Concurrent Achilles tendon vibration and tibial nerve stimulation to estimate persistent inward current strength in motoneurons
Accepted: 22 October 2021
HTML: 2
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
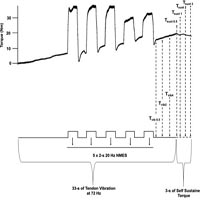
Vibratory (Tvib) and sustained (Tsust) torque responses to concurrent Achilles tendon vibration and neuromuscular electrical stimulation applied over the muscle belly (vib+stim) are used as indicators of motoneuron facilitation and, theoretically, persistent inward current strength. However, neuromuscular electrical stimulation (NMES) applied to the nerve trunk may potentiate motoneuronal excitability more than muscle belly NMES, yet it remains unclear whether NMES applied over the nerve evokes robust Tvib and Tsust responses when used during the vib+stim protocol. This study tested whether a nerve-targeted vib+stim protocol elicits Tvib and Tsust responses in the ankle plantar flexors with acceptable intra- and inter-session reliability. Fifteen men performed the vib+stim protocol with NMES applied over the tibial nerve three times across two sessions; twice in a single session (5-min apart) to test intrasession reliability and then again after 48 h to test intersession reliability. Intraclass correlation coefficients (ICC3,1), within-participant coefficients of variation (CV) and pairwise comparisons were used to verify relative and absolute reliability as well as systematic bias. Thirteen men presented Tvib and Tsust responses (response rate of 87%). Intrasession Tvib and Tsust ICCs were >0.73 but inter-session ICCs were <0.5. Although no systematic bias was detected (p>0.05), both intra- and inter-session CVs were large (>10%) for Tvib and Tsust. The Vib+stim protocol with NMES applied over the nerve evoked Tvib and Tsust in almost all participants, but presented a large intra- and inter-session variability. The method does not appear to be effective for assessing motoneuron facilitation in the plantar flexors.
Trajano GS, Seitz LB, Nosaka K, Blazevich AJ. Can passive stretch inhibit motoneuron facilitation in the human plantar flexors? J Appl Physiol. 2014;117(12):1486-1492. DOI: https://doi.org/10.1152/japplphysiol.00809.2014
Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle Nerve. 2005;31(2):135-156. doi:10.1002/mus.20261. DOI: https://doi.org/10.1002/mus.20261
Lee RH, Heckman CJ. Paradoxical Effect of QX-314 on Persistent Inward Currents and Bistable Behavior in Spinal Motoneurons In Vivo. J Neurophysiol. 1999;82(5):2518-2527. DOI: https://doi.org/10.1152/jn.1999.82.5.2518
Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127(10):2247-2258. DOI: https://doi.org/10.1093/brain/awh243
Kirk EA, Rice CL. Contractile function and motor unit firing rates of the human hamstrings. J Neurophysiol. 2017;117(1):243-250. DOI: https://doi.org/10.1152/jn.00620.2016
Del Vecchio A, Negro F, Holobar A, Casolo A, Folland JP, Felici F, Farina D. You are as fast as your motor neurons: speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. J Physiol. 2019 May;597(9):2445-2456. Epub 2019 Mar 1. DOI: https://doi.org/10.1113/JP277396
Johnson MD, Heckman CJ. Interactions between focused synaptic inputs and diffuse neuromodulation in the spinal cord. Ann N Y Acad Sci. 2010;1198(1):35-41. DOI: https://doi.org/10.1111/j.1749-6632.2010.05430.x
Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist. 2008;14(3):264-275. DOI: https://doi.org/10.1177/1073858408314986
Vandenberk MS, Kalmar JM. An evaluation of paired motor unit estimates of persistent inward current in human motoneurons. J Neurophysiol. 2014;111(9):1877-1884. DOI: https://doi.org/10.1152/jn.00469.2013
Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for Plateau Potentials in Tail Motoneurons of Awake Chronic Spinal Rats With Spasticity. J Neurophysiol. 2001;86(September 2000):1972-1982. DOI: https://doi.org/10.1152/jn.2001.86.4.1972
Perrier J-F, Rasmussen HB, Christensen RK, Petersen AV. Modulation of the intrinsic properties of motoneurons by serotonin. Curr Pharm Des. 2013;19(stage 28):4371-4384. DOI: https://doi.org/10.2174/13816128113199990341
Hyngstrom AS, Johnson MD, Heckman CJ. Summation of excitatory and inhibitory synaptic inputs by motoneurons with highly active dendrites. J Neurophysiol. 2008;99(4):1643-1652. DOI: https://doi.org/10.1152/jn.01253.2007
Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol. 2009;102(4): 2026-2038. DOI: https://doi.org/10.1152/jn.00151.2009
Kirk BJC, Trajano GS, Pulverenti TS, Rowe G, Blazevich AJ. Neuromuscular Factors Contributing to Reductions in Muscle Force After Repeated, High-Intensity Muscular Efforts. Front Physiol. 2019;10(June):1-17. DOI: https://doi.org/10.3389/fphys.2019.00783
Magalhães FH, Kohn AF. Vibration-induced extra torque during electrically- evoked contractions of the human calf muscles. J Neuroeng Rehabil. 2010;7(26):1-17. DOI: https://doi.org/10.1186/1743-0003-7-26
Bergquist AJ, Clair JM, Collins DF. Motor unit recruitment when neuromuscular electrical stimulation is applied over a nerve trunk compared with a muscle belly: Triceps surae. J Appl Physiol. 2011;110(3):627-637. DOI: https://doi.org/10.1152/japplphysiol.01103.2010
Bergquist AJ, Wiest MJ, Collins DF. Motor unit recruitment when neuromuscular electrical stimulation is applied over a nerve trunk compared with a muscle belly: Quadriceps femoris. J Appl Physiol. 2012;113(1):78-89. DOI: https://doi.org/10.1152/japplphysiol.00074.2011
Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: Systematic variations in rhythmic firing patterns. J Neurophysiol. 1998; 80(2):572-582. DOI: https://doi.org/10.1152/jn.1998.80.2.572
Mizuno T. Changes in joint range of motion and muscle–tendon unit stiffness after varying amounts of dynamic stretching. J Sports Sci. 2017; 35(21):2157-2163. DOI: https://doi.org/10.1080/02640414.2016.1260149
Opplert J, Babault N. Acute effects of dynamic stretching on mechanical properties result from both muscle-tendon stretching and muscle warm-up. J Sport Sci Med. 2019;18(2):351-358.
Weir JP. Quantifying Test-Retest Reliability Using the Intraclass Correlation Coefficient and the SEM. J Strength Cond Res. 2005;19(1):231. DOI: https://doi.org/10.1519/15184.1
Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155-163. DOI: https://doi.org/10.1016/j.jcm.2016.02.012
Atkinson G, Nevill AM. Statistical Methods For Assessing Measurement Error (Reliability) in Variables Relevant to Sports Medicine. Sport Med. 1998;26(4):217-238. DOI: https://doi.org/10.2165/00007256-199826040-00002
Hopkins WG. Measures of Reliability in Sports Medicine and Science. Sport Med. 2000;30(1):1-15. DOI: https://doi.org/10.2165/00007256-200030010-00001
Hopkins WG. How to Interpret Changes in an Athletic Performance Test. Sportscience. 2004;8:1-7.
Flanagan EP. The effect size statistic-applications for the strength and conditioning coach. Strength Cond J. 2013;35(5):37-40. DOI: https://doi.org/10.1519/SSC.0b013e3182a64d20
Collins DF. Central Contributions to Contractions Evoked by Tetanic Neuromuscular Electrical Stimulation. Exerc Sport Sci Rev. 2007;103(1):102-109. DOI: https://doi.org/10.1097/jes.0b013e3180a0321b
Kitago T, Mazzocchio R, Liuzzi G, Cohen LG. Modulation of H-reflex excitability by tetanic stimulation. Clin Neurophysiol. 2004;115(4):858-861. DOI: https://doi.org/10.1016/j.clinph.2003.11.029
Leung H, Latella C, Lamon S, Hendy AM. The reliability of neurological measurement in the vastus medialis: Implications for research and practice. Front Psychol. 2018;9(OCT):1-10. DOI: https://doi.org/10.3389/fpsyg.2018.01857
Merlet AN, Cattagni T, Cornu C, Jubeau M. Effect of knee angle on neuromuscular assessment of plantar flexor muscles: A reliability study. PLoS One. 2018;13(3):1-16. DOI: https://doi.org/10.1371/journal.pone.0195220
Trajano GS, Taylor JL, Orssatto LBR, McNulty CR, Blazevich AJ. Passive muscle stretching reduces estimates of persistent inward current strength in soleus motor units. J Exp Biol. 2020;223(September). DOI: https://doi.org/10.1101/2020.07.31.230029
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2021.10045
https://doi.org/10.4081/ejtm.2021.10045



