Introduction
Largemouth bass is a freshwater carnivorous fish that is widely preferred by the farmers and consumers because of its high economic value and delicious meat.1 According to the statistics of FBMA, the production of largemouth bass in China has increased from 185941 tons in 2010 to 802486 tons in 2022.2 With the intensification of aquaculture, the immune system of largemouth bass has been severely weakened, resulting in outbreaks of bacterial, parasitic, and fungal diseases.3 To combat these problems, many therapeutic agents are applied to the water, and used in largemouth bass farming, including copper (Cu).4,5 Cu is widely used as an algaecide, fungicide, and parasiticide in aquaculture.6 Waterborne Cu has been widely used to treat external bacteria (e.g., columnaris disease), parasites (e.g., white spot disease), and fungal (e.g., water mold infection) diseases in largemouth bass.3,4 However, this has led to Cu overuse, resulting in excessive Cu build-up in largemouth bass aquaculture.3 It is well known that excessive Cu in water often causes various negative effects on aquatic animals, such as growth retardation, fish mortality, and tissue damage.5 Based on this, it is particularly important to determine the toxicity and appropriate level of Cu in largemouth bass aquaculture water.
The toxicity test is an important method to determine the tolerance of animals to certain toxic substances and the appropriate dose of toxic substances in fish farming.7 The 96-h median lethal concentration (LC50) trial is the most common approach in toxicity tests.8 The intestine and kidneys are important organs for the fish to maintain normal metabolism and growth. However, at present, there are few studies on Cu toxicity and its effects on the intestinal and renal health of largemouth bass juveniles. Therefore, in this study, we intended to use the 96-h LC50 trial to assess the acute toxicity of Cu. Additionally, the relationship of Cu to intestinal and renal health of largemouth bass, as well as the recommended dose of Cu in aquaculture water, were studied from the perspective of oxidative stress using a chronic toxicity experiment. The results of this investigation will offer theoretical support for the healthy development of largemouth bass culture.
Materials and Methods
Experimental fish and the stock solution
Juvenile largemouth bass were provided by a fish farm in Guangzhou, China. Prior to the experiment, fish were exposed to laboratory settings for two weeks and were fed a commercial pelleted floating diet (Dongyufeng No.0, 48% crude protein, 5% crude fat, and 3 mg/kg Cu) (Zhejiang Dongyu Biological Technology Co., Ltd., Huzhou, China).
To create a stock solution of Cu (31.77 g/L), CuSO4·5H2O (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) (124.84 g) was dissolved in distilled water using a volumetric flask (1000 mL). The Cu concentrations required for the experiment were achieved by diluting the stock solution with distilled water.
Experimental design
The 96-h acute waterborne Cu toxicity test was carried out in accordance with OECD guideline 203.9 180 experimental fish (2.58 ± 0.03 g) were evenly distributed to 18 tanks (20 L) and then subjected to different Cu concentrations: 0, 3, 6, 9, 18, and 30 mg/L (estimated Cu contents were 0.0 ± 0.0, 2.8 ± 0.2, 5.4 ± 0.6, 10.2 ± 0.8, 16.3 ± 1.7, and 27.2 ± 2.4 mg/L, respectively). Each treatment (Cu concentration) had three duplicates. To maintain the waterborne Cu concentrations, fresh water that has been aerated and has the appropriate Cu content was added to the system on a regular basis. Throughout the acute toxicity test, the fish were not fed, and dead fish were taken out of the fish tank every day. Death was defined as the absence of gill movement and a reaction to mild prodding. The 96-h LC50 was calculated using Finney’s Probit analysis method10 based on the fish death rates in each treatment.
In the exposure study, six Cu concentrations (0, 51.3, 164, 513, 1640, and 5130 μg/L) were selected, with the highest concentration being 40% of the 96-hour LC50.11 To keep the Cu concentration as consistent as possible, each tank’s water was refreshed and re-dosed on a daily basis using the stock solution. Mean Cu concentrations in tank water samples of 0, 51.3, 164, 513, 1640, and 5130 μg Cu/L treatments were 0.030 ± 0.011, 50.5 ± 2.8, 161.0 ± 3.1, 519.3 ± 8.4, 1630 ± 10.8, and 5142.0 ± 42.6 μg/L, respectively. 270 largemouth bass (2.69 ± 0.02 g) were randomly and evenly distributed among 18 tanks (20 L) (three tanks for each Cu concentration). The fish were fed by hand to apparent satiety twice (10:00 and 17:00) daily with the commercial pelleted floating feed for 30 days. The photoperiod and water parameters throughout the experiment were as follows: photoperiod 12 h light/12 h dark, dissolved oxygen 8.0 ± 1.2 mg/L, water temperature 25.0 ± 0.5 °C, pH 8.2 ± 0.4, ammonia less than 0.1 mg/L, alkalinity 157 ± 17 mg/L, total hardness 166 ± 6 mg/L, and conductivity 450 ± 30 μS/cm.
Sampling
On the 30th day of the experiment, all fish were starved for 24 h. Then 6 fish from each tank were sampled and anesthetized with 50 mg/L benzocaine. Intestine and kidney samples were collected on ice-cold plates, rapidly frozen in liquid nitrogen, and stored at -80 °C until analysis of oxidative-related parameters.
All of the studies were carried out in accordance with China’s official protocol for the care and use of laboratory animals. The Institutional Animal Care and Use Committee at Henan University of Science and Technology approved both the study protocol and all experimental techniques.
Measurement of Cu content in the feed and water samples
The microwave digestion method was used to assess Cu contents in feed and water.12 Samples (approximately 0.5 g for feed and 2 mL for water) were first digested by HNO3 (70%) in the Berghof mws-3 microwave system. All digested samples were cooled to room temperature before being diluted to 10 mL with distilled water in 10 mL volumetric flasks. An atomic absorption spectrometer (PerkinElmer, Inc. Waltham, USA) was used to determine the Cu content at 324.8 nm.
Analysis of oxidative-related parameters
Samples of intestine and kidney were homogenized with 0.68% physiological saline (tissue weight:saline weight = 1 g:9 mL). The homogenate was centrifuged at 1000 g for 10 min. Then the supernatant was collected and stored at 4 °C until the analysis of oxidative-related parameters.
Both contents of malondialdehyde (MDA), reduced glutathione (GSH), and total soluble protein and activity levels of total superoxide dismutase (T-SOD), catalase (CAT), and glutathione peroxidase (GPx) were determined by commercial kits (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China).
Statistical analysis
The probit analysis in IBM SPSS Statistics 20.0 software was used to calculate the 96-h LC50 based on the fish mortality at the acute toxicity test. For the 30-day exposure test, all data were expressed as means ± standard errors of means (SEM) and analyzed with one-way analysis of variance using SPSS 20.0. Duncan’s multiple range test was used to examine mean differences at a significance level of 5%.
Results
Lethal concentration of copper in largemouth bass
Table 1 shows the relationship between Cu concentrations and fish mortality at the 96-h acute waterborne Cu exposure test. In the control group, no dead fish were found. With the increase of waterborne Cu concentration, dead fish number and fish mortality rate increased. 80% of fish were dead when waterborne Cu concentration was 30 mg/L.
Table 2 demonstrates the probit analysis of fish mortality at different Cu concentrations. Observed responses were close to expected responses (Table 2).
Table 3 shows the estimated LC values of waterborne Cu and their 95% upper and lower confidence limits at the 96-h acute toxicity test. The 96-h LC50 of waterborne Cu was 12.78 mg/L at the 95% confidence level, with upper and lower limits of 8.60 mg/L and 21.43 mg/L, respectively.
The regression line between fish mortality and the log values of waterborne Cu generated by the software SPSS 20.0 is shown in Fig. 1. R2 linear =0.976 suggested that experimental data fitted the predicted regression very well.
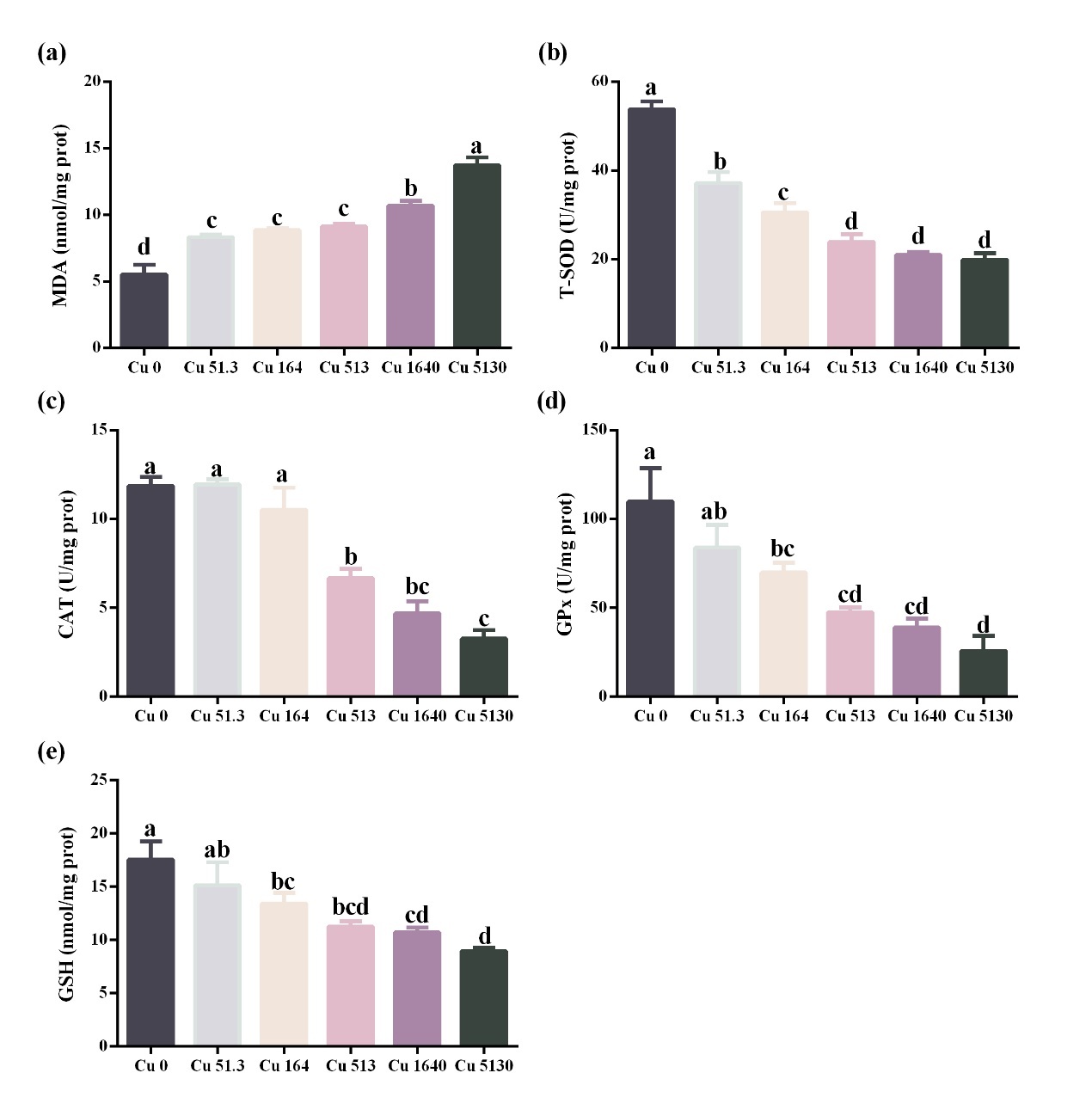
Oxidative response in the intestine
Cu concentrations over 51.3 μg/L substantially increased MDA levels while decreased T-SOD activity levels when compared to the control (P < 0.05). GPx and GSH demonstrated similar changes. Compared to that in the unexposed group, GPx activity levels and GSH contents were significantly reduced when Cu concentrations were at and above 164 μg/L (P < 0.05). In addition, fish in the groups with Cu levels greater than 513 μg/L showed lower CAT levels than the control (P < 0.05) (Fig. 2).
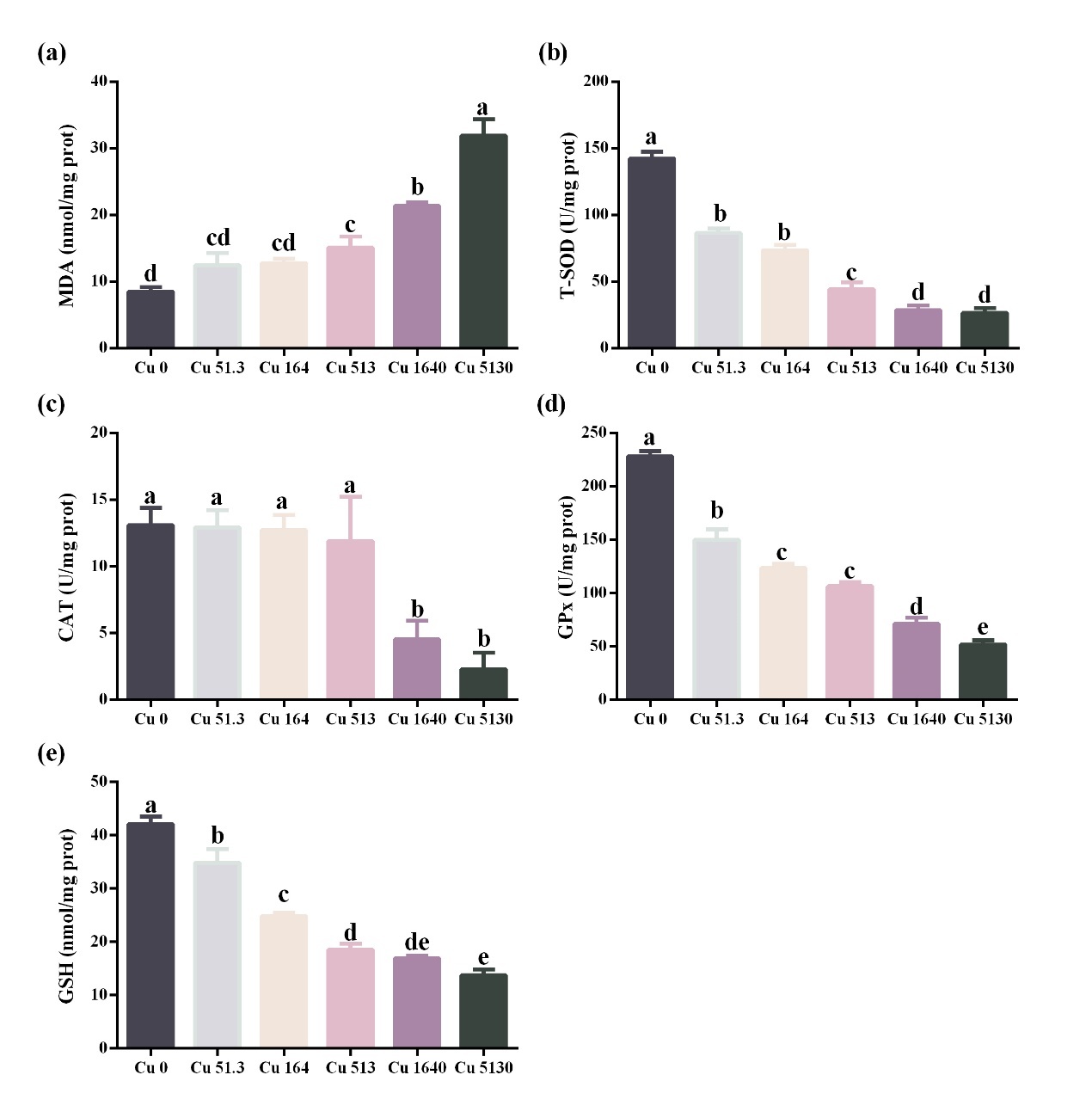
Oxidative response in the kidney
Compared to the control, fish exposed to Cu concentrations at and above 51.3 μg/L demonstrated lower T-SOD and GPx activity levels and GSH contents (P < 0.05). However, only high Cu concentrations induced significant changes in MDA contents (Cu at and above 513 μg/L) and CAT activity levels (Cu at and above 1640 μg/L) in the kidney (P < 0.05) (Fig. 3).
Discussion
In aquaculture, Cu is often used as algaecides, fungicides, and parasiticides.13 However, excess Cu in water bodies has been shown to significantly reduce fish growth and health.14 Therefore, it is necessary to determine the appropriate Cu level in water for the healthy development of aquatic animals. The 96-h LC50 test is often used to assess the potential susceptibility and mortality of a species to toxic substances.6,9 In this study, the 96-h LC50 assay was used to assess the tolerance of largemouth bass to waterborne Cu. Cu toxicity in water is affected by a variety of factors, such as the acclimation period of fish to Cu, water chemical parameters (e.g., water hardness, alkalinity, and pH), species and growth stage of the tested aquatic animals, and Cu types (e.g., copper nanoparticles and copper sulfate).6 In general, an increase in the acclimation period to waterborne Cu, water alkalinity or hardness, and fish size will reduce the toxicity of Cu.6 In this experiment, the 96-h LC50 for juvenile largemouth bass was 12.8 mg/L, which was consistent with the results in juvenile Nile tilapia (Oreochromis niloticus) (12.0 mg/L)15 and juvenile Indian knife fish (Notopterus notopterus) (12.0 mg/L). However, in butterfish (Poronotus triacanthus) (0.5 mg/L for 96-h LC50),16 channel catfish (Ictalurus punctatus) (0.05 mg/L for 96-h LC50),17 and rohu (Labeo rohita) (0.80 mg/L for 96-h LC50),18 the 96-h LC50 of waterborne Cu is ranged from 0.05 to 0.80 mg/L, which is much smaller than the results of the present study. Differences in water chemical parameters and fish species may account for this phenomenon.
The intestine and kidneys play critical roles in maintaining fish health. The intestine plays an important role in the digestion and absorption of nutrients, salt and water balance, and barrier function and immunology in fish (Dawood 2021). The kidney mainly functions in osmoregulation, acid-base regulation, and immunity in fish.19 Previous studies have shown that oxidative stress can damage intestinal and renal functions and threaten the health of aquatic animals.20,21 MDA is a typical marker of oxidative stress.22 In this study, MDA contents in the gut and kidney were significantly higher when Cu concentration was at and above 513 μg/L, indicating the occurrence of oxidative stress in these tissues. To avoid the negative effects of oxidative stress, fish develop antioxidant defense systems, including enzymatic (e.g., T-SOD, CAT, and GPx) and non-enzymatic antioxidants (e.g., GSH).23 However, these indexes decreased significantly when Cu concentration was above 513 μg/L in the intestine and 1640 μg/L in the kidney, indicating that high waterborne Cu concentration reduced the antioxidant capacity of the gut and kidney. Consistent with our results, studies in Rhynchocypris lagowski24 and grouper (Acrossocheilus fasciatus)25 showed that high waterborne Cu exposure caused oxidative stress and decreased antioxidant capacity in the intestine and kidney. The causes of oxidative stress induced by Cu can be attributed to the following three points. Firstly, Cu is a transition metal and can act as a catalyst for the production of reactive oxygen species (e.g., superoxide anion and hydroxyl radical) through the Fenton reaction.26 Secondly, Cu weakens fish antioxidant defense systems by inhibiting antioxidant enzymes and depleting cellular GSH.27 Thirdly, Cu inhibits the mitochondria electron-transfer chain and stimulates the formation of reactive oxygen species.28 Based on the results of renal and intestinal oxidative stress, it is recommended that the Cu concentration in the cultured water for largemouth bass farming should be less than 51.3 μg/L.
Conclusion
The 96-h LC50 of Cu in the form of CuSO4 for largemouth juveniles (2.6 g) was 12.8 mg/L. Waterborne Cu exposure can increase oxidative stress and decrease the antioxidant capacity of the intestine and kidney in largemouth bass when Cu concentration is at and above 513 μg/L. Based on the results of the present study, Cu content in the water for largemouth bass culture is recommended to be less than 51.3 μg/L.
Acknowledgments
This work was supported by the Doctoral Scientific Research Foundation of Henan University of Science and Technology (13480087; 13480088), the National Natural Science Foundation of China (NSFC, no. 32202952), Henan Provincial Science and Technology Research Project (222102320144), and the Innovation and Entrepreneurship Training Program for College Students in Henan University of Science and Technology (2023438, 2023442).
Authors’ Contribution
Conceptualization: Junhao Zhang (Equal), Shiyang Gao (Lead). Methodology: Junhao Zhang (Equal), Na Zhao (Equal), Zihao Meng (Equal), Mengkang You (Equal). Writing – original draft: Junhao Zhang (Equal), Yuchao Huang (Equal). Resources: Na Zhao (Lead). Formal Analysis: Ke Wang (Equal), Songlin Dai (Equal), Zhenyang Zhang (Equal). Investigation: Ke Wang (Equal), Songlin Dai (Equal), Zhenyang Zhang (Equal). Writing – review & editing: Weijun Chen (Equal), Shiyang Gao (Lead). Funding acquisition: Weijun Chen (Equal), Shiyang Gao (Lead). Supervision: Shiyang Gao (Lead).
Competing of Interest
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
All experiments and handling of the animals were conducted according to the research protocols approved by the Institutional Animal Care and Use Committee, Henan University of Science and Technology.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
Data are available upon reasonable request.