Epidemiological, Clinical and Evolutionary Aspects of Human African Trypanosomiasis in Children in Nola ()
1. Introduction
Human African trypanosomiasis (HAT), also known as “sleeping sickness”, is a parasitic infection that is almost always fatal if left untreated [1] . The World Health Organization (WHO) has classified it as a neglected tropical disease, and estimated in 2017 that around 65 million people were at risk of contracting it [2] . A major scourge of black Africa in the first half of the 20th century, human African trypanosomiasis (HAT) had become a relatively rare disease by the early 1960s, thanks to eradication campaigns [3] . The incidence of the disease then declined, thanks to intensive surveillance and control efforts in endemic areas. As a result, WHO keeps exhaustive records of all reported cases. In 2018, the number of notified cases was historically low (<1000) [1] .
The Central African Republic (CAR) has three historical outbreaks of HAT, including Nola, located in the south-west of the country, in the Sangha-Mbaéré prefecture [4] . Numerous epidemiological surveys carried out in this outbreak have shown that it has remained very active, often with epidemic outbreaks [4] [5] [6] . Historical data on human African trypanosomiasis in CAR show that in 1945, the country had the sad record of 25,000 trypanosomiasis cases out of the 50,000 in French Equatorial Africa [7] . The fight against the great endemics had reduced this number to 84 patients in 1968 for all outbreaks [8] . But the disease re-emerged dramatically in 1980, rising from less than 80 annual cases before 1981 to between 100 and 700 cases in subsequent years [8] . The epidemiological survey carried out after the recent military-political crisis in 2015 showed a prevalence rate of 1.12% [9] . It is well known that in hyper-endemic situations, pediatric forms of the disease are commonplace, whether transmission is from mother to child or concomitant contamination of the child and his parents gathered in the same site at the mercy of tsetse fly bites [10] . Diagnosis in children is often made only after the onset of neurological signs [11] . The Central African Republic (CAR) is a landlocked country whose economy is based on agriculture and livestock farming, mainly in rural areas. Its economic development therefore depends on good health coverage for the rural population [12] . The aim of this study was to describe the epidemiological, clinical and evolutionary aspects of human African trypanosomiasis in children in rural Central Africa.
2. Materials and Methods
2.1. Study Site
Our study took place in the paediatric wards of the Nola prefectural hospital, located in south-western CAR between 3˚26' north latitude and 15˚52' longitude. The commune of Nola is located in the transition zone between an equatorial Congolese and a subtropical climate, with an almost bimodal rainfall pattern: the rainy season runs from March to November, and the dry season from December to February. Recorded temperatures include monthly averages of 24.7˚C in July and 26.7˚C in March (Map 1). The above map clearly shows the geographical location of the town of Nola and the problem of the central hospital’s isolation from the natural constraints of the two river crossings.
2.2. Ethics
Our study protocol was approved by the local ethical and scientific council. Informed consent from the children’s parents or legal guardians was required prior to inclusion in the study.
2.3. Type and Timing of Study
This was a descriptive and analytical cross-sectional study, covering a fifteen-month period (January 1, 2017 to March 30, 2018) and conducted according to the protocol of the National Program for the Control of Human African Trypanosomiasis [13] [14] .
2.4. Study Population
It consisted of all children seen in pediatric consultation or hospitalization at Nola prefectural hospital during the study period.
![]()
Map 1. Location of Nola prefectural hospital and access problems.
2.5. Inclusion Criteria
We included in the study all children seen as outpatients or admitted as inpatients to the pediatric wards of Nola prefectural hospital during the study period, in whom the diagnosis of HAT had been confirmed by parasitological or serological examination.
2.6. Procedure
On pre-established survey forms, we collected data relating to epidemiological (age, sex, commune of origin), clinical and paraclinical (CATT, trypanosome testing) characteristics, treatment (pentamidine, melarsoprol), course (cure, relapse, death, sequelae), cerebrospinal fluid (CSF) study, numbering of white CSF.
2.7. Case Screening
For all children seen in consultation or hospitalized with suspected THA, according to the criteria of the Central African Republic trypanosomiasis control program [15] . The first step was to carry out a serological cardagglutination test with stained trypanosomes (CATT) [16] on whole blood, enabling an initial screening. The second step, in CATT-positive children (CATT+), involved palpation and lymph node puncture (ppg). If trypanosomes were present (T+ on ppg), the diagnosis of HAT was confirmed. The third stage involved CATT titration (dilution of CATT on successive dilutions) if no trypanosomes were present at ppg (T− at ppg). If titration > 1/8 (specificity close to 100%), continue with trypanosome testing; if titration < 1/8: subject considered free of trypanosomes. The fourth stage consisted of parasitological testing of venous blood if CATT > 1/8 by centrifugation in a capillary heparin tube (CTC). If CTC was negative, parasitological tests were carried out using the mini Anion Exchange Centrifugation Technique (mAECT) [17] [18] . At the end of these four steps, the children were classified into four categories:
- healthy children: CATT negative, or CATT positive but T− and CATT titration < 1/8,
- sick children: T+ at ppg, CTC or mAECT,
- sick children: CATT > 1/8,
- serological children: CATT = 1/8, T−, subjects classified as “to be followed” [18] .
In all cases, a child was declared ill only if the trypanosome was demonstrated microscopically, either by examination of the lymph node juice or in the blood by mAECt, and was included in the study. Once cases were included in the study, a lumbar puncture was performed immediately, and the disease stage was defined on the basis of the number of leukocytes present and/or the presence of trypanosomes in the CSF. Staging criteria were those advocated by WHO (2014). All children whose cytorachy was between 0 - 5 elements/mm3 elements/mm3 were classified in the first stage (lymphaticosanguineous). Children classified as second stage (meningoencephalitis), were those whose cytorachy was greater equal to 6 elements/mm3 [19] . After phase diagnosis, treatment with pentamidine or melarsoprol was initiated, followed by monitoring.
2.8. Data Processing and Analysis
Data were encoded in Excel 2010. Statistical analysis was performed using Epi Info version 7 software. Chi2 and correlation tests were applied.
3. Results
3.1. Descriptive Results
3.1.1. Epidemiological Data
During the study period, the Nola prefectural hospital recorded 40 cases of HAT among the 2446 infants and children hospitalized (Diagram 1).
1) Sex and age
Of the 40 cases collected, 40% (n = 16) were male and 60% (n = 24) female, i.e. a sex ratio of 0.66. The median age of the children was 8.65 ± 12.48 years, with extremes [6 months - 14 years]. Children under 5 years of age accounted for 15% (n = 6) versus 85% (n = 34) of those over 5. The distribution of children by age group shows that children aged 0 to 4 represented 15% (n = 6), those aged 5 to 9 42.5% (n = 17) and those aged 10 to 14 42.5% (n = 17).
2) Distribution of children according to year and household (Communes) of origin
The year 2018 recorded 52.5% (n = 21) cases versus 47.5% (n = 19) cases in 2017. Children came from the Bilolo household in 60% (n = 24/40) of cases, followed by the Nola commune in 30% (n = 12/40) of cases. The Salo commune household was less active, with 10% (n = 4) of cases.
3) Children’s daily activities
In our series, 82.50% (33/40) of children were of school age. Of these, 75.75% (25/33) actually attended school, versus 24.24% (n = 8/25) who worked with their parents on the plantations (15.15%, 5/33), in the gold/diamond mines (3.03%, n = 1/33) and 6.06% (n = 2) alternated between fishing, field work and animal husbandry. The 75.75% (25/33) of children attending school also practised other activities outside school hours, such as hunting 32% (n = 8/25), honey gathering 28% (n = 7/25) and 40% (n = 10/25) regularly collected firewood or practised other activities related to the exploitation of natural resources.
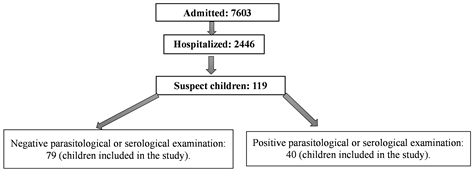
Diagram 1. Flow of children.
3.1.2. Clinical Data
1) History and functional signs
A previous history was found in 15% (n = 6) of children - 7.5% (n = 3) sickle cell disease, 5% (n = 2) HIV, and 2.5% (n = 1) diabetes.
Functional signs included fever 82.5% (n = 33), nocturnal insomnia 75% (n = 30), daytime somnolence 67.5% (n = 27), headache 65% (n = 26), polyarthralgia 62.5% (n = 25), consults 52.5% (n = 21), physical asthenia 37.5% (n = 15), tremor 27.5% (n = 11), pruritus 25% (n = 10), dizziness 12.5% (n = 5), trypamide 7.5% (n = 3), bulimia 7.5% (n = 3) and delirium in 2.5% (n = 2), noting that the same child could have one or more symptoms. The reasons for consultation are shown in Figure 1.
4) Physical examination data
The physical examination revealed hepatosplenomegaly 65% (n = 26), adenopathy 52.5% (n = 21), paresthesia 30% (n = 12), decreased cutaneous-abdominal reflexes 25% (n = 10), hyperesthesia 20% (n = 8), decreased osteoarticular reflexes 17.5% (n = 7), jaundice 17.5% (n = 7), extrapyramidal hypertonia 15% (n = 6), and depression 2.5% (n = 1). It should be noted that the same child could have one or more physical signs. For the 21(n = 52.50%) children with adenopathies, the location was axillary in 22.50% (n = 9), cervical in 20% (n = 8), subclavicular in 5% (n = 2) and inguinal in 5% (n = 2). Examination findings are shown in Figure 2.
3.1.3. Paraclinical Data
Data from cerebrospinal fluid analysis showed that 67.5% (n = 27) of children had a WBC count between 0 - 5/µl and 32.5% (n = 13) a WBC count ≥ 6 /µl. Of the 27 (67.5%) children with WBC counts of 0 - 5/µl, 65% (n = 26) had no trypanosomes in the CSF, versus 2.5% (n = 1) with trypanosomes present in the CSF. Among the 13 (n = 32.5%) children with leukocyte counts ≥ 6/µl, 20% (n = 8) had trypanosomes present in the CSF versus 12.5% (n = 5) with absence of trypanosomes in the CSF. The distribution of children according to CSF results is shown in Table 1.
![]()
Figure 1. Distribution of children by reason for consultation. *The same child could have one or more symptoms.
![]()
Figure 2. Distribution of children by reason for consultation. NB: the same child could have one or more physical signs. For the 21 (n = 52.50%) children with adenopathies, the location was axillary in 22.50% (n = 9), cervical in 20% (n = 8), subclavicular in 5% (n = 2) and inguinal in 5% (n = 2).
![]()
Table 1. Distribution of children according to CSF results.
3.1.4. Phase Diagnosis and Treatment
65% (n = 26) of the children were in the lymphatico-sanguineous phase (first phase), while 35% (n = 14) were in the meningoencephalic phase (second phase). Treatment of the children depended on phase orientation: 65% (n = 26) were treated with Pentamidine and 35% (n = 14) received combined treatment (Eflornitine + Nifurtimox).
3.1.5. Progression
Progression was favorable in 75% (n = 30) of cases. Complications occurred in 25% (n = 10) of cases, with neurological sequelae in 15% (n = 6) and death in 10% (n = 4).
3.2. Analytical Results
3.2.1. Relationship between Severity and Socio-Demographic Characteristics
There was a relationship between age under 5, Bilolo’s household, children’s history and disease severity.
3.2.2. Relationship between Sequelae and Socio-Demographic and Clinical Characteristics
There was an association between age under 5, gender, household, year of infection, children’s activity, history and occurrence of sequelae.
4. Discussion
4.1. Epidemiological Aspects
HAT is a disease that afflicts the rural populations of sub-Saharan Africa, where the tsetse fly (or glossina) proliferates, the vector of the trypanosomes that cause it [19] . The WHO Roadmap on Neglected Tropical Diseases set a target date of 2020 for the elimination of HAT as a public health problem, and projected the total interruption (zero cases) of transmission in Africa by 2030 [20] [21] [22] . To achieve this goal, coordinated international efforts are needed between WHO, national HAT programs and non-governmental organizations. The Central African Republic has five (5) active outbreaks of HAT, including Nola, located in the south-west of the country, in the isolated and landlocked prefecture of Sangha-Mbaéré, where access is difficult [4] [5] . The combined efforts of the National HAT Control Program, set up in 1990, the WHO and non-governmental organizations led to the stabilization of the HAT epidemic in the Nola outbreak in 2000. The intensification of regular surveillance (active and passive screening, followed by treatment to reduce the parasite reservoir), the creation of outpatient treatment centers (Nola commune, Bilolo commune) and vector control using Gouteux-Lancien conical traps impregnated with the insecticide deltamethrin, carried out in endemic villages, broke the chain of transmission and brought the HAT endemic under control in the Nola and Bilolo foci [23] [24] [25] . All these actions, coupled with epidemiological surveillance, resulted in the Nola and Bilolo outbreaks becoming inactive between 2007 and 2011, with no reported cases of HAT [4] . The evolution of the HAT epidemic between 1991 and 2008 in the Nola and Bilolo outbreak is shown in Figure 3.
An epidemiological survey carried out in 2015 after the socio-militaro-political crisis that shook CAR in 2013 noted a possible re-emergence of the Nola and Bilolo outbreak with an HAT incidence rate of 1.12% [9] . Our study bears witness to this re-emergence of the Nola and Bilolo outbreak, with 40 cases recorded between January 2017 and March 2018. However, we must remain cautious and consider that the 40 cases in our series are probably underestimated, due to the fact that our study was carried out in a paediatric hospital setting. This passive medical survey covers only a tiny area of the Nola sub-prefecture. What’s more, diagnosis is often delayed, as symptoms are often misleading in children living in rural tropical environments, where any fever initially suggests malaria,
![]()
Figure 3. Répartition annuelle des cas en fonction des types de dépistage [4] .
and its persistence suggests poor treatment or typhoid fever [11] [26] [27] . A retrospective study covering a period of 33 years (1971 to 2004) noted 606 (18.1%) cases of children under 15 in the Nola and Bilolo households, an average of 18.36 children per year [7] . This average value is lower than the number of cases found in our series, where from one year to the next the number of cases was clearly on the rise, with 19 cases in 2017 and 21 cases in 2018. This shows the scale of the phenomenon after the socio-militaro-political crisis of 2013, which is probably the catalyst for the re-emergence of HAT in Nola. A strong risk of HAT re-emergence had already been suspected by other authors after the socio-political crisis experienced by Côte d’Ivoire between 2000 and 2011 [28] [29] . In our series, the Nola outbreak comprised three infestation zones, with the epicentre of HAT located in the commune of Bilolo, which accounted for 60% (n = 24) and 59% of the total 40 cases. Our results corroborate those of Mbelsso, who noted that Bilolo accounted for 59% of all cases [7] . Other authors have also confirmed Bilolo as the epicenter of the disease [4] [5] . This could be explained by the favorable conditions associated with the natural environment. Like all forest areas, Bilolo enjoys a sub-equatorial climate, with hot, humid winds typical of the southwestern part of the Central African Republic, and an average annual rainfall of 1520 mm. Recorded temperatures include monthly averages of 24.7˚C in July and 26.7˚C in March. The relief of the Bilolo commune is made up of a succession of hills with low-lying areas of over 40 meters. There are two main plant formations: dense humid forest and dense semi-deciduous forest. Trees can reach heights of 60 to 70 meters and diameters of 3 to 4 meters. A large part of these forests remains untouched. The commune of Bilolo is abundantly watered by swamps and rivers [9] . All these conditions are conducive to maintaining the existing tsetse fly population in Bilolo, and to increasing their density. This finding corroborates the conclusions of several authors [30] [31] [32] [33] . However, the outbreak in the commune of Salo, which is generally inactive, recorded 10% (n = 4) of cases.
School-age children were the most affected by HAT, at 82.5% (n = 33/40). The burden of HAT in this age group can be explained by the fact that, in rural Africa, children, whether attending school or not, are a great help to their families, often working with their parents or engaging in other activities, such as bathing, gathering honey, collecting wood, hunting and fishing, as shown by the results of our series. All this increases their exposure to tsetse fly bites. Contact time between children and tsetse flies was highest among the 24.24% (n = 8/25) of schoolchildren who had deserted the classroom, either for lack of support from their parents, who have been hard hit by the region’s economic crisis. Either because of a lack of infrastructure in the schools, in particular the two nursery schools in the town of Nola and the school at the CDV center, which were completely looted by the Séléka. The SCED school has been in ruins since 2013 (see Plate 1). Although education in the Central African Republic is compulsory from age 6 to 15 and free of charge, [14] [15] more than 3,000 children in the SCED village are deprived of this right for lack of infrastructure. The few children who want an education have to cross a 7 km path to study in Belamboké, increasing the risk of exposure to tsetse flies along the way [34] . The rest of the school-age children in this village who are supposed to be in classrooms are forced to go to tsetse fly habitats to work alongside their parents. This finding reinforces the conclusions of UNICEF, which estimates that 31% of Central African children aged between 5 and 17 work, despite the fact that this is forbidden by the Central African National Labor Code [35] . In his study, Camara recorded 27.08% (n = 26/96) of children, 65.38% (n = 17) aged 10 to 14 and 34.62% (n = 9) aged 5 to 9, with no children under 4 [36] .
4.2. Clinical Aspects
Despite the existence of these HAT foci, the situation appears to be less worrying

Plate 1. Pygmy habitats and school situation of SCED village children.
than in the Central African capital (Bangui), to such an extent that this condition is ignored by most practitioners and health personnel caring for children. The same is true of the historical focus of Nola, as our study shows, where many of the 35% cases (n = 14) were diagnosed late in the second phase (meningoencephalitis). This is due to the fact that, in an area where the possibilities of bacterial, viral or parasitic contamination are multiple and permanent, fever (82.5%), convulsions (52.5%) and adenopathies (52.50%), whether isolated or associated, do not a priori suggest trypanosomiasis. On the other hand, the association with any of the signs of nocturnal (75%) and/or diurnal (67.5%) wakefulness or sleep disturbances, asthenia/prostration (37.5%), motor disorders (27.5%) or psychic disorders (2.5%), generally draws the attention of practitioners in endemic zones to think of HAT. Moreover, one of the characteristics of paediatric HAT is the intertwining of the clinical signs of the two phases of the disease, due to the rapid transition from the first to the second phase [37] [38] . Other authors emphasize the undoyance of clinical signs in the paediatric setting, ranging from more specific signs such as hypersomnia, psychic disorders and the very marked physical asthenia found by Triolo et al. [38] [39] [40] . Non-specific, misleading signs common to many tropical pathologies, yet classic of HAT, such as: sudden onset of infection or rapid onset of febrile coma, headache, pruritus, hepatosplenomegaly [3] [37] [38] [40] [41] [42] . These signs were also present in our children in varying proportions, although it should be noted that in Africa, these signs only attract the attention of clinicians at a late stage.
4.3. Developments
HAT screening during the study period was essentially passive, due to the scarcity of survey campaigns. Delayed diagnosis is a factor in poor prognosis. Moreover, this prognosis depends mainly on the stage of the disease, and appears to be worse when the trypanosome is detected in the CSF [43] . In our series, treatment resulted in an uncomplicated cure in 75% of cases. However, as the children were not followed up for more than a year, the possibility of neurological sequelae cannot be ruled out [44] [45] . Complications were present in 25% (n = 10) of cases, with neurological sequelae in 15% (n = 6) and death in 10% (n = 4). In a Cameroonian study, almost half the children had sequelae, 12% remained severely handicapped due to a combination of several disorders, and 58% were unaffected [39] . The case-fatality rate was 15.7% in Kinshasa [37] , and 2.5% in Fontem, Cameroon [38] . The study carried out in Dakar revealed 4 deaths out of a total of 14 children [41] .
5. Conclusion
Human African trypanosomiasis is still common among children in Nola, especially those who work alongside their parents in forestry activities. Sequelae remain high, especially in the second phase of the disease. Public awareness campaigns and vector control measures are needed to reduce the impact of the disease.