Introduction
Largemouth bass (Micropterus salmoides) was introduced in China from America in 1983. It is a thriving highly economical freshwater-farmed species that has been widely cultivated in China due to its nutritious and delicious meat.1 Under intensive aquaculture conditions, the growth rate of largemouth bass is very fast and susceptible to pathogens due to environmental stresses such as poor water quality and farm treatment. These diseases lead to severe economic losses in largemouth bass breeding.2 The major pathogens in largemouth bass include bacterial pathogens such as Nocardia and Aeromonas hydrophila (A. hydrophila), viral pathogens such as Largemouth bass ranavirus (LMBRaV), and parasitic pathogens such as Trichodina, Apiosoma blanchard, and Ichthyophthirius.3 Therefore, many studies have investigated the pathogenicity, detection and diagnostic technologies, prevention and control measures, medicine, and vaccines to prevent these diseases.4 Antibiotics or drugs are often used to control diseases in aquaculture. However, these treatments face various challenges, including potential drug resistance, immune system suppression, environmental hazards, and food safety problems.5 Nonetheless, no effective and commercialized vaccine or drug has been developed for disease control of largemouth bass.6
Functional feed or feed additives isolated from natural herbs can enhance aquatic animal health and improve the water environment in aquaculture.7 The application of naturel-identical compounds in feed additives to stimulate the immune system of fish and prevent and control diseases has emerged as a research hotspot.6 Plant sterols (phytosterols) are naturally occurring plant constituents that are structurally similar to cholesterol. They are active components present in plants and have a variety of pharmacological activities, such as anti-inflammation, anti-diabetes, and anti-cancer properties. Natural plant-derived steroid compounds are novel feed additives that regulate immunity and growth in animals.7 Phytosterols mainly include β-sitosterol, stigmasterol, rapeseed oil sterols, and rapeseed sterols. Among them, β-sitosterol is the most abundant and plays an essential role in physiological processes . β-sitosterol was found to exert significant anti-inflammatory effects in animals.8 β-sitosterol also resulted in several effects in mice, including increased body weight, improved colon morphology, and reduced expression of proinflammatory factors.9 It reduced Pseudomonas aeruginosa lung infection and inflammation in mice,10 and dramatically increased the expression of antimicrobial proteins in intestinal epithelial cells in mice.9 However, studies on the effects of β-sitosterol on fish are very limited. One piece of research reported that β-sitosterol enhanced the intestinal immune function of large yellow croakers (Larimichthys crocea) infected with A. hydrophila.11 Furthermore, β-sitosterol was found to exert certain anti-inflammatory and antioxidant effects in zebrafish inflammation induced by copper sulfate.12 Therefore, the current study was designed to investigate the effects of β-sitosterol supplementation on growth performance, antioxidant ability, and disease resistance in largemouth bass. In addition, different doses were tested to determine the optimal dose. The results are expected to provide evidence facilitating the development of natural functional feed for largemouth bass, thereby improving its health for the sustainable development of the breeding industry.
Materials and Methods
Fish and experimental diets
Largemouth bass were purchased from one farm of Wuhan, Hubei Province, China. All largemouth bass were kept in fifteen recirculating aquaculture systems and fed with commercial largemouth bass feed (Tongwei, China) for 14 days before the experiment. The largemouth bass (weight 37.30 ± 0.26 g) were randomly assigned to five feeding treatments, each consisting of three replicates and 60 largemouth bass per replicate. β-sitosterol was purchased from Yuanye Bio-Technology Co., Ltd. (Shanghai, China), which was rolled into powder and mixed with flour before being added to commercial feed. Five different concentrations of β-sitosterol (0, 20, 40, 80, and 160 mg/kg) were added to the commercial feeds according to the previous study results.13,14 The prepared supplemented diet was labeled as control (0), T1 (20), T2 (40), T3 (80), and T4 (160). The largemouth bass were kept in oxygenated water at a temperature of 25 ± 1°C. The fish were fed with the prepared supplemented diet three times a day for 28 days until the end of the experiment. All the experimental procedures were approved by the Yangtze River Fisheries Research Institute’s Animal Experimental Ethical Committee (YFI 2022-Mengyan-0412).
Sample collection
Blood samples were collected for serum biochemical analysis, and tissue samples for histological analysis, digesting and antioxidant enzyme activity analysis, and gene expression analysis. After the feeding experiment, largemouth bass were anesthetized with MS-222 (100 mg/L, Sigma, USA) and weighed. Nine largemouth bass from each experimental group were randomly selected and blood was collected from the tail vein into 1.5 mL centrifugal tubes. The collected blood was kept at 4°C for 2 h and then centrifuged at 3000 rpm for 10 min. The supernatant was obtained and preserved at -80°C. For histological analysis, the tissues from the midgut and liver were soaked in a 4% paraformaldehyde solution. In addition, the liver and intestinal tissues were carefully removed and preserved at -80°C for the intestinal digesting and antioxidant enzyme activity assay. At days 3, 7, 14, 21, and 28 of the feed experiment, the head kidney and spleen tissues from three largemouth bass in each tank were collected and kept in TRIzol (Invitrogen, USA) to extract RNA for the gene expression analysis.
Growth performance
The growth performance of weight gain rate (WGR), hepatosomatic index (HSI), viscerosomatic index (VSI), condition factor (CF), and feed conversion ratio (FCR) were evaluated to assess the effects of dietary β-sitosterol supplementation on the growth performance of largemouth bass. The calculation formulae for these parameters were obtained from a previously published study15: WGR (%) = 100 × (Wt - W0)/W0; FCR (%) = 100 × F/ (Wt - W0); CF (g/cm3) = 100 × Wt/ (body length)13; HSI (%) = 100 × liver weight/Wt; VSI (%) = 100 × visceral weight/Wt. In these formulae, Wt and W0 represent the final weight and initial weight of the largemouth bass, and the F indicates the total quantity of feed consumed.
Serum biochemical analysis
In this study, the serum biochemical analysis indicators included glucose (GLU), total cholesterol (TCHO), triglyceride (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein (TP), and albumin (ALB). These parameters were measured using different commercial kits (Dibao, China) and an Olympus 600 automatic biochemical analyzer (Olympus, Japan).
Intestinal digestive and antioxidant enzyme activities analysis
The levels of trypsin, amylase, and lipase in the intestine, and superoxide dismutase (SOD), malondialdehyde (MDA), total cholesterol (TCHO), and total cholesterol (TCHO) in the liver were determined to assess the intestinal digestive and antioxidant enzyme activities of largemouth bass. They were detected by the corresponding detection test kits (Jiancheng, China), and analyzed using a UV spectrophotometer (Thermo, USA) and microplate reader (Biotek, USA).
Immune-related gene expression analysis
Total RNA was isolated from tissue samples collected from the head kidney and spleen, conserved in TRIzol reagent following the instructions. Subsequently, the total RNA was reverse-transcribed into cDNA using a reverse transcription kit (TaKaRa, China). Immune-related genes were selected based on previously published studies, including tumor necrosis factor-α (TNF-α), myxovirus resistance (Mx) and interleukin-1 beta (IL-1β),16 interferon regulatory factor 3 (IRF3) (XM_038735465.1), and interleukin-10 (IL-10).17 In this study, β-actin was used as an internal reference gene.16 The primer sequences are listed in Table 1.
Challenge test
Aeromonas hydrophila and largemouth bass ranavirus (LMBRaV) were isolated and stored in our laboratory.3 Largemouth bass were infected to evaluate the effects of β-sitosterol on disease resistance. After the supplemented feeding experiment, thirty fish were chosen randomly and infected with 0.2 mL A. hydrophila suspension (1.0 × 107 colony forming unit CFUs/mL) intraperitoneally. Another thirty fish were chosen randomly and infected with 0.1 mL LMBRaV of 1 × 103 TCID50 via intraperitoneal injection. In addition, thirty fish were injected with phosphate-buffered saline to establish the mock group. The deaths in each group were recorded daily. To ensure that largemouth bass were killed by A. hydrophila and LMBRaV, the diseased fish were collected, and pathogens were detected.
Results
Effects of dietary β-sitosterol supplementation on growth performance
A 100% survival rate was achieved in all experimental groups during the experiment period. All the growth parameters results of largemouth bass are displayed in Table 2. The final weight increased in all experimental groups, with the T2 group displaying the most significant increase compared to the control group (P < 0.05). Weight gain rate (WGR) and condition factor (CF) demonstrated a similar increase compared to the control group (P < 0.05). In contrast, a decreasing trend was observed for the feed conversion ratio (FCR), viscerosomatic index (VSI) and hepatosomatic index (HSI) across the β-sitosterol treatment groups (P < 0.05).
Results of serum biochemical analysis
Serum biochemical indices of β-sitosterol supplementation to largemouth bass feed were displayed in Figure 1. The glucose (GLU) levels were significantly increased in the β-sitosterol treatment groups compared to the control group (P < 0.05). Moreover, the total cholesterol (TCHO) and alanine aminotransferase (ALT) were significantly decreased in β-sitosterol treatment groups compared to the control group (P < 0.05). The alkaline phosphatase (ALP), total protein (TP), and albumin (ALB) levels exhibited the same increase when compared to the control group.
Analysis of intestinal digestive and antioxidant enzyme activities
The results of intestinal digestive and antioxidant enzyme activities are displayed in Figure 2. The results indicated that β-sitosterol supplementation can lead to changes in intestinal digestive and antioxidant enzyme activity. As shown in Figure 2A, total cholesterol (TCHO) and malondialdehyde (MDA) were significantly decreased in β-sitosterol treatment groups compared to the control group (P < 0.05). Meanwhile, superoxide dismutase (SOD) was increased following β-sitosterol supplementation but showed no statistical significance (P > 0.05). There were no significant differences in serum triglyceride (TG) contents among the treatment groups (P > 0.05). However, trypsin, lipase, and amylase were significantly increased following β-sitosterol treatments compared to the control group. The most significant increase was in the T2 group (P < 0.05) (Figure 2B).
Histopathological analysis
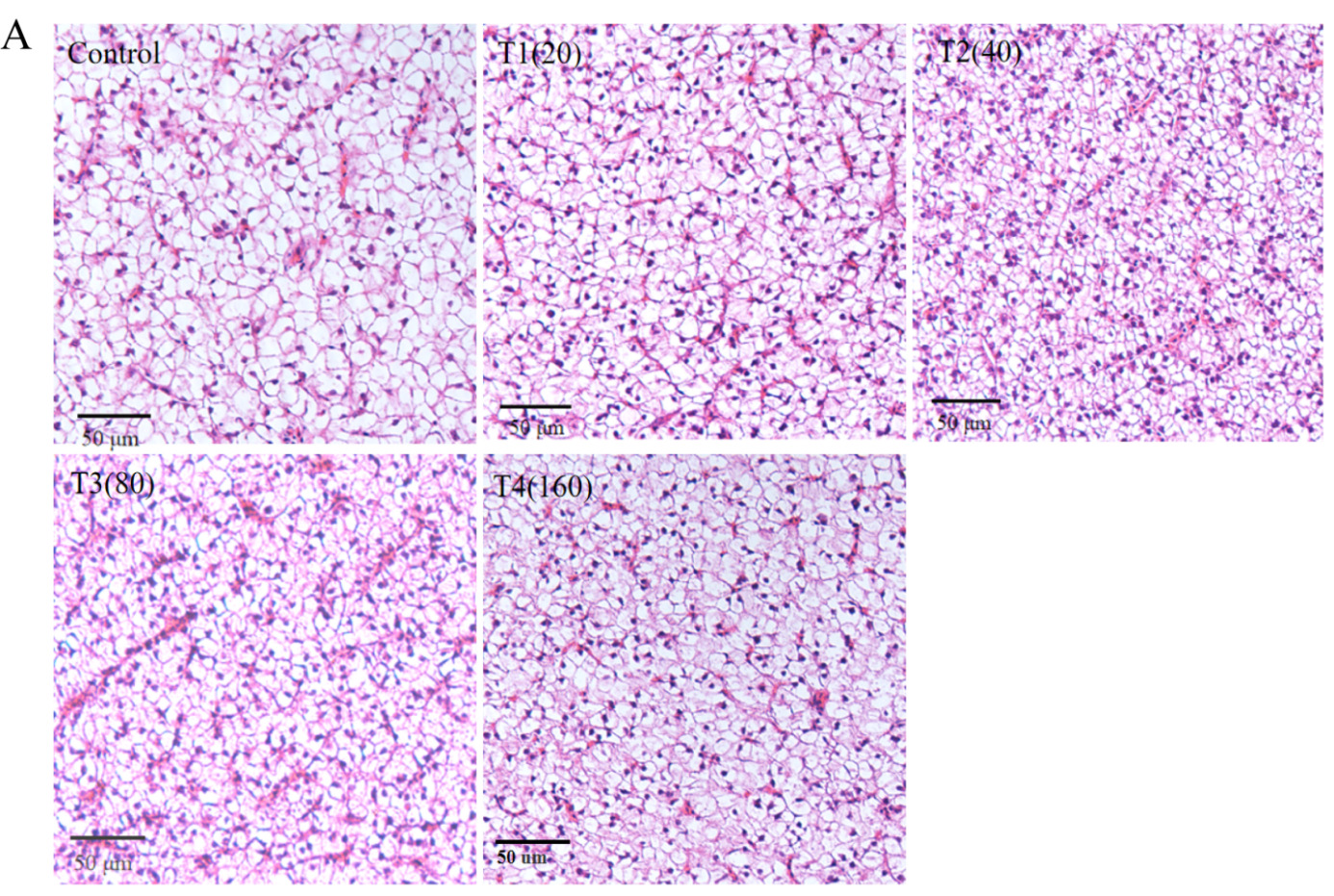
Histopathological analysis indicated that all groups of liver tissues exhibited no abnormal changes. Compared to the control group, the β-sitosterol supplementation groups showed clear cell-to-cell boundaries, and no inflammatory infiltration or hepatocellular damage was observed (Figure 3A). In terms of intestinal tissue structure, the β-sitosterol supplementation treatment groups exhibited normal microstructure and dense microvilli (Figure 3B). β-sitosterol supplementation groups demonstrated increased intestinal villus length and muscularis propria thickness compared to the control group (Figure 3C).
Analysis of the expression of immune-related genes
The expression levels of immune-related genes (IL-10, IL-1β, TNF-α, IRF-3, and Mx) were measured in the largemouth bass’s head kidney and spleen on days 3, 7, 14, 21, and 28. Decreased expression levels of IL-1β and TNF-α were observed in all β-sitosterol treatment groups, with the most significant reduction found in the head kidney on day 14 and in the spleen on day 21 (P < 0.05). The expression level of IL-10 was elevated in all β-sitosterol treatment groups, with the highest in the head kidney on day 14 and in the spleen on day 21 (P < 0.05). IRF3 and Mx expression levels were higher in all β-sitosterol treatment groups; the most significant increase was observed in the head kidney on day 3 and in the spleen on day 14 (Figure 4).
Challenge test
To investigate the protective effect of sterol on largemouth bass, the fish were infected with LMBRaV and A. hydrophila to carry out the infection experiments. During the challenge tests, no death occurred in the mock group. In the LMBRaV challenge test, largemouth bass began to die from day 3 in the control group (commercial feed without β-sitosterol supplement) and from day 4 in the β-sitosterol treatment groups. The LMBRaV cumulative survival rate reached 20% in the T2 group (Figure 5A). In the A. hydrophila challenge test, all largemouth bass died on day 6 in the control group, whereas the survival rates for T1, T2, T3, and T4 groups were 30, 40, 40 and 35%, respectively (Figure 5B). These findings indicated that compared to the control group, the β-sitosterol treatment groups had higher protective efficiency than largemouth bass. β-sitosterol supplementation displayed higher protection against A. hydrophila than LMBRaV.
Discussion
With the development of aquaculture and aquafeed additives, fish diets supplemented with effective plant extracts can enhance growth performance, alter gut microbiota, improve immune and oxidative functions, and control bacterial infections, thereby enhancing healthy breeding.2,6 This study showed that adding β-sitosterol to the largemouth bass feed can improve the growth performance, antioxidant ability, intestinal structure, immune-relative gene expression, and disease resistance. The results not only expand the use of β-sitosterol but also provide a potentially functional plant-derived feed additive for the healthy development of the largemouth bass breeding industry.
Many studies have reported that phytosterols can improve animal growth performance. Adding 75 mg/kg β-sitosterol to broiler feed significantly increased the body weight and feed intake.18 Supplementing phytosterols to the feed of weaned piglets could increase their intestinal villus length.19 In tilapia fish, the addition of 20 and 40 mg/kg phytosterols to feed significantly increased the final weight, weight gain rate (WGR), and specific growth rate and reduced the feed conversion ratio (FCR).20 In the present study, the β-sitosterol supplement groups demonstrated superior weight gain rate (WGR) and condition factor (CF), and lower feed conversion ratio (FCR) compared to the control group (only commercial feed). Meanwhile, the T2, T3, and T4 groups exhibited significantly increased intestinal villus length and muscularis propria thickness of largemouth bass. Supplementation with 40 mg/kg showed the best results. The findings led to a similar conclusion to previous studies, indicating that β-sitosterol exerts a positive effect on growth performance in animals.
Previous studies have also shown that phytosterols impact animal blood biochemical indexes and digestive enzyme activity, which is conducive to animal growth, digestion, and absorption. Digestive enzyme activity has a direct impact on the ability to absorb and digest nutrients, and is an essential measure of fish growth performance. Alkaline phosphatase (ALP) is closely related to the transfer of phosphate groups and calcium and phosphorus metabolism. β-sitosterol can promote hepatic glycogenolysis.21 The addition of β-sitosterol to rat feed increased serum glucose (GLU).22 Furthermore, adding 40 mg/kg phytosterol to the basal diet of broiler chickens resulted in significantly elevated levels of trypsin, lipase, and amylase activities.23 Phytosterols (particularly β-sitosterol) can enhance the synthesis of animal proteins. In this study, the changes in serum biochemical indices, intestinal morphology, and antioxidant enzyme activities when adding β-sitosterol in largemouth bass commercial feed were investigated. The results revealed increased serum biochemical indices, including glucose and alkaline phosphatase. The intestinal villus length and muscularis propria thickness increased too, accompanied by the digestive enzyme activities, which all can promote digestion and absorption of nutrients. Meanwhile, the levels of total cholesterol, aspartate aminotransferase, and alanine aminotransferase in serum decreased to reduce liver damage. In addition, β-sitosterol supplementation can enhance the antioxidant capacity of largemouth bass by decreasing malondialdehyde and elevating superoxide dismutase. In fish, antioxidant enzymes play an essential role in the antioxidant system. β-sitosterol provides protection against oxidative stress by regulating antioxidant enzymes and free radicals.24 Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are often used as specific indicators of impaired liver function. Total cholesterol (TCHO) reflects the body’s lipid metabolism. In addition, superoxide dismutase (SOD) activity showed an increasing trend in β-sitosterol treatment groups in tilapia.20 β-sitosterol also reduced total cholesterol (TCHO) in the serum and liver. Collectively, these findings suggest that dietary β-sitosterol supplementation promoted the growth performance of largemouth bass by affecting the intestinal morphology and serum biochemical factors, increasing the hepatic antioxidant capacity to improve the absorption of nutrients.
Abundant evidence suggests that certain pro-inflammatory cytokines (like TNF-α and IL-1β) and anti-inflammatory cytokines (like IL-10) play a role in an organism’s inflammatory processes. Mx, the Interferon (IFN)-inducible gene, acts as a first line of defense against viral infection by blocking early steps in the viral replication cycle. In a previous study, large yellow croakers were fed an optimal dose of sitosterol, which resulted in reduced IL-1β levels.11 Previous studies reported that Pseudomonas aeruginosa lung infection and inflammation in mice were alleviated by β-sitosterol.10 Moreover, β-sitosterol significantly increased the production of antimicrobial peptides in intestinal epithelial cells and reduced the survival of intracellular Salmonella typhimurium in mice.9 In the present study, the expression levels of IL-10, IL-1β, TNF-α, IRF-3, and Mx presented different fluctuation levels in β-sitosterol treatment groups. Surprisingly, the β-sitosterol supplementation in feed had a relatively lower protection rate against A. hydrophila (40%) or LMBRaV (20%) infection in largemouth bass. This may be attributed to the immune-related gene expression, or that the effects of β-sitosterol are not as prominent in disease resistance as they are in regulating growth in largemouth bass. Nevertheless, these findings require further research for confirmation.
In conclusion, in this study, dietary supplementation of 20-160 mg/kg of β-sitosterol improved growth performance, disease resistance, and antioxidant enzyme activity in largemouth bass, revealing an optimal dose of 40 mg/kg. β-sitosterol is an effective plant extract that promotes healthy fish growth.
Acknowledgments
This research was funded by the National Key Research Development Program of China (2023YFD2400704), the Technical Innovation Special Project of Hubei Province (2022BBA0054, 2023BBB122), the Central Public-interest Scientific Institution Basal Research Fund (YFI 202207, 2023TD46), the National Natural Science Foundation of China (32202980) and National Freshwater Aquatic Germplasm Resource Center (FGRC18537).
Authors’ Contribution
Writing – original draft: Yangyang Xing (Equal), Liping Zhang (Equal). Software: Yangyang Xing (Lead). Validation: Yangyang Xing. Project administration: Liping Zhang (Lead). Visualization: Liping Zhang (Lead). Conceptualization: Mingyang Xue (Lead). Investigation: Mingyang Xue (Lead). Methodology: Wei Liu (Lead). Formal Analysis: Nan Jiang (Lead). Funding acquisition: Yiqun Li (Lead). Data curation: Jianwu Chen (Lead). Writing – review & editing: Yan Meng (Lead).
Competing of Interest – COPE
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Conduct Approval – IACUC
All the experimental procedures were approved by the Yangtze River Fisheries Research Institute’s Animal Experimental Ethical Committee (YFI 2022-Mengyan-0412).
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.