Secreted key regulators (Fgf1, Bmp4, Gdf3) are expressed by PAC1-immunopositive retinal ganglion cells in the postnatal rat retina
Accepted: 2 March 2022
HTML: 17
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
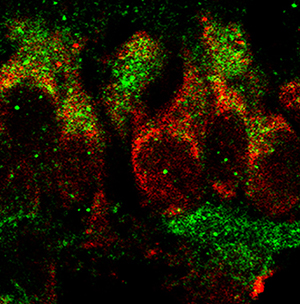
Identified as a member of the secretin/glucagon/VIP superfamily, pituitary adenylate cyclase-activating polypeptide (PACAP1-38) has been recognized as a hormone, neurohormone, transmitter, trophic factor, and known to be involved in diverse and multiple developmental processes. PACAP1-38 was reported to regulate the production of important morphogens (Fgf1, Bmp4, Gdf3) through PAC1-receptor in the newborn rat retina. To follow up, we aimed to reveal the identity of retinal cells responsible for the production and secretion of Fgf1, Bmp4, and Gdf3 in response to PACAP1-38 treatment. Newborn (P1) rats were treated with 100 pmol PACAP1-38 intravitreally. After 24 h, retinas were dissected and processed for immunohistochemistry performed either on flat-mounted retinas or cryosections. Brn3a and PAC1-R double labeling revealed that 90% of retinal ganglion cells (RGCs) expressed PAC1-receptor. We showed that RGCs were Fgf1, Bmp4, and Gdf3-immunopositive and PAC1-R was co-expressed with each protein. To elucidate if RGCs release these secreted regulators, the key components for vesicle release were examined. No labeling was detected for synaptophysin, Exo70, or NESP55 in RGCs but an intense Rab3a-immunoreactivity was detected in their cell bodies. We found that the vast majority of RGCs are responsive to PACAP, which in turn could have a significant impact on their development or/and physiology. Although Fgf1, Bmp4, and Gdf3 were abundantly expressed in PAC1-positive RGCs, the cells lack synaptophysin and Exo70 in the newborn retina, thus unable to release these proteins. These proteins could regulate postnatal RGC development acting through intracrine pathways.
Ethics Approval
All procedures were approved by the Ethical Committee of the University of Pécs, Hungary, and the Animal Health and Animal Welfare Directorate of the National Food Chain Safety Office of the Hungarian State (BA/35/51-58/2016)Rights
TKP2021-EGA-16 has been implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the TKP2021-EGA funding scheme. The work has also been supported by EFOP 3.6.-2-16-2017-0008 and NKFIH 119 289.How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2022.3373
https://doi.org/10.4081/ejh.2022.3373






