar, S.M. Talesh, Three-component one-pot synthesis of 2, 3-dihydroquinazolin-4 (1H)-one derivatives in 2, 2, 2-trifluoroethanol, Comptes Rendus Chimie 15(9) (2012) 779-783.
M.R. Nafchi, R. Ebrahimi-kahrizsangi, Synthesis of Zn-Co-TiO2 nanocomposite coatings by electrodeposition with photocatalytic and antifungal activities, Journal of Composites and Compounds 3(9) (2021) 213-217.
C.K. Khatri, S.M. Potadar, G.U. Chaturbhuj, A reactant promoted solvent free synthesis of 3, 4-dihydropyrimidin-2 (1H)-thione analogues using ammonium thiocyanate, Tetrahedron Letters 58(18) (2017) 1778-1780.
S. Mohammadi, Z. Mohammadi, Functionalized NiFe2O4/mesopore silica anchored to guanidine nanocomposite as a catalyst for synthesis of 4H-chromenes under ultrasonic irradiation, Journal of Composites and Compounds 3(7) (2021) 84-90.
Y. Cui, C. Li, M. Bao, Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones/thiones, Green Processing and Synthesis 8(1) (2019) 568-576.
N.S. Pawar, P.N. Patil, R.N. Pachpande, An Efficient Synthesis and Antibacterial Activity of Some Novel 3, 4–Dihydropyrimidin-2-(1H)-Ones, Chemistry Proceedings 8(1) (2021) 37.
A. Mobinikhaledi, A. Yazdanipour, M. Ghashang, A green one-pot Biginelli synthesis of 3, 4-dihydropyrimidin-2-(1H)-ones catalyzed by novel Aurivillius nanostructures under solvent-free conditions, Reaction Kinetics, Mechanisms and Catalysis 119(2) (2016) 511-522.
Published
How to Cite
License
Copyright (c) 2022 The University of Georgia Publishing House (UGPH)

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors will be asked, upon acceptance of an article, to transfer copyright of the article to the Publisher. This will ensure the widest possible dissemination of information under copyright laws. The submitted materials may be considered for inclusion but can not be returned.
Licensing: The JCC articles are licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source (appropriate citation), provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
*Author rights
As an author you (or your employer or institution) have certain rights to reuse your work.


