Review and Analysis: Fate of Arsenic Applied to Canal Shipping Lane Vegetation and United States Military Base Grounds in the Panama Canal Zone ()
1. Introduction
1.1. Arsenic in the World’s Drinking Water
The problem of arsenic (As) contamination of drinking water resources is globally recognized. Other environmental toxic contaminants in drinking water have been reported in all 20 Latin American countries including Panama. Arsenic is naturally released into the environment and mobilized by humans and livestock via drinking water or the food chain. Arsenic rich (arsenite (III)) water ascending from deep geo-thermal reservoirs can contaminate freshwater sources. Mining and related activities have increased the mitigation challenges [1] . As is transported by rivers from mining sites over long distances and often reaches the coastal environments. There is a lack of a long-term strategy to remediate As problem. Data on the As sources, speciation, mobilization, mobility and pathways are lacking and it is imperative for determining the public and private quality of a water source.
Arsenic in Panama water resources has only been studied marginally via several monitoring programs for restoring the groundwater and surface systems; however, the results have not officially been published [1] . Panamanian exposure to As in drinking water data is very scarce. Some studies investigating trace metals, including As in rocks, sediments and water have been carried out in the Colón Province, Bahia Damas, Gulf of Chiriqui, Azuero peninsula, and Bocas de Toro. The University of Cartagena and the University of Panama, Colombia are conducting an ongoing study on “Trace Elements and Toxicity of Sediments” in the Panama Channel basin [2] .
There are several silver, copper, and gold deposits in the territory that have been exploited since colonial times. In one of the largest deposits of gold-bearing Cu-porphyry in the world, the Petaquilla, deposit southeast of Panama City in Donoso, Colón Province. Corral et al. [3] found “As concentrations in drilled surface samples varied from 15,000 - 28,000 µg·kg−1 in sulfide ores and 15,000 - 3,700,000 µg·kg−1 in oxide ores. The results show that sulfide ores have also the highest concentration of Cu, Zn, As (2.74%) and Cd in the whole-rock data [3] . The mining concession contains headwaters are used for agricultural purposes on the national level, producing milk, beef, rice, vegetables and fruits” [4] . Human exposure to As from gold tailing dams and other pollution contaminants studies are limited [1] .
In the Bay of Panama, increasing industrial growth has raised concern about metalloids pollution and heavy metals and their impact on the marine ecosystem. Heavy metals (Cd, Cu, As, Zn, and Hg) in sediments and tissues of the corals were analyzed by Berry et al. [5] [6] . Neary [7] conducted another study assessing contents of As and trace metals in Panama water resources. Ross studied marine turtles in Panama and related implications for human consumption. An important source of food and income in Panama is turtles and their eggs. Median As contents were 0.12 ± 0.06 mg/kg−1 and 0.12 ± 0.04 mg·kg−1 for Olive Ridley and Green eggs. According to WHO, organic As species are rapidly excreted by the human body, which are abundant in seafood, are less harmful than inorganic species.
1.2. Source of Arsenic: Wetland Vegetation and Hyacinth Control in Panama Canal
The Panama Canal shipping lanes in (Figure 1) [8] the newly created Lake Gatun (Figure 2), were periodically covered by floating plants including hyacinths [9] [10] [11] . It was apparent, in 1913, the water hyacinth had to be controlled. Gatun Lake, a feeder lake was formed by impounding the waters of the many rivers and streams behind Gatun Locks and Dam (Figure 3). In March of 1914, the destruction of the water hyacinth in the Panama Canal, Lake Gatun and its tributaries began. The white arsenic was commercially available in 1914 and could be ordered and used separately by any Federal Agency [8] [11] [12] or the Panama Canal Zone Company. White As was shipped to Panama Canal Zone ports, Balboa or Cristobal, and used on the Panama Canal Zone and Lake Gatun wetland vegetation (Figure 4).
Commercially available white arsenic was purchased to eliminate the floating plants. White arsenic was mixed with soda bicarbonate and water [10] [11] and applied to the floating plants. In 1914, white arsenic was commercially from multiple sources including the Panama Canal Zone Company or the Naval Depot Supply Federal and Stock Catalog. The Federal Standard Stock Catalog (Procurement Division of the Treasury) was not established until 1935. This arsenic and soda was then sprayed on floating plants including hyacinths. An outfit consisting of a 10 m steam launch with a quarter boat and a pump boat was used [10] [11] . The launch supplied steam to the pump boat and for transportation of men and towing of supplies. The arsenic mixture used was made up of
![]()
Figure 1. Construction in the early 1910s of a lock on the Panama Canal. Reprinted with permission from Editor of Open Journal of Soil Science.
![]()
Figure 2. Panama Canal Zone map showing the Panama Canal, Lake Gatun, military bases and Panama City. Reprinted with permission from Editor of Open Journal of Soil Science.
soda, arsenic, and water in proportions of 0.45 kg soda, 0.45 kg of arsenic, and 68 liters of water. The arsenic desiccated and killed the water plants and the arsenic rich residues to the bottom of Panama Canal and Lake Gatun. Bottom feeding fish could feed on the decomposing vegetation and bio-accumulate the arsenic. Bigger fish and birds ate these bottom feeding fish and then humans ate the fish and birds. This soda-arsenic mixture was used on the Panama Canal shipping lane vegetation until 1935.
The water soluble arsenate and arsenite were disbursed throughout the lake and canal plant residues, waters, and sediments [13] . Since arsenic has no half-life, the arsenic remained trapped in the reservoir water, plants (Figure 5) and sediments until such time as the arsenic rich water was released to both oceans via the locks and dam system. After 1935, the white arsenic and soda usage was discontinued and replaced by other herbicides including a solution of copper sulphate and water which was used until 1948 [9] [10] .
The reason for the switch from white arsenic and soda to a copper sulphate solution was not provided. Apparently, there was either concern about the raising
![]()
Figure 3. Gatun Locks sign dated 1913 at the Panama Canal dam. Reprinted with permission from Editor of Open Journal of Soil Science.
![]()
Figure 4. Islands in Lake Gatun which were created in 1913 as a result of Gatun dam. Reprinted with permission from Editor of Open Journal of Soil Science.
![]()
Figure 5. Forested islands and peninsula uplands in Lake Gatun. Reprinted with permission from Editor of Open Journal of Soil Science.
arsenic levels in the Lake Gatun water or copper sulphate was better at controlling wetland vegetation. With the creation of 2,4-D during WWII and the commercial availability of 2,4-D, by 1948, copper sulphate was replaced. One of the reasons for the herbicide switch related to poisoning of local livestock and wildlife by the copper sulphate. The 2,4-D was also more effective for wetland vegetation control than copper sulphate and is still in use today [10] .
1.3. White Arsenic
The top producer of white arsenic (70%) in the world is China followed by Russia, Morocco and Belgium. Most arsenic refinement operations in both U.S. and Europe have been closed as a result of environmental concerns. Arsenic is also found in smelter dust from lead, gold and copper refinement [13] [14] . The European Union (EU), US Environmental Protection Agency (EPA), and World Health Organization (WHO) all recognize arsenic contamination as a significant threat to human health [15] . The WHO guideline for processed drinking water is 10 ug/L and the untreated water standard is 100 ug/L. Ingestion includes eating products from animals, including meat, that were fed with arsenic feed additives and food crops (grain) that grown with arsenic-laced irrigation water [16] . Another source of As was drinking water from arsenic rich groundwater wells and canal.
Food and agricultural researchers thought for years that organic arsenic [16] [17] would never become toxic inorganic arsenic. However, now there is considerable doubt about this agricultural practice [18] [19] . For decades, chickens in the U.S. were given compounds with organic arsenic in them to make the meat redder, plumper, and to prevent or treat specific diseases. The chemical and chicken industries had insisted the arsenic in the feed was organic. However, the livers of chickens fed with organic arsenic compounds were found to contain more arsenic than the control group. Researchers concluded that organic arsenic had been converted (transformed) into the toxic inorganic arsenic. Olson and Cihacek [13] reported: “after a release of a 2011 peer-evaluated FDA study 89 forms of 102 arsenic compounds which could potentially be converted from organic to inorganic arsenic were removed from the market. Arsenic is a heavy metal and thought to be a carcinogen and dangerous. WHO suggests 10 ug/L is the safe drinking water standard. Many countries ignore this standard since it takes decades for people to begin to show symptoms of As poisoning”.
1.4. Cacodylic Acid
Olson and Cihacek found [13] : “cacodylic acid, C2H2AsO2, is created by reducing disodium methyl arsenate with sulfur dioxide and converting the sodium salt to the resultant Arseno methane. The solubility in water of both sodium salt and acid are extremely high. Cacodylic acid (Figure 6), is water soluble and non-volatile, but being an organic (C- or carbon-containing) compound, it decomposes rapidly to non-soluble, relatively non-toxic, inorganic arsenical compounds in soil
![]()
Figure 6. Cacodylic acid structure. Reprinted with permission from Editor of Open Journal of Soil Science.
and water. The chemical compound is stable in sunlight. Chemical and physical properties of cacodylic acid effect the fate in the soil and plants. Cacodylic acid is a contact herbicide and only kills tissues with chemical symptoms appearing within two days since it lacks mobility. It is not effective if rain falls within a few hours of the treatment. Sub-lethal doses induce defoliation, malformed inflorescence, and fewer seeds. In plant tissue, cacodylic acid appears to undergo limited breakdown” [13] .
“Since it contains C in its chemical structure, soil microflora degrades cacodylic acid. Under aerobic conditions the breakdown is slow but is much more rapid under anaerobic and flooded conditions. The ultimate environmental fate is a change from organic to inorganic arsenate which occurs primarily in soil. Soils naturally contain 5 ppm of arsenic in the inorganic form. Plants absorb cacodylic acid from the soil more readily than inorganic arsenic. Evidence suggests that crops do not suffer injury on soils which was previously treated. However, excessive rates on land unusually rich in phosphates can cause injury to sensitive plants such as peanuts and rice. In humans, toxicity rating of cacodylic acid is 3, or medium toxicity. Toxicological data for Ansar 138 (16.8% arsenic) and Phytar 560G (15% arsenic) are similar to cacodylic acid” [13] .
1.5. Use of Cacodylic Acid on Panama Canal Military Bases
For security reasons, 14 United States military areas, including eight bases, were established in the Panama Canal Zone [20] [21] . The flora and fauna needed to be controlled to make life bearable for the military personnel on these tropical jungle bases. The 2010 U.S. Medical Department and Center and School sub-course [22] and 1967 U.S. Army grounds manual [23] gave specific instructions on how grounds crews should deal with each type of weed. Chemicals and pesticides flowed, either attached to the sediment or in solution, into Lake Gatun via subsurface flow and surface runoff and. These herbicides, including cacodylic acid (arsenic), can be bio-accumulated in birds and fish and enter the human food supply [13] . The extent of the current arsenic contamination in Lake Gatun, the Panama Canal channel and former Panama Canal Zone U.S. military base grounds is unknown. Systematic sampling of the Lake Gatun or the Panama Canal sediments and soil sampling of former military bases, chemical disposal sites, and sediment is needed to determine if restoration and mitigation are required.
After 1948, the United States military base commanders had the ability [22] [24] to requisition cacodylic acid (arsenic based) from the Federal Supply Catalog for use on the military bases grounds in the Panama Canal Zone. Cacodylic acid was shipped to Panama Canal Zone ports, including Cristobal and Balboa, and distributed to the military bases. This herbicide was available for ordering and use at 800 world-wide US military bases. Cacodylic acid was used to control narrow leaf grasses. Panama Canal Zone cacodylic acid herbicide shipping records still exist. Almost all the Agent Blue (with the active ingredient cacodylic acid), the arsenic based herbicide used during the Vietnam War for food denial, was shipped through the Panama Canal (Figure 7). Often the shipping records for “Agent Blue” were labeled as “cacodylic acid” [8] [12] . Cacodylic acid, Physar 560G, Ansar 138 could be purchased from the Federal Supply Catalog by any Panama Canal Zone Base Commander.
The objective of this study is to determine the fate of arsenic: 1) applied between 1914 and the 1935 to Panama Canal shipping lane hyacinth and other wetland vegetation and 2) cacodylic acid (arsenic) sprayed from the 1948 to 1999 on the US military base grounds in the Panama Canal Zone.
2. Findings
2.1. Herbicide Testing in Panama Canal Zone
Cacodylic acid has been widely used in developed countries in forestry and agriculture for many years prior to the Vietnam War with few known risks to human health [25] [26] . In 1960s and 1970s the US military tested tactical herbicides in Panama Canal Zone [22] [24] . The most likely herbicides tested were probably cacodylic acid, 2.4-D and 2,4,5-T with unknown amounts of dioxin TCDD) [24] . Cristobal and Balboa ports were the port destinations in the Panama Canal Zone which received herbicide shipments for testing and use on military base grounds. Fort Detrick scientists, DOD, CIA, and USDA may have secretly tested cacodylic
![]()
Figure 7. Tactical herbicides sprayed from a vehicle. Reprinted with permission from Editor of Open Journal of Soil Science.
acid, in Panama Canal Zone [24] . Other known herbicide test sites included Fort Drum, New York and Korea DMZ were not tropical forests so it seems logical that some testing would have been done in at a tropical site.
The only remaining US official record, not requiring declassification, found to date confirming the testing of herbicides in Panama was published in the proceedings of the 92nd National Convention of the American Legion. The Director of National Legislative Commission [24] stated the following at the 92nd National Convention of the American Legion. Report to: Convention Committee on Veteran Affairs and Rehabilitation (on pages 201 and 202 of the proceedings of Wednesday, September 1, 2010):
“WHEREAS, VA was congressionally mandated by Public Law 102-4 (1991) to contract with the National Academy of Sciences (NAS) to review existing peer-reviewed research on herbicides—to include their components—exposure and medical evidence on related health effects; and WHEREAS, A committee convened by the Institute of Medicine (IOM) of the National Academies to study the health effects of herbicide exposure had little information about the exposures the Vietnam veterans encountered and recommended in its 1994 biennial report that VA, upon discovering the feasibility of a valid exposure reconstruction model, facilitate epidemiological studies; and WHEREAS, The Secretary of VA, in response to the recommendation, requested that IOM convene a separate committee to oversee development and evaluation of herbicide exposure models for use in studies of Vietnam veterans; and WHEREAS, The IOM committee determined—in 2003 that a model created by researchers from Columbia University’s Mailman School of Public Health demonstrated the feasibility of a valid exposure-reconstruction model of Vietnam veterans’ herbicide exposure and recommended that VA and other governmental agencies promote more epidemiological studies of veterans by non-governmental groups and independent researchers; and WHEREAS, The Department of Veterans Affairs has still not funded the major epidemiology study mandated by public law; and WHEREAS, In view of the current absence of alternative judicial recourse to remedy the injustice perpetuated on so many disabled Vietnam veterans, their families and survivors, The American Legion will continue to monitor other pending dioxin related suits. WHEREAS, Information has been released by the Department of the Defense on numerous locations other than Vietnam where the herbicide was tested, sprayed, stored, including: testing at Fort Drum, New York, in 1959; spraying in the Panama Canal Zone in the 1960s and 1970s and in the Korean Demilitarized Zone (DMZ) in 1968-69; and the storage of unused herbicide on Johnston Atoll in the Pacific from 1972 78; and WHEREAS, VA has recently identified more units that were exposed to herbicide at the Korean DMZ [24] ”.
Since Agent Orange was not available for testing in the early 1960s, the herbicides tested in the tropical climate in Panama would most likely have been Agent Purple (with dioxin), Agent White (perchloric acid), and Agent Blue with cacodylic acid (arsenic). The CIA laboratory, Fort Detrick, located Fredrick, Maryland, was responsible for the creation and testing of cacodylic acid (Agent Blue). If the scientists at Fort Detrick, the CIA laboratory did test cacodylic acid (Agent Blue) in Panama, it was a top secret program, and these documents are still classified. Panama has not been identified by DOD and CIA, on the public record, as a location where herbicides were tested, sprayed, or stored.
The only official US government statement, on the existing record, related to the spraying in the Panama Canal Zone in the 1960s and 1970s was made by a Communication from Director, National Legislative Commission [24] in his presentation at the 92nd National Convention of the American Legion. Report to: Convention Committee on Veteran Affairs and Rehabilitation (on pages 201 and 202 of the proceedings of Wednesday, September 1, 2010). If the Director of National Legislative Commission statement acknowledging the testing of herbicides in Panama was not true, the National Convention of the American Legion records would probably have been changed and corrected. Since the CIA was responsible for the spraying of herbicides during the Second Indochina War (Laos, Cambodia and South Vietnam) prior to the official start of the American-Vietnam war in 1965 it seems logical that CIA laboratory at Fort Detrick spray records for Panama would have been classified (as were the spray records in Laos, Cambodia and South Vietnam) and apparently remain classified to this day. After 60 years, these CIA Fort Detrick Research Laboratory records for Panama need to be declassified and released to the media and public. Why have these records not been declassified for more than 60 years? Perhaps, FOIA request might help obtain these documents, even if redacted, that would acknowledge the CIA and Fort Detrick testing of herbicides, including cacodylic acid, in Panama Canal Zone.
2.2. Panama Vietnam Era Veterans Exposure to Cacodylic Acid and Arsenic
Many Vietnam Era veterans while serving in Panama, came in contact with cacodylic acid and arsenic that was sprayed on military bases in the Panama Canal Zone. Military personnel applied cacodylic acid residues by using sprayers on back packs, trucks (Figure 7), helicopters (Figure 8) and boats. The military personnel, serving in the Panama Canal Zone (Figure 9), were told that the herbicides, including cacodylic acid, were harmless. The US soldiers and civilians herbicide handlers (Figure 10), did not need to wear protective gear such as facemasks, googles, gloves and suits. The cacodylic acid came in contact with the skin of military personnel and ground crews while they were spraying cacodylic acid. The used and almost empty herbicide barrels containing cacodylic acid were washed and poured out on the ground or buried by handlers often without protective gear (Figure 10). The rinse water, from cleaning the barrels, was poured on the soil surface and was either transported off-site during monsoon rains and into the waterways or leached into the soil and groundwater.
![]()
Figure 8. Cacodylic acid being sprayed by arial sprayers. Reprinted with permission from Editor of Open Journal of Soil Science.
![]()
Figure 9. Ships going through a lock near the southern entrance of the Panama Canal. Reprinted with permission from Editor of Open Journal of Soil Science.
![]()
Figure 10. Tactical herbicides barrels being buried by military personnel. Photo Credit: ResearchGate. Reprinted with permission from Editor of Open Journal of Soil Science.
2.3. Effects of Cacodylic Acid and Arsenic on Animal and Human Health
Watson et al. [16] found “that life expectancies of animals exposed to cacodylic acid were reduced to less than ten percent of the unexposed animal population. The lethal concentration for rats of cacodylic acid is 3.5 µg/L. Soldiers with prolonged exposure to cacodylic acid had a garlic odor in their breath which is one of the common noticeable symptoms of arsenic poisoning”. Other research studies [25] [26] have shown the human liver absorbs 40% of the cacodylic acid. High bioaccumulation of arsenicals in the body and extreme levels of arsenicals are detrimental to human health and crops.
Cognetta et al. [27] found “the most common manifestation of As exposure in susceptible individuals is bilateral palmoplantar hyperkeratosis. Arsenic is one of the known causes of acquired palmoplantar keratoderma (PPK)”. Cognetta et al. [27] reported “a unique case of palmoplantar hyperkeratotic lesions and numerous squamous cell carcinoma in situ (SCCIS) which developed in a Vietnam veteran approximately 15 years after exposure to As (cacodylic acid) during the Vietnam War”. Cognetta et al. [27] stated “It is possible that exposure to Agent Blue (cacodylic acid), ANSAR 138 (powder) and PHYTAR 560G (liquid)) herbicides all containing cacodylic acid (arsenic) contributed to the development of these lesions. Though a causal link between Agent Blue (cacodylic acid) and skin cancer cannot be established, increased awareness of this potential exposure may prompt further study to improve our understanding of Agent Blue and its role in cutaneous oncology.” During the last 50 years, the Panamanian’s living in the Panama Canal Zone have continued to ingest and bio-accumulate As (natural and manufactured) via their food supply and drinking water. However, not all of the ingested As remains in the human body. The major proportion can be excreted as organic forms of As. The portion that bio-accumulates is not excreted. Arsenic is transported by blood to different organs in the body and can bio-accumulate in humans and animals [28] .
The routes of entry of cacodylic acid into the human body are ingested, inhalation, absorption through the skin and eye contact. Cacodylic acid is more readily absorbed into the bloodstream when inhaled as an aerosol [29] . It bio-accumulates in and is excreted by nails, skin, and hair and is metabolized by the liver and [28] . The dimethyl arsenic (DMAA) are metabolites of inorganic arsenic formed intra-cellular by most living organisms exposed to natural sources and do not bind strongly to human molecules. Consequently, the acute toxicity is less than that of the inorganic arsenicals [30] . Recent studies [31] [32] of the trivalent organic arsenicals have shown metabolic products of inorganic arsenic to be more toxic than the parent compound. This includes endocrine, immune, and epigenetic effects as well as inhibition of oxidative stress [33] [34] . An examination of arsenic levels in hair, urine, and toenails can be made to analytically determine arsenic poisoning. Kapaj and Pederson [35] found “individual communities relying on groundwater sources for drinking water should consider a program to document arsenic levels. Since arsenic poisoning of humans can occur by the gradual accumulation of small doses until the lethal levels are reached, the use of cacodylic acid and other organic arsenicals pose a long-term danger” [35] . Gastro-intestinal effects are less common than neurological symptoms over prolonged exposure to organic arsenicals [22] . Cacodylic acid may cause weakness of the hands and feet or parathesis [30] [36] . Repeated skin contact may cause hyperpigmentation and keratosis. Malnourished people are more susceptible to arsenic-related skin lesions [27] [37] .
2.4. Arsenic in Panama Canal Zone Drinking Water
Although the chronic and acute effects of organic arsenicals are not as severe as those of inorganic arsenicals, organic arsenicals still have a significant impact on human health. Future studies many uncover more currently unknown or unproven health effects. The present public health concern to human exposure to arsenic was linked with the consumption of drinking water rich in arsenic. This is a result of the alluvial sediments on the floodplains being rich in anthropic and natural arsenic [1] [31] [38] . Current experimental and epidemiological studies and have attempted to identify the specific mechanism of arsenic carcinogenicity. This has led to the question of whether it is an epigenetic carcinogen. Because of genetic polymorphism in the human population and due to the complexity of the mechanism of toxicity on the molecular level both options continue to remain viable [30] . However, recent studies [18] [31] [32] [39] show that the trivalent organic arsenicals are metabolic products of inorganic arsenic could be more toxic that the parent compound.
Additional anthropic sources include [8] , U.S. military use on bases located in Panama Canal Zone, sewage and industrial sources, and wastewater treatment discharge into the waterways have raised the As levels in the Panama Canal Zone groundwater. Arsenic does not have a half-life and once added to the Panama Canal Zone environment, since 1914, was not destroyed. As could only be eliminated when the water-soluble arsenic in the lake and canal water flowed to the Atlantic and Pacific Oceans [1] . In addition, As can be volatized in wetlands, incinerated and released into the air or transported in the harvested grain. However, most of the water-soluble arsenic was retained in the food crop soils and/or leached into the groundwater or transported in runoff water to Lake Gatun. The water plants or wells along the Panama Canal then return the As rich groundwater to the land surface for urban and agricultural uses. Fish ingest significantly more arsenic than plant food (grain) crops but As is converted to less harmful organic As that is excreted in large quantities from the animal and human bodies. The more toxic inorganic As in the grain which is consumed is more difficult for humans to excrete and can bio-accumulated.
Cacodylic acid is considered to have very low toxicity for mammals. Cacodylic acid is a highly soluble organic arsenic compound that readily breaks down in the soil into water-soluble arsenic. In 2009, the USEPA issued a cancellation order to phase out and eliminate the use of organic arsenical pesticides by 2013. The only exception of monosodium methane arsonate [MSMA] a broadleaf weed herbicide for use on cotton. The highest exposure in insecticide manufacturing was usually found during, screening, drying, mixing, drum-filling and bagging operations. During these operations, reported arsenic concentrations ranged from 0.5 - 45 mg/m3. The As in the sediments may be associated with iron oxyhydroxides and released into the groundwater by reductive dissolution of iron. The oxidation of sulfide phases could also release arsenic to the groundwater, but sulfur concentrations in sediments were below 1 mg/g. The World Health Organization guideline for the safety limit of arsenic is 10 ug/L in drinking water.
3. Results
3.1. Arsenic
Olson and Cihacek [13] found “arsenic, a natural element with an atomic number of 33, is present in the biosphere, hydrosphere, pedosphere and atmosphere. Arsenic is the 12th most common element in the earth’s crust, 12th most abundant element in the human body, and 14th most abundant in seawater. There are four oxidation states of arsenic: −3, 0, +3 and +5. Gaseous arsine, in the form of AsH3, is characteristic of the −3 oxidation state and elemental arsenic is characteristic of the 0-oxidation state. The most common As species are arsenite [As (III)] which is characteristic of the +3 oxidation state; and arsenate [As (V)] which is characteristic of the +5 oxidation state. The most readily available oxidation states for bioaccumulation are the +3 and +5 oxidation states but can be ingested in the As (−3) form by inhalation” [13] .
Arsenic, as the crystalline oxides As2O5 and As2O3, is readily soluble and hygroscopic in water to form acidic solutions [40] [41] [42] . Arsenic salts are weak acids called arsenates and the most abundant arsenic contaminants in drinking water and groundwater of the thousands of Panamanian people living in the Panama Canal Zone including the Chagres River Delta. When As-containing biomass is burned, at burn temperatures below 400˚C, these arsenic oxides can decompose and form As-containing aerosols. Even at temperatures below 200˚C, this decomposition and formation of aerosol compounds can be aided by the presence of carbon (charcoal). During smoke exposure, airborne ash, particulate As-containing aerosols, can also contain inhalable As [13] .
Olson and Cihacek [13] reported “arsenic is a natural constituent of water, soil, animals (Figure 11) and plants. The average arsenic content in soil is 5 ppm but can vary from 1 to 40 ppm while fresh and sea water contain between 0.003 to 0.05 ppm. Crystalline rock has an average 2.0 ppm, table salt 2.71 ppm, and most edible parts of plants are between 0.1 and 1.0 ppm but sometimes as high as 3 ppm and higher on a dry weight basis. Arsenic is water soluble but rarely found in its elemental form, rather, it forms compounds called arsenicals. Arsenicals are detected in more than 200 different minerals. Arsenicals are often
![]()
Figure 11. American crocodile in Lake Gatun. Reprinted with permission from Editor of Open Journal of Soil Science.
associated with complex sulfurous minerals made up of sulfur, gold, iron, copper, silver, nickel, antimony and cobalt due to the anionic ion structure being similar to sulfate (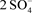 ). Arsenic is a chemical element which occurs in many minerals. Arsenic and its compounds including the trioxide are used in insecticides and pesticides. Arsenical herbicide use is declining due to the toxicity of arsenic and its compounds. Arsenic is the 53rd most common element in nature comprises about 0.00015% of the Earth’s crust. Typical background concentrations of arsenic are about 100 mg/kg in the soils, usually less than 10 ug/L in freshwater and 3 ng/m3 in the atmosphere” [13] .
). Arsenic is a chemical element which occurs in many minerals. Arsenic and its compounds including the trioxide are used in insecticides and pesticides. Arsenical herbicide use is declining due to the toxicity of arsenic and its compounds. Arsenic is the 53rd most common element in nature comprises about 0.00015% of the Earth’s crust. Typical background concentrations of arsenic are about 100 mg/kg in the soils, usually less than 10 ug/L in freshwater and 3 ng/m3 in the atmosphere” [13] .
3.2. What Is the Difference between Organic and Inorganic Arsenic?
Schwarcz [18] determined that “Arsenic atoms can combine with other elements atoms to form two types of compounds, ‘inorganic’ and ‘organic’. When arsenic atoms are attached to a carbon atom it forms a highly complex, non-toxic organic arsenic molecule. The inorganic arsenic compounds do not contain carbon, are simple molecules, but are highly toxic. The reason organic arsenicals are non-toxic is because arsenic atoms are tightly bound with bio-molecules including proteins. Groundwater can contain inorganic arsenic in the form of arsenite or arsenate in which arsenic is bound to oxygen atoms. The organic and inorganic forms of arsenic can be found in soil, water and food around the world. Inorganic arsenic is not usually in water and food that we ingest but is found in the soil [13] . Organic arsenic is not thought to be harmful except in high doses. However, inorganic arsenic is highly poisonous and is a known carcinogen” [18] .
4. Discussion
The addition of white arsenic to control wetland vegetation, including hyacinth, would have increased the arsenic levels in Lake Gatun water. Similarly, arsenic levels in the ground water were increased with the use of cacodylic acid on the US military bases. In many places in the Panama Canal Zone there are high levels of both anthropic and/or natural arsenic, which exceed the WHO standard of 10 μg/l, in the drinking water.
After the construction of the Panama Canal the vegetation control program would have added arsenic to the water in Lake Gatun and the Panama Canal shipping lanes (Figure 9). After 1914, the bio-accumulation of arsenic in the food chain, including birds, fish, and animals, would adversely affected human health of the people living in the Panama Canal Zone who eat local foods and drinking the arsenic rich water from Lake Gatun.
4.1. Fate of Cacodylic Acid Applied to Tropical Soils
For the last 50 years, the National Academy of Sciences (NAS) Part A: The Effects of Herbicides in South Vietnam. Summary and Conclusion report dated 1974 [43] was the “final word” on the fate of Agent Blue and its active component cacodylic acid in the environment including water and soil. The NAS report stated: “Cacodylic acid breaks down in the soil and thought to remain tightly bond as arsenate (+5) to soil compounds and particles”.
The 1974 National Academy of Sciences Part A: Summary and Conclusions report [43] states: “Cacodylic acid, the active component in Agent Blue, is a non-selective herbicide killing a wide variety of herbaceous plants. It is a non-volatile, highly soluble organic compound which is broken down in soil, mostly to inorganic arsenate bound as insoluble compounds which also exist naturally in the soil. Acute and chronic toxicity studies in a variety of animals indicates a low to medium toxicity rating. No teratological studies, nor toxicity studies in man have been reported”. While the author has great respect for NAS scientists and their research and field work in South Vietnam during the Vietnam War, its scope was limited. The NAS study (1971-1972) was conducted after President Nixon ordered the stop of herbicide spraying and completed just before he ordered the withdraw of soldiers from the American Vietnam War in January 1973 [13] . Due to the unstable political environment on the ground, the study was conducted mostly from the air. This provided little chance for scientist boots on the ground. The arsenic was not bound to the soil matrix and immobilized as originally thought by NAS scientists 50 years ago.
Soil scientists [44] determined the arsenate and arsenite were actually water soluble in the soil root zone and could leach into and pollute the Mekong ground water. By 1952 Buckman and Brady [44] had already established the fact that soils have a cation exchange capacity and that water soluble cations, such as As+3 (arsenite) and As+5 (arsenate), were retained on soil particle surfaces, especially those surfaces of minerals and soil organic matter. The cations are adsorbed to soil surfaces by the electrostatic interaction between the negative charge of the particle surfaces and the cations positive charge. The As cations retained a shell of water molecules and did not form direct and permanent chemical bonds with the surface [45] . The NAS findings, that arsenic is bound to the soil matric and is immobile, needs to be updated and corrected.
Young [46] in a recently (2022) revised chapter re-stated the 1970s NAS findings and more recent Institute of Medicine information: “Agent Blue was an organic arsenical as cacodylic acid and sodium cacodylate, and once in contract with the soil it would have been bound within the soil matrix making it immobile, but slowly converting to volatile alkyl arsine and released into the atmosphere. In rice fields sprayed with Agent Blue, anaerobic conditions would have dominated and the conversion of the cacodylic acid and sodium cacodylate to alkyl arsine would have been even faster, 61% in 24 weeks. The phytotoxic properties of cacodylic acid were quickly inactivated on contact with moist soils”. These statements are misleading and out-of-date. Soil scientists have long known that arsenic (arsenate and arsenite) was water soluble in the soil root zone and often leached into the groundwater.
The arsenic rich groundwater in topical soils including the Mekong Delta in South Vietnam has been pumped, since the late 1970s, to the surface via 700,000 tube wells for use in rice paddies, shrimp ponds and to meet the hold water needs, including drinking water, for 15 to 20 million Vietnamese living on the Mekong Delta [13] . The Vietnamese now have elevated arsenic levels in their bodies as a result of eating local foods and drinking the As rich groundwater. The arsenic was not tightly bound to the soil matrix and immobilized as scientists at the National Academy of Science had claimed in 1970s. The Institute of Medicine information, based on out-of-date science and cited by Young [46] , also needs to be updated and corrected.
Olson and Cihacek [13] found that: “Arsenic exists in four forms including two water soluble forms arsenite (+3) and arsenate (+5), which is a water soluble arsenic salt, and much of the water soluble arsenic was not tightly bound and leached from the root zone into the groundwater potentially contaminating the groundwater”.
4.2. Health Effects of Cacodylic Acid on Animals and Humans
The 1974 National Academy of Sciences Part A: Summary and Conclusions report [43] states: “Acute and chronic toxicity studies in a variety of animals indicates a low to medium toxicity rating. No teratological studies, nor toxicity studies in man seem to have been reported”.
Young in a recently revised chapter [46] stated: “The toxicity of the active ingredients, the acid and sodium salt of cacodylic acid, were considered low (LD50 of 2600 mg/kg in the rat). In man, these active ingredients were rapidly excreted unchanged in the urine. Reviews of the limited available studies by the Institute of Medicine concluded that cacodylic acid was likely not a carcinogen, teratogen, or a mutagen in man.” In the 1970s, cacodylic acid is an organic form of arsenic which is considered to be harmless to animals and humans [43] by National Academy of Sciences and the Institute of Medicine. However, later research has shown that inorganic arsenic can be converted by chickens into inorganic arsenic which is toxic and a carcinogen. Recent U.S. Food & Drug Administration research [47] study found “the effects of feeding chickens organic arsenic (non-toxic) supplements and their ability to convert it into inorganic arsenic (toxic Group-A carcinogen). As a result of these findings the use of organic rich chicken feed was banned in the United States. The feed had been used to make chickens more marketable (plumper, redder and prevent certain chicken diseases). Arsenic is a heavy metal and thought to be a carcinogen and dangerous”.
Arsenic poisoning of organisms, including humans and animals, occurs when exposed to As quantities larger than needed. Millions of people living on Southeast Asia deltas, including the Mekong Delta, are affected by arsenic contamination of groundwater. The US Environmental Protection Agency (EPA) considers arsenic, in all forms, a significant risk to human health [48] and is classified as a Group-A carcinogen. On its Hazardous Substances Superfund sites list [49] , the U.S. Agency for Toxic Substances and Disease Registry (ATSDR) ranks arsenic No. 1. Synthetic arsenates include cupric hydrogen arsenate, calcium arsenate, and lead hydrogen arsenate. These 3 synthetic compounds, used prior to, during and after the Vietnam War time period, have also been used in agricultural insecticides, herbicides, and poisons. The list is based on overall toxicity and potential for frequency of occurrence and human exposure at National Priority List Superfund sites. This list ranks chemicals using a formula or algorithm that ranks potential public health hazards on a points-scaled system [49] . The human body is not immune to potential caused by arsenic.
5. Conclusions
Floating plants, including hyacinth, started to clog the shipping lanes through Lake Gatun after the damming of the Chagres River to create the Lake Gatun for the Panama Canal. A mixture of white arsenic, soda and water was sprayed from boats on the floating vegetation from 1914 to 1935. The arsenic, which has no half-life and is water soluble, was deposited in Lake Gatun waters and sediments. Over time, the arsenic levels in Lake Gatun should have declined as a result of the discharge of canal water into the Atlantic and Pacific Oceans [1] . However, water testing is needed to check the arsenic levels and if above 10 μg/l the drinking water should be treated.
The As hotspots in Panama Canal Zone need to be cleaned up [8] . The As levels, including both natural and anthropic sources, in the surface and groundwater of the Panama Canal Zone, need to be reduced to meet WHO drinking water standard (10 ug/L). The canal and groundwater is the drinking water source for the millions of people living in the Panama Canal Zone and Panama City. High As levels in the water used for agriculture can also contribute to the As contamination of the food supply, including sea food. Some of the As can bio-accumulate in animals and humans. As levels in the Panama Canal Zone hotspots, surface water and groundwater need to be mitigated regardless of whether it is from anthropic sources or is naturally occurring As.
Most of the As spikes in the Panama Canal Zone surface water and groundwater are from both anthropic sources and natural alluvial sediment sources. Anthropic sources [1] include past use of As to control wetland vegetation, industrial sources, use of herbicides (cacodylic acid, ANSAR 138 (powder) and PHYTAR 560G (liquid)) on military bases and perimeters, smelting by-products; sewage and wastewater treatment discharges into the Panama Canal and Lake Gatun have added to the As levels in the soil, surface water and groundwater. The As spikes and levels in the Panama Canal Zone soils, surface water and groundwater need to be mitigated [8] , especially when used as a drinking water source. Inorganic arsenic in plant food (grain) can be more problematic than As in sea food including fish which is often organic arsenic. The uptake of trace amounts of inorganic As in plant food is indeed a critical human health and food security issue.
The As is water-soluble and can flow into the Atlantic or Pacific Oceans via the Panama Canal [1] . The As rich ground water on the US military base perimeters can remained in the saturated soil root zone, runoff or was leached into the groundwater. The As levels in anthropic sources, from herbicides (cacodylic acid, ANSAR 138 (powder) and PHYTAR 560 (liquid)) applications, spilling, spraying and dumping hotspots in Panama Canal Zone, are more concentrated (spikes) than the natural As concentration in the alluvial sediments and soils.
The extent of the current arsenic contamination in Lake Gatun, the Panama Canal channel and former Panama Canal Zone U.S. military base grounds is unknown. Systematic sampling of the Lake Gatun or the Panama Canal sediments and soil sampling of former military bases, chemical disposal sites, and sediment is needed to determine if restoration and mitigation are required.
Acknowledgements
This research study was conducted with the support and approval of the Merry Band of Retirees Research Committee. The team includes five US Vietnam veterans, two US Vietnam Era veterans, two US Army veterans, and four Agricultural College Professors. Our team mission is to conduct soil, water, agricultural and natural resource management scientific research; the synthesis and analysis of current and historical documents and scientific evidence relevant to the legacies of war, especially the US Vietnam War; and the preparation and publication of peer reviewed papers of interest and value to those who lead and served in the US military, especially Vietnam Era veterans, their families and the general public. The legacies of the US Vietnam War had impacts far beyond front line veterans; encompassing civilian and military personnel who manufactured, transported and handled the tactical herbicides--arsenic-based Agent Blue and Agent Orange (and other 2,4,5-T herbicides) contaminated with the dioxin TCDD; those who came in contact with contaminated aircraft and other equipment; and the residual effects of these chemicals on southern Vietnam soil and water and the health of people who continue to work these lands for their living.
NOTES
*Kenneth R. Olson is U. S. Army Vietnam Era Veteran and Professor Emeritus of Soil Science.