Comparison of IgG and Neutralizing Antibody Response After Pfizer BioNTech COVID-19 Vaccine Among Iraqi Individuals
DOI:
https://doi.org/10.51173/jt.v5i2.1267Keywords:
SARS-CoV2, COVID-19, IgG Level, Neutralizing Antibody, VaccinationAbstract
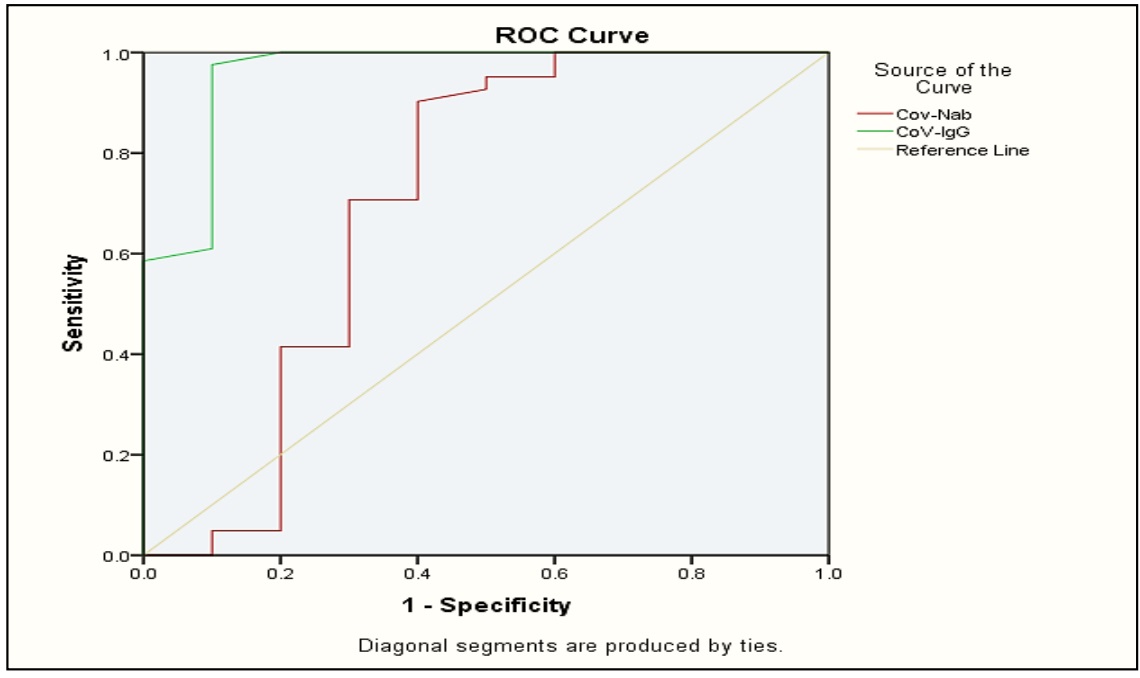
SARS-C0V-2 has quickly caused a pandemic. The distribution of vaccines is presently underway in an effort to stop the viral transmission and stop fatalities, several vaccinations have been created to decrease COVID virus disease, in 2019, the development of vaccine-induced population immunity is an important global strategy. Review of the anti-spike protein receptor-binding domain (S-RBD) a person's level of immune response to antibody strategy can be used to assess SARS-CoV-2 viral infection in 2019. Their efficacy, safety, and immunogenicity in various population are not thoroughly understood. The objective of this study was to evaluate the vaccinations adverse effects as well as determine the immunogenicity of the messenger ribonucleic acid (mRNA) BNT162b2 vaccines through the production of IgG and neutralizing antibodies against the protein s subunit. A total of 41 vaccinated individuals with Pfizer-BioNTech COVID-19 vaccine, as well as (10) non vaccinated were included in the study. Measurements of Neutralizing antibody (Nab) levels and CoV-IgG levels were tested by fluorescence Immunochromatographic assay. IgG and Nab levels showed a significant difference between its level in the sera of vaccinated and controls (p<0.05). This means the majority of immunization recipients between the ages of 18 -50 years can develop an immunological response to the SARS-Cov-2 vaccine.
Downloads
References
JM Dan, J Mateus, Y Kato, KM Hastie, ED Yu, CE Faliti, A Grifoni, SI Ramirez, Haupt, A Frazier, C Nakao, V Rayaprolu, SA Rawlings, B Peters, F Krammer, V Simon, EO Saphire, DM Smith, D Weiskopf, A Sette, S Crotty. “Immuno-logical memory to SARS-CoV-2 assessed for up to 8 months after infection”. Science 371: eabf4063. 2021 https://doi.org/10.1126/science.abf4063.
MR Shurin, A Morris, A Wells, SE Wheeler. “Assessing immune response to SARS-CoV-2 infection. Immunotargets” Ther 9:111–114. 2020 https://doi.org/10.2147/ITT.S264138.
HB Najeb, AJ Mohamed, EM Rasheed Effect of Covid-19 Vaccine on Women's Fertility Hormones. Journal of Techniques, ISSN: 2708-8383, Vol. 4, No. Special Issue, November 2022, Pages 139-144.
S Kashte, A Gulbake, SF El-Amin III, A Gupta - A. COVID-19 vaccines: Rapid development, implications, challengesandfutureprospects. Human Cell 2021, 34, 711–733.
EE Walsh, RW Frenck, AR Falsey, N Kitchin, KNeuzil, A Gurtman, S Lockhart, M J. Mulligan, R Bailey, K A. Swanson, P Li, K Koury, W Kalina, D Cooper, C F-Garfias, PY Shi, Ö Türeci, K R. Tompkins, K E. Lyke, V Raabe, P R. Dormitzer,K U. Jansen, U Şahin, and W C. Gruber. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. New England gournal of Medicine. 2020; 383:2439-2450.
L Dai, GF Gao - G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82.
Iwasaki, A.; Omer, S.B. Why and how vaccines work. Cell 2020, 183, 290–295. https://doi.org/10.1016/j.cell.2020.09.040.
Z Wang, F Schmidt, Y Weisblum, F Muecksch, CO Barnes, S Finkin, c,Viant,cGaebler. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection.Nature2021,595,426–431.
Z Wang, F Muecksch, D Schaefer-Babajew, S Finkin. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection.Nature2021,595,426–431.
Y Wu, F Wang, C Shen, W Peng, D Li, C Zhao, Z Li, S. Li, Y. Bi, Y. Yang, “A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2”. Science, 368, 1274–1278. 2020.
E Dolgin. “How COVID unlocked the power of RNA vaccines”. Nature;5 89:189–91. 2021.
Food and Drug Administration. “Pfizer COVID-19 vaccine emergency use authorization”. U.S. Food and Drug Administration, Silver Spring, MD. 2020.
Food and Drug Administration. “Moderna COVID-19 vaccine emergency use authorization”. U.S. Food and Drug Administration, Silver Spring, MD. 2020.
CO Barnes, CA Jette, ME Abernathy, KA Dam, SR Esswein, HB Gristick, AG Malyutin, NG Sharaf, KE Huey-Tubman, YE Lee, DF Robbiani, MC Nussenzweig, AP West, PJ Jr, Bjorkman. “SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies”. Nature 588:682–687. 2020. https://doi.org/10.1038/s41586-020-2852-1.
B Lo Sasso, R V Giglio, M Vidali, C Scazzone, G Bivon, C M Gambino, A M Ciaccio, L Agnello and M Ciaccio, “Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 mRNA BNT162b2 Vaccine”. Diagnostic 11(7):1135 Jun-2021.
C. RydyznskiModerbacher, S.I. Ramirez, J.M. Dan, A. Grifoni, K.M. Hastie, D. Weiskopf, “Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity Cell, 183, pp. 996-1012.e19 2020.
B Lo Sasso, C.M Gambino, N Scichilone, R. V Giglio, G Bivona, C. Scazzone, R. Muratore, S. Milano, M Barbagallo, L. Agnello, “Clinical Utility of Midregional Proadrenomedullin in Patients with COVID-19”. Lab. Med. 2021.
C.M Gambino, B Lo Sasso, C. Colomba, R. V Giglio, L. Agnello, G. Bivona, M. Ciaccio, “Comparison of a rapid im-munochromatographic test with achemiluminescence immunoassay for detection of anti-SARS-CoV-2 IgM and IgG”. Biochem.Med., 30(3): 030901. 2020.
L Baden,. et al., “Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine”. N. Engl. J. Med. 384, 403–416 2021.
F. Polack, et al. “Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine” . N. Engl. J. Med. 383, 2603–2615 2020.
AS Abd, IA AL-Rayahi A Study of Some Biochemical Parameters in COVID-19 Patients with Diabetes
Mellitus: Comparative Study. Journal of Techniques, ISSN: 2708-8383, Vol. 4, No. Special Issue, November 2022, Pages 1-6.
M.J. Mulligan, K.E Lyke, N. Kitchin, J. Absalon, A. Gurtman, S. Lockhart, K. Neuzil, V. Raabe, R. Bailey, K.A Swanson, et al. “Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults” . Nature, 586, 589–593. 2020.
D.S Khoury, D. Cromer, A. Reynaldi, T.E. Schlub, A.K. Wheatley, J.A. Juno, K. Subbarao, S.J Kent, J.A Triccas, M.P Davenport, “Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection.” Nat.Med., 27, 1205–1211, 2021.
F Wu, A Wang, M Liu, Q Wang, J Chen, S Xia, Y Ling, Y Zhang, J Xun, L Lu, S Jiang, H Lu, Y Wen, J Huang 2020 “Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications” doi: https://doi.org/10.1101/2020.03.30.20047365.
A R. Demonbreun, A Sancilio, M P. Velez, D T. Ryan, R Saber, L A. Vaught, N L. Reiser, R R. Hsieh, R T. D’Aquila , B Mustanski , E M. McNally , T W. McDade . “ Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals.” E Clinical Med. Vol. 38 101018 2021.
F Krammer, K Srivastava, H Alshammary, AA Amoako, MH Awawda, KF Beach, et al. “Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine” N Engl J Med, 384 , pp. 1372 1374, 2021 10.1056/nejmc2101667.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Najat Saeed Hussein, Izzat Abdulsatar Al-Rayahi, Salwa S. Muhsin, Redhwan Abdul Kareem Alameer

This work is licensed under a Creative Commons Attribution 4.0 International License.











