Synthesis, characterization and application of a bio-derived ZnO nano-powder from spinacia oleracea leaf extract for the removal of BOD and COD from tannery wastewater
DOI:
https://doi.org/10.52493/j.cote.2021.1.08Keywords:
ZnO-nanopowders, Bio-derived, Bio-sorbents, Tan liquor, Green synthesisAbstract
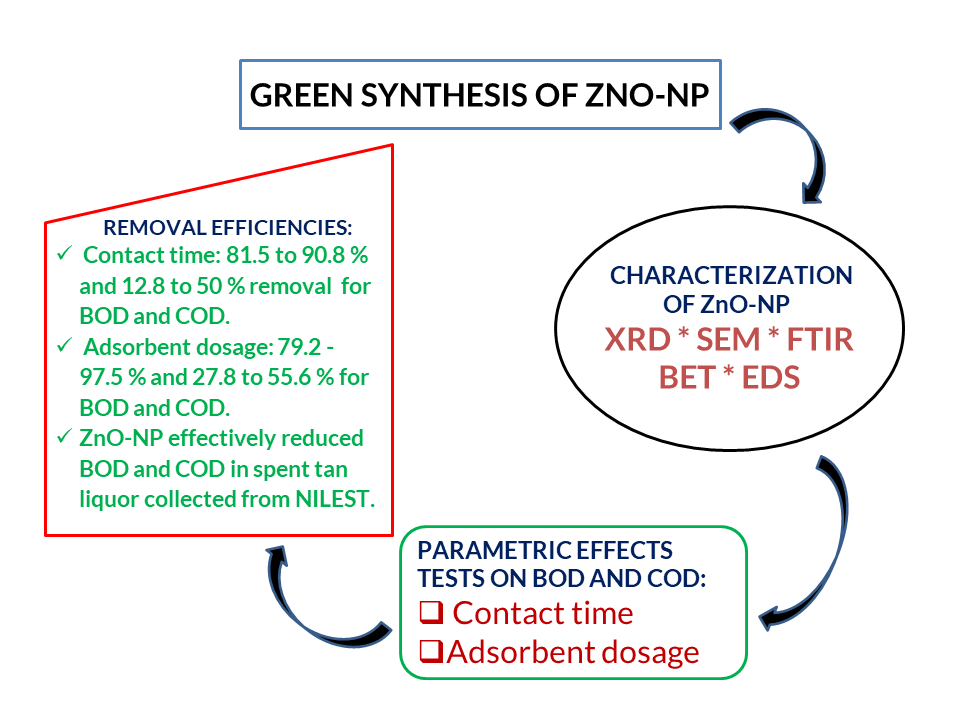
Biochemical Oxygen Demand (BOD) and Chemical Oxygen Demand (COD) as biological and chemical pollutants of wastewater are renowned environmental problems. A study of the performance of BOD and COD degradation via adsorption was undertaken using Bio-derived ZnO nanopowder (ZnO-NP), synthesized using leaf extract of Spinacia oleracea, and Zn (NO3)2 at 500 0C, following a simple and green approach. XRD, SEM, FTIR, EDS and BET analysis were used to characterize the nano-adsorbent. EDS spectrum recorded elemental weight compositions of 67.33% and 32.67% for Zn and O, while the FTIR absorption peaks revealed the presence of Zn-O–H and Zn-O. Surface area analysis revealed the mesoporous structure of the ZnO-NPs. The reduction efficiency of the ZnO-NPs was evaluated in the presence of raw tannery wastewater by application of treatment time and adsorbent dosage as parametric factors; results obtained were compared to environmental regulatory limits (WHO and NESREA). Contact times reported removal efficiencies of 81.5-90.8 % and 12.8-50 % for BOD and COD, while adsorbent dosage-influenced BOD and COD removal at an optimum contact time of 30 minutes was found to be 79.2 - 97.5 % and 27.8 to 55.6 % respectively. This study reveals that ZnO nanopowder is better applied as BOD reductants over COD for spent tan liquor.
References
Amin, M. T., Alazba, A. A., & Manzoor, U. (2014). A review of removal of pollutants from water/wastewater using different types of nanomaterials. Advances in Materials Science and Engineering, 2014. https://doi.org/10.1155/2014/825910
Andrew R. (2019). Processing, structure, and properties of alumina ceramics, Woodhead Publishing Series in Biomaterials, Alumina Ceramics, Woodhead Publishing, Pgs 71-121.
APHA, AWWA, WEF. (1998). Standard methods for examination of water and waste water. American Public Health Association, Washington, D.C.
Ashraf, M. A., Peng, W., Zare, Y., & Rhee, K. Y. (2018). Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale research letters, 13(1), 1-7. https://doi.org/10.1186/s11671-018-2624-0
Bali, U., Çatalkaya, E. Ç., & Şengül, F. (2003). Photochemical degradation and mineralization of phenol: a comparative study. Journal of Environmental Science and Health, Part A, 38(10), 2259-2275. https://doi.org/10.1081/ESE-120023373
Brame, J. A., & Griggs, C. S. (2016). Surface area analysis using the Brunauer-Emmett-Teller (BET) method: scientific operation procedure series: SOP-C. https://hdl.handle.net/11681/20339
Burkhardt, W., & Calci, K. R. (2000). Selective accumulation may account for shellfish-associated viral illness. Applied and Environmental Microbiology, 66(4), 1375-1378. https://doi.org/10.1128/AEM.66.4.1375-1378.2000
Chandrasekharan, N., & Kamat, P. V. (2000). Improving the photoelectrochemical performance of nanostructured TiO2 films by adsorption of gold nanoparticles. The Journal of Physical Chemistry B, 104(46), 10851-10857. https://doi.org/10.1021/jp0010029
Deepracha, S., Vibulyaseak, K., & Ogawa, M. (2019). Complexation of TiO2 with clays and clay minerals for hierarchically designed functional hybrids. In Advanced Supramolecular Nanoarchitectonics (pp. 125-150). William Andrew Publishing. https://doi.org/10.1016/B978-0-12-813341-5.00010-3
Deng, Y., Feng, C., Tang, L., Zeng, G., Chen, Z., & Zhang, M. (2019). Chapter 5 - Nanohybrid photocatalysts for heavy metal pollutant control. In Nanohybrid and Nanoporous Materials for Aquatic Pollution Control (pp. 125-153). Elsevier. https://doi.org/10.1016/B978-0-12-814154-0.00005-0
Devi, R. (2010). Innovative technology of COD and BOD reduction from coffee processing wastewater using avocado seed carbon (ASC). Water, Air, and Soil Pollution, 207(1), 299-306. https://doi.org/10.1007/s11270-009-0137-2
Dutta, A. K., Maji, S. K., & Adhikary, B. (2014). γ-Fe2O3 nanoparticles: an easily recoverable effective photo-catalyst for the degradation of rose bengal and methylene blue dyes in the waste-water treatment plant. Materials Research Bulletin, 49, 28-34. https://doi.org/10.1016/j.materresbull.2013.08.024
Edelstein, A. S., & Cammaratra, R. C. (Eds.). (1998). Nanomaterials: synthesis, properties and applications. CRC press. https://doi.org/10.1201/9781482268591
El-Saliby, I. J., Shon, H., Kandasamy, J., & Vigneswaran, S. (2008). Nanotechnology for wastewater treatment: in brief. Encyclopedia of life support system (EOLSS), 7. https://www.eolss.net/Sample-Chapters/C05/E6-144-23.pdf
Fawell, J., & Nieuwenhuijsen, M. J. (2003). Contaminants in drinking waterEnvironmental pollution and health. British medical bulletin, 68(1), 199-208. https://doi.org/10.1093/bmb/ldg027
Feka, D. P. (2017). adsorptive treatment of tannery effluent with granulated activated carbon. An MSc thesis submitted to the department of chemistry, college of sciences, Federal University of Agriculture Makurdi, Nigeria.
Ferroudj, N., Nzimoto, J., Davidson, A., Talbot, D., Briot, E., Dupuis, V., ... & Abramson, S. (2013). Maghemite nanoparticles and maghemite/silica nanocomposite microspheres as magnetic Fenton catalysts for the removal of water pollutants. Applied Catalysis B: Environmental, 136, 9-18. https://doi.org/10.1016/j.apcatb.2013.01.046
Gubicza, J. (2012). 8 - Relationship between microstructure and hydrogen storage properties of nanomaterials. In J. Gubicza (Ed.), Defect Structure in Nanomaterials (pp. 301-332). Woodhead Publishing. https://doi.org/10.1533/9780857096142.301
Gupta, V. K., Khamparia, S., Tyagi, I., Jaspal, D., & Malviya, A. (2015). Decolorization of mixture of dyes: a critical review. https://www.sid.ir/en/journal/ViewPaper.aspx?id=441797
Gupta, V. K., Tyagi, I., Sadegh, H., Ghoshekandi, R. S., & Makhlouf, A. H. (2017). Nanoparticles as adsorbent; a positive approach for removal of noxious metal ions: a review. Science Technology and Development, 34(3), 195-214. https://doi.org/10.3923/std.2015.195.214
Itodo, A. U., Khan, M. E., & Feka, D. P. (2017). On the adsorptive detoxification of chrome tan liquor: kinetics, thermodynamics and mode of transport. nature, 4, 5. https://doi.org/10.9734/AJOCS/2017/32728
Itodo, U., Khan, M., Feka, D., & Ogoh, B. (2018). Tannery wastewater evaluation and remediation: Adsorption of trivalent chromium using commercial and regenerated adsorbents. Journal of Water Technology and Treatment Methods, 1(1), 1-105. https://doi.org/10.31021/jwt.20181105
Jarvis, P., Jefferson, B., & Parsons, S. A. (2006). Floc structural characteristics using conventional coagulation for a high doc, low alkalinity surface water source. Water research, 40(14), 2727-2737. https://doi.org/10.1016/j.watres.2006.04.024
Jiuhui, Q. U. (2008). Research progress of novel adsorption processes in water purification: a review. Journal of environmental sciences, 20(1), 1-13. https://doi.org/10.1016/S1001-0742(08)60001-7
Kansal, S. K., Lamba, R., Mehta, S. K., & Umar, A. (2013). Photocatalytic degradation of Alizarin Red S using simply synthesized ZnO nanoparticles. Materials letters, 106, 385-389. https://doi.org/10.1016/j.matlet.2013.05.074
Krishna, Y. S., Sandhya, G., & Babu, R. R. (2018). Removal of heavy metals Pb (II), Cd (II) and Cu (II) from waste waters using synthesized chromium doped nickel oxide nano particles. Bulletin of the Chemical Society of Ethiopia, 32(2), 225-238. https://doi.org/10.4314/bcse.v32i2.4
Kumar, K. Y., Muralidhara, H. B., Nayaka, Y. A., Balasubramanyam, J., & Hanumanthappa, H. (2013). Hierarchically assembled mesoporous ZnO nanorods for the removal of lead and cadmium by using differential pulse anodic stripping voltammetric method. Powder technology, 239, 208-216. https://doi.org/10.1016/j.powtec.2013.02.009
Kyzas, G. Z., & Matis, K. A. (2015). Nanoadsorbents for pollutants removal: a review. Journal of Molecular Liquids, 203, 159-168. https://doi.org/10.1016/j.molliq.2015.01.004
Lubick, N., & Betts, K. (2008). Silver socks have cloudy lining/Court bans widely used flame retardant. Environmental Science & Technology; 42(11), 3910. https://doi.org/10.1021/es0871199
Mousavi, S. M., Mahjoub, A. R., & Abazari, R. (2015). Green synthesis of ZnO hollow sphere nanostructures by a facile route at room temperature with efficient photocatalytic dye degradation properties. RSC advances, 5(130), 107378-107388. https://doi.org/10.1039/C5RA19507A
Nagpal, M., & Kakkar, R. (2019). Use of metal oxides for the adsorptive removal of toxic organic pollutants. Separation and Purification Technology, 211, 522-539. https://doi.org/10.1016/j.seppur.2018.10.016
Oladipo, A. A., Adeleye, O. J., Oladipo, A. S., & Aleshinloye, A. O. (2017). Bio-derived MgO nanopowders for BOD and COD reduction from tannery wastewater. Journal of water process engineering, 16, 142-148. https://doi.org/10.1016/j.jwpe.2017.01.003
Ramasami T., Rajamani S. and Raghava rao J. (2015). Pollution control in leather industry: Emerging technological options; Paper presented at the International Symposium on Surface and Colloidal Science and its elevance to soil pollution, Madras.
Rehman, A., & Anjum, M. S. (2010). Cadmium uptake by yeast, Candida tropicalis, isolated from industrial effluents and its potential use in wastewater clean-up operations. Water, Air, and Soil Pollution, 205(1), 149-159. https://doi.org/10.1007/s11270-009-0062-4
Rodríguez, C., Briano, S., & Leiva, E. (2020). Increased Adsorption of Heavy metal ions in multi-walled carbon nanotubes with improved dispersion stability. Molecules, 25(14), 3106. https://doi.org/10.3390/molecules25143106
Sadegh, H., Shahryari-ghoshekandi, R., & Kazemi, M. (2014). Study in synthesis and characterization of carbon nanotubes decorated by magnetic iron oxide nanoparticles. International Nano Letters, 4(4), 129-135. https://doi.org/10.1007/s40089-014-0128-1
Saikia, I., Hazarika, M., & Tamuly, C. (2015). Synthesis, characterization of bio-derived ZnO nanoparticles and its catalytic activity. Materials Letters, 161, 29-32. https://doi.org/10.1016/j.matlet.2015.08.068
Sarma, G. K., Gupta, S. S., & Bhattacharyya, K. G. (2019). Nanomaterials as versatile adsorbents for heavy metal ions in water: a review. Environmental Science and Pollution Research, 26(7), 6245-6278. https://doi.org/10.1007/s11356-018-04093-y
Sawyer, C.N., McCarty, P.L., & Parkin, G.F. (2000). Chemistry for Environmental Engineering 4th Edition. Tata McGraw-Hill Publishing Company Limited.
Shamsizadeh, A., Ghaedi, M., Ansari, A., Azizian, S., & Purkait, M. K. (2014). Tin oxide nanoparticle loaded on activated carbon as new adsorbent for efficient removal of malachite green-oxalate: non-linear kinetics and isotherm study. Journal of Molecular Liquids, 195, 212-218. https://doi.org/10.1016/j.molliq.2014.02.035
Singh, S., Barick, K. C., & Bahadur, D. (2013). Fe 3 O 4 embedded ZnO nanocomposites for the removal of toxic metal ions, organic dyes and bacterial pathogens. Journal of Materials Chemistry A, 1(10), 3325-3333. https://doi.org/10.1039/c2ta01045c
Talam, S., Karumuri, S. R., & Gunnam, N. (2012). Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. International Scholarly Research Notices, 2012. https://doi.org/10.5402/2012/372505
Tamuly, C., Hazarika, M., Das, J., Bordoloi, M., Borah, D. J., & Das, M. R. (2014). Bio-derived CuO nanoparticles for the photocatalytic treatment of dyes. Materials Letters, 123, 202-205. https://doi.org/10.1016/j.matlet.2014.03.010
Theron, J., Walker, J. A., & Cloete, T. E. (2008). Nanotechnology and water treatment: applications and emerging opportunities. Critical reviews in microbiology, 34(1), 43-69. https://doi.org/10.1080/10408410701710442
United Nations Industrial Development Organization (UNIDO) (2016). Pollutants in tannery effluents; sources, description, environmental impact.
Wei, X., Zhu, G., Fang, J., & Chen, J. (2013). Synthesis, characterization, and photocatalysis of well-dispersible phase-pure anatase TiO2 nanoparticles. International Journal of Photoenergy, 2013. https://doi.org/10.1155/2013/726872
Zare, K., Najafi, F., & Sadegh, H. (2013). Studies of ab initio and Monte Carlo simulation on interaction of fluorouracil anticancer drug with carbon nanotube. Journal of Nanostructure in Chemistry, 3(1), 1-8. https://doi.org/10.1186/2193-8865-3-71
Liu, Z., Wu, Y., Chen, J., Li, Y., Zhao, J., Gao, K., & Na, P. (2018). Effective elimination of As (iii) via simultaneous photocatalytic oxidation and adsorption by a bifunctional cake-like TiO 2 derived from MIL-125 (Ti). Catalysis Science & Technology, 8(7), 1936-1944. https://doi.org/10.1039/C8CY00125A
Downloads
Published
Issue
Section
License
Copyright (c) 2021 Danauta Paschal Feka, Amaya Jobin Habila, Kyauta Francis, James Dama Habila, Moses Yohanna Bammai

This work is licensed under a Creative Commons Attribution 4.0 International License.


