- Center on Health Equity and Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Diagnosis and Management of Iron Deficiency Anemia in Inflammatory Bowel Disease
Abstract
Inflammatory bowel disease (IBD) is associated with extraintestinal manifestations in more than one-quarter of patients. Anemia is one of the most common concerns. Patients with IBD and comorbid iron deficiency anemia (IDA) are at risk for hospitalization and surgery, and IDA impacts health-related quality of life. IDA in IBD is often underdiagnosed and undertreated. Prompt treatment has the potential to improve patient quality of life and clinical outcomes. Although the treatment goals for IDA in IBD are well-defined, selecting a treatment is not as straightforward. Traditional oral iron replacement therapies are generally only recommended in patients with mild anemia who do not have active inflammatory disease. Novel oral iron formulations may circumvent some of the limitations associated with traditional oral products.
Am J Manag Care. 2021;27(suppl 11):S211-S218. https://doi.org/10.37765/ajmc.2021.88714
Introduction
Inflammatory bowel disease (IBD) is an overarching term used to describe chronic relapsing gastrointestinal (GI) inflammatory disorders, which may cause mucosal ulceration and bleeding, specifically Crohn disease (CD) and ulcerative colitis (UC). The incidence and prevalence of IBD are increasing with Westernized nations having the highest rates.1 Pathogenesis is multifactorial, including genetic, lifestyle, immune, and intestinal microbiota factors.2 IBD is associated with extraintestinal manifestations in more than 25% of patients, including hematologic or renal effects.2,3 The most common causes of anemia in IBD include iron deficiency, which is present in more than half of all cases, as well as vitamin B12 deficiency, and anemia of chronic disease.2,4
Patients with IBD have a higher incidence of anemia compared with a matched cohort without IBD, with an incidence of 92.8 per 1000 person-years compared with 51.2 per 1000 person-years.5 Patients with IBD also have an almost 2-fold increase in duration of anemia, compared with those without: 52 days versus 27 days per year. This prevalence is greater in females than males, and in CD compared with UC.4,6
IDA significantly affects patient quality of life, and in patients with IBD is associated with higher rates of hospitalization and surgery.2,4 Unfortunately, IDA is underdiagnosed and undertreated in patients with IBD. For example, results of a Veterans Affairs study found more than one-third of patients with anemia and UC were not tested for IDA. Moreover, among those who were diagnosed with IDA, 25% did not receive iron replacement therapy.7 The purpose of this document is to review the diagnosis and management of IDA in patients with IBD.
Iron Homeostasis and Effect of Inflammation
Iron, an essential component of hemoglobin (Hb), is required for various cellular functions, including enzymatic processes, DNA synthesis, and mitochondrial energy generation.8 Iron homeostasis is regulated systemically by multiple mechanisms, but without an excretion regulation pathway, iron status is controlled primarily by dietary intake, intestinal absorption, and iron recycling.1,9 Patients with IBD may experience iron loss due to intestinal bleeding, and iron depletion may be compounded by the fact that patients with IBD may have disordered eating and often experience malabsorption related to duodenal disease or inflammation.
The impact of inflammation is mediated primarily via hepcidin, a small protein secreted by hepatocytes. Hepcidin negatively regulates ferroportin, the major cellular iron exporter found predominantly on the intestinal epithelium, macrophages, and hepatocytes.9,10 Ferroportin enables the transport of iron absorbed via the intestine into circulation, and also mediates iron release from other cells.10,11 In the presence of iron, hepcidin binds to ferroportin and induces its internalization and degradation by lysosomes, ultimately hindering absorption and export of iron with a net result of depressing serum iron levels.10,11 Typically hepcidin is upregulated in the presence of high iron concentrations and downregulated by iron deficiency, erythropoiesis, hypoxia, and endocrine signals.8 However, patients with active inflammatory diseases such as IBD often have elevated hepcidin levels suppressing iron absorption even in states of iron deficiency.
Signs and Symptoms of IDA
IDA can range from mild (Hb 10 to 13 grams/dL) to severe (Hb <8 grams/dL).12 The symptoms of iron deficiency are nonspecific, including fatigue, weakness, headache, and dyspnea on exertion. Severe IDA may present with tachycardia, a functional systolic heart murmur, syncope, dyspnea at rest, or angina.1,8
Monitoring Iron Status and Diagnosing IDA
Anemia is defined by the World Health Organization as a blood Hb concentration below 13 grams/dL for males and 12 grams/dL for females.12 Laboratory evaluation is necessary to establish iron deficiency as the cause of anemia. Serum ferritin, the storage molecule for iron, is less than 30 µg/L in patients with isolated iron deficiency. Transferrin, the carrier molecule for iron, is typically elevated, while serum iron and the transferrin saturation (TSAT) are low. TSAT below 20% is required for the diagnosis of IDA.1,13
No single assay is sufficient for diagnosing iron deficiency in patients with concomitant inflammation. If findings indicative of inflammation are present (eg, elevated C-reactive protein), the threshold for IDA is a ferritin less than 100 µg/L.1,2,14 Iron-restricted erythropoiesis can occur with even higher ferritin levels. Transferrin levels may be normal or reduced in chronic inflammatory states.2 Serum and urinary hepcidin concentrations are significantly decreased in isolated IDA but are often increased in patients with IBD and IDA.1,2 Additional markers may aid in diagnosing iron-restricted erythropoiesis in the setting of chronic inflammatory disease, such as soluble transferrin receptor, which is typically elevated, and a reduced reticulocyte Hb level. Thus, clinicians should consider a constellation of laboratory parameters related to iron for diagnosis of iron restriction or deficiency, and these same values can also be used to evaluate response to therapy.
Once a patient receives a diagnosis of IDA, appropriate treatment strategies must be selected. Elevated C-reactive protein, interleukin-6, or hepcidin are consistent with ferroportin downregulation, which may preclude adequate absorption of oral iron and bolster the role of intravenous (IV) iron replacement.1,13
Management of IDA in IBD
Patients diagnosed with IDA, regardless of overt clinical symptoms, should be promptly treated with an emphasis on replenishing iron stores and addressing the underlying cause.15,16 The goals of IDA treatment include normalization of Hb and iron stores and improving quality of life.2
Although there is a clear consensus among experts worldwide on overall treatment goals, there are various approaches for iron replenishment in patients with IBD. For example, in Europe, IV iron has become the preferred first-line therapy for patients with IBD and active disease, while in the United States, oral iron is still preferred in certain guidelines.17 Both routes have demonstrated clinical efficacy. Adverse effects (AEs) associated with oral iron therapies are often dose dependent, and may be minimized with newer oral iron products.18-20 Considerations with IV replacement therapy include the need for administration by a healthcare provider as well as cost, which may impact access to care.
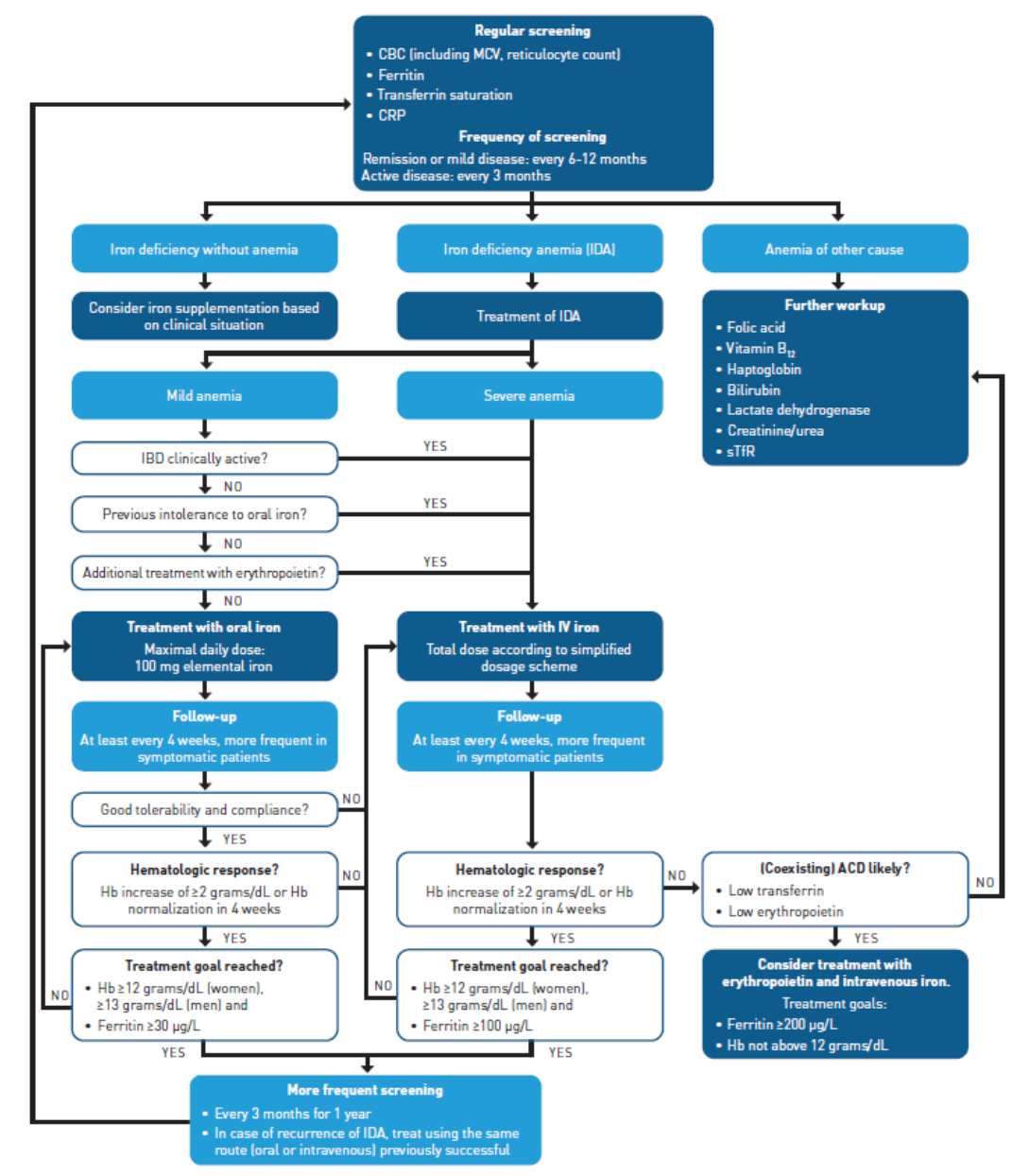
An algorithm from Niepel and colleagues provides some guidance for screening and treatment of patients with IDA in IBD based on the 2015 European Consensus Guidelines, as well as the 2007 Crohn’s & Colitis Foundation Guidelines (Figure21). Specifically in patients with IBD and anemia, several factors must be taken into account, including degree of intestinal inflammation, history of GI surgery, and potential vitamin deficiency or dietary restrictions. Specifically in patients with duodenal disease or in those taking a proton pump inhibitor, the ability to absorb iron may be impaired.21
ACD, anemia of chronic disease; CBC, complete blood count; CRP, C-reactive protein; Hb, hemoglobin; IBD, inflammatory bowel disease; IDA, iron deficiency anemia; IV, intravenous; MCV, mean corpuscular volume; sTfR, soluble transferrin receptor.
Oral Iron
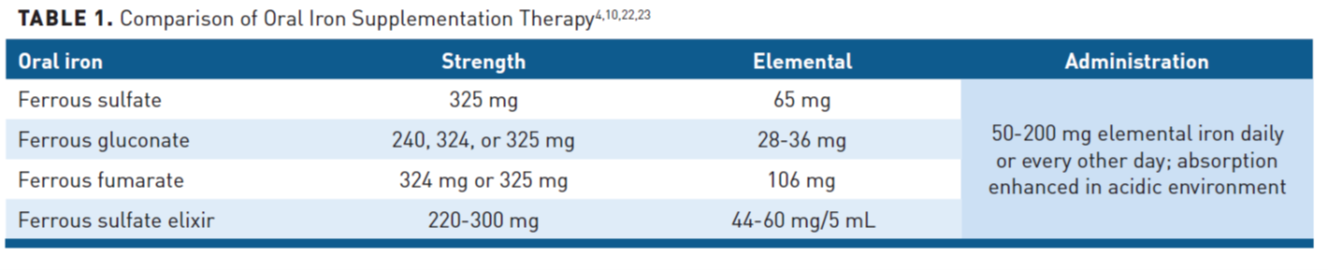
Oral iron is a commonly prescribed treatment for iron replacement due to low cost with easy access and administration (Table 14,10,22,23).Oral iron is recommended in patients with mild IDA, and for most patients without active IBD unless there is a contraindication or intolerance.1,24,25 Oral iron products require an acidic environment for optimal absorption and as a polyvalent cation, it has notable drug interactions that must also be considered. An increase of 2 grams/dL of Hb may be observed within 4 weeks of treatment initiation, but oral treatment for 6 months is typically required to fully replenish iron stores.2,15
Clinical efficacy and safety of oral iron in patients with IBD have been demonstrated in multiple studies.10,26 A 2009 study of 100 patients reported results on 78 patients with mild anemia (Hb >10 grams/dL) and inactive disease, finding that 89% of participants had complete normalization of Hb, with only 5.1% being intolerant and requiring IV iron.27 Moreover, IBD activity did not increase, and quality of life improved as Hb levels normalized.
Ferrous iron. Due to bioavailability, ferrous iron preparations (ie, ferrous sulfate, ferrous gluconate, ferrous fumarate) are most commonly used.10 The optimal oral dose of iron for patients with IDA has not been fully elucidated; however, as only 10% to 25% is absorbed, it is recommended not to exceed 200 mg of oral iron daily to minimize AEs, which are often dose dependent and may suppress ferroportin.10 An alternate-day single-dose has been shown to be more effective than daily dosing, as iron absorption is decreased with more frequent dosing due to increased serum hepcidin and its inhibitory effects on iron transfer.28 Patients should be counseled to take on an empty stomach if tolerated, or with either 250 mg ascorbic acid or a glass of orange juice to improve absorption.23
Ferric maltol (FM) is a newer oral iron preparation created from a stable complex of ferric iron (Fe3+) with trimaltol.29 FM is less toxic to the GI tract mucosa than ferrous preparations. The Food and Drug Administration (FDA) approved ferric maltol in 2019 for IDA, but it is not yet commercially available.29 FM has been used in the European Union for IDA treatment since 2016.30 The efficacy and tolerability of FM in IDA in IBD was examined in a randomized, double-blind, placebo-controlled trial that included patients with disease remission or nonsevere disease activity and those whose treatment of other ferrous oral preparations previously failed.18 More participants who received FM achieved Hb normalization within 12 weeks, 66% compared with 13% in the placebo group, but the lack of an active comparator was a limitation. Subsequent studies have shown acceptable safety profiles for FM, with most patients achieving Hb normalization within 3 months.26 Preliminary clinical trial data demonstrated FM demonstrates similar improvement in quality of life compared with IV ferric carboxymaltose.31 Although the proportion of responders for Hb increase was not statistically significantly different, the study may have been underpowered and the difference was particularly noticeable among patients with an Hb less than 9.5 at baseline (80% vs 32%).32
Sucrosomial iron is an oral formulation of ferric iron enveloped in a phospholipid bilayer designed to overcome ferroportin inhibition.33 Sucrosomial iron has greater bioavailability and lower dose requirements compared with ferrous sulfate, but is not currently available in the United States. This iron does not directly contact the GI mucosa; therefore, it has lower GI impact and decreased reports of associated AEs.34 A pilot interventional study in 30 patients with nonsevere IBD and mild IDA had a 96.6% study completion rate, and 80% of participants took all scheduled doses of sucrosomial iron.33
Despite the well-established safety profile, affordability, and ease of administration, oral iron preparations have multiple challenges and barriers to their use. As mentioned, the high proportion (75%-90%) of unabsorbed iron within the GI tract frequently results in intolerability and discontinuation of therapy in up to 20% of patients.4,13,16,24 The most common AEs associated with oral iron therapies include constipation, nausea/vomiting, and abdominal pain.4 Dose-dependent AEs may be minimized by delayed-release or enteric-coated preparations; however, these products may inadvertently reduce absorption, which occurs predominantly in the duodenum.10 In addition, free iron in the GI tract may provoke oxidative stress and has the potential to exacerbate the inflammatory state of IBD.4 Mucosal inflammation may worsen iron malabsorption, thus compounding the state of iron deficiency. Finally, oral iron may disrupt gut microbiota composition, which plays a role in IBD disease activity.35
IV Iron
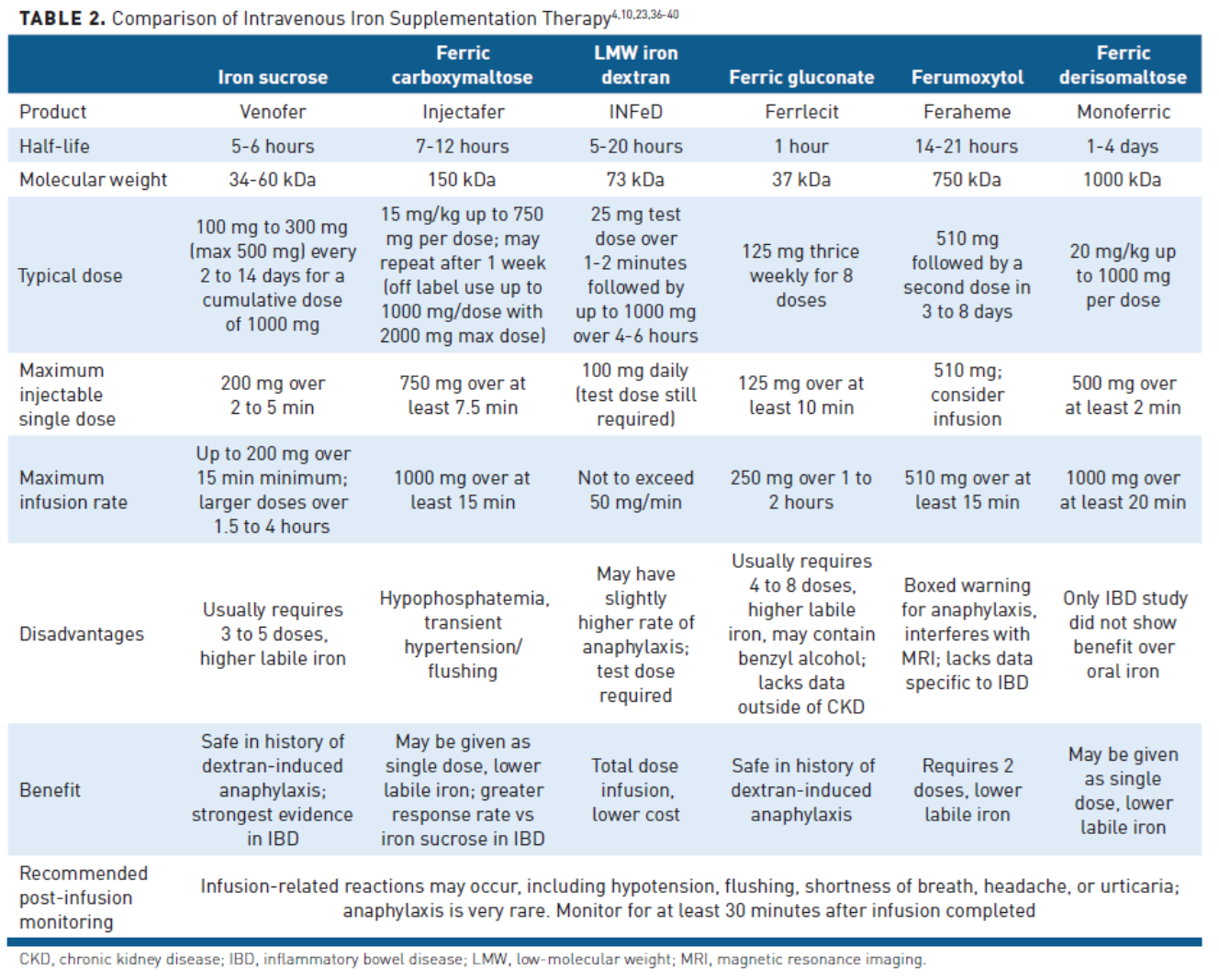
IV iron is recommended in the 2015 European consensus guideline as first-line treatment for IDA in IBD among patients with moderate to severe anemia (Hg <10 grams/dL), in active IBD, when oral iron preparations are ineffective or not tolerated, or in patients who require erythropoiesis-stimulating agents.25 Compared with oral iron salts, IV iron bypasses the inflammation-induced blockade of iron absorption and may be given in larger doses, allowing for faster iron repletion.2 A comparison of IV iron preparations is available in Table 2.4,10,23,36-40
Despite advantages, IV iron is expensive, and administration may be less convenient as it must be administered by a healthcare professional. Of note, all IV iron products have been associated with infusion-related reactions or very rarely anaphylaxis, so monitoring for 30 minutes after administration is complete is recommended. Several studies conducted in patients with IBD demonstrated that IV iron was more effective in increasing Hb with lower treatment-related AEs.41 Furthermore, an observational study by Stein et al showed that IV iron improved clinical scores, decreased hospitalizations, and reduced total healthcare costs.42
Iron sucrose is the most studied IV preparation in patients with IBD.4,43 It has a molecular weight of 34 to 60 kDa and a short half-life of 5 to 6 hours.44,45 Typically, it is administered as 100 to 200 mg over 30 minutes 2 to 3 times weekly, but larger doses up to 500 mg can be diluted and infused slowly.4,45 Multiple treatment sessions are required to replenish iron stores fully.45 AEs such as dyspnea, tachycardia, and transient hypotension may occur if iron sucrose is infused too quickly (faster than 4 mg/min) or greater than 7 mg/kg is given as a single dose.4 In randomized trials, patients receiving iron sucrose are more likely to achieve resolution of anemia and normalization of ferritin compared with those receiving oral iron. An association has also been demonstrated between quality of life and iron treatment response rate (65%-75%), which is typically seen in the first 4 to 8 weeks.4 Iron sucrose is also safe to use in pregnant women during the second and third trimesters and postpartum.44
Ferric carboxymaltose (FCM) is another IV iron preparation that has been extensively studied and used in patients with IBD.21 It has a molecular weight of 150 kDa and is highly stable; therefore, 1000 mg over 15 minutes can be safely administered in 1 or 2 infusions separated by a week.4,13,46 FCM has demonstrated noninferiority to oral iron and was more likely to achieve Hb normalization than iron sucrose 200 mg doses up to twice weekly in patients with IBD. It has an acceptable safety profile and patients are adherent to therapy.47,48 Preliminary clinical trial data indicate the potential to be more effective than oral ferric maltol for increasing Hb.32 One unique and common AE associated with this formulation is prolonged hypophosphatemia. Patients may be asymptomatic, but hypophosphatemia may be observed within 24 hours of administration, and typically peaks within 1 to 2 weeks.49 Multiple infusions may result in hypophosphatemia, and rarely, may cause osteomalacia.49,50
Iron dextran is one of the oldest formulations of IV iron. Low-molecular-weight iron dextran (73 kDa) is safer (2-12–fold lower risk of AEs) compared with the high molecular weight products historically used.4 The risk for anaphylaxis is similar to the newer IV iron preparations, but a test dose should be administered. The major advantages of this product include the ability to give a total dose infusion at one time and a lower cost than other IV iron products. In patients with IBD, iron dextran showed greater increase in Hb in a case-matched comparison with oral treatment and raised Hb substantially by 4 weeks in patients refractory to other therapies.51,52
Ferric gluconate is a free iron formulation with a molecular weight of 37 kDa.46,53 Ferric gluconate has been studied primarily in patients with chronic kidney disease undergoing dialysis.4 The typical dose is 125 mg 3 times weekly for up to 8 doses.53 Hypotension, tachycardia, dyspnea, and edema are the most common AEs.4 More studies are needed to elucidate further the role of ferric gluconate in patients with IBD and anemia.
Ferumoxytol is a novel IV iron product designed to minimize immunologic sensitivity.54 Ferumoxytol has a molecular weight of 750 kDa, and should be diluted in 50 to 200 mL of normal saline and infused over 15 minutes or longer.36,55 It is indicated in patients with IDA with an intolerance or an unsatisfactory response to oral iron and patients with chronic kidney disease (CKD).36 Ferumoxytol does not require a test dose.55 Ferumoxytol can also fully replenish iron stores with 2 administrations.54-56 Studies with ferumoxytol do not specify results in patients with IBD, and there is no direct comparison with oral therapy. The most common AEs noted in patients with CKD who received ferumoxytol were nausea, dizziness, hypotension, and peripheral edema.36 More studies are needed to understand the role of ferumoxytol in treating patients with IBD and IDA.
Ferric derisomaltose is an iron carbohydrate complex of ferric hydroxide and derisomaltose with a molecular weight of 155,000 Da.39 It is approved in patients who have an intolerance or an unsatisfactory response to oral iron, and non−hemodialysis-dependent CKD. FDA approval was granted based on 2 randomized, open-label, actively controlled clinical trials in 3050 patients with IDA in various etiologies and CKD.57,58 Noninferiority to iron sucrose was established in both trials regarding Hb increase from baseline to week 8. The only study in IBD was not able to establish a benefit of a 1000-mg dose compared with oral iron, and the authors suggested higher doses might be necessary, particularly in patients with Hb less than 9 grams/dL and elevated C-reactive protein. It was well tolerated and can still be considered in patients who are not candidates for oral therapy.59
Each of the IV iron preparations is considered effective in the treatment of iron deficiency60; however, in patients with IBD, ferric carboxymaltose and iron sucrose have the most substantial clinical evidence.61 While the unique AE of hypophosphatemia must be considered, ferric carboxymaltose may be considered if a high iron dose and quick iron store replenishment are required.47,62
IV iron preparations differ based on cost, formulary/purchasing agreements, dosing and administration time, and the number of visits required for iron replenishment.60 The agents can also be distinguished by iron assimilation into stable iron complexes or release as labile or semi-labile iron. Newer IV iron preparations are associated with infusion reactions (eg, urticaria, palpitations, dizziness, neck/back spasm) in fewer than 1% of patients, and usually do not progress to more serious reactions.60,63 It is important to monitor patients and provide education on expectations before IV iron administration.
IDA relapse in IBD is common, occurring in more than 50% of patients within 10 to 12 months.64 Therefore, Hb indices and iron status should be monitored every 3 months for a minimum of 1 year after anemia correction and then every 6 to 12 months once Hb normalization and replenishment of iron stores occur.25 The FERGImain study evaluating FCM and the PROCEED extension trial assessing iron isomaltoside showed that IDA recurrence may be prevented in patients with IBD with IV iron treatment every 2 to 3 months based on ferritin levels.65,66
If rapid recurrence of IDA occurs after anemia correction, the patient should be further evaluated for subclinical inflammation.60,67 IDA refractory to IV iron should similarly be evaluated for other etiologies or ongoing inflammation. Notably, only patients with quiescent to moderately active IBD are included in iron replacement studies, and newer medications under development, such as hepcidin antagonists and prolyl hydroxylase inhibitors, may expand the treatment portfolio for patients with IBD and IDA in the future.
Conclusions
In summary, IDA is the most common extraintestinal manifestation of IBD, and it is underdiagnosed and undertreated. As IDA is known to decrease quality of life and is associated with hospitalizations and healthcare costs, it is essential that patients are screened, diagnosed, and treated appropriately. Due to the underlying pathogenesis of IBD, oral iron is not appropriate in all clinical scenarios. Newer IV iron preparations significantly improve iron stores compared with oral iron. Various factors must be considered when selecting a formulation, including dosing, potential AEs, administration time, and patient-specific barriers to access.
Author affiliation: Nicole Bohm, PharmD, BCPS, is an associate professor and clinical pharmacy specialist, internal medicine, Department of Clinical Pharmacy and Outcomes Sciences, College of Pharmacy, Medical University of South Carolina, Charleston, SC.
Funding source: This activity is supported by an educational grant from American Regent.
Author disclosure: Dr Bohm has the following relevant financial relationships to disclose: consultant for Wolters Kluwer and TRC Pharmacist’s Letter.
Authorship information: Analysis and interpretation of data,drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Address correspondence to: bohm@musc.edu
Medical writing and editorial support provided by: Brittany Hoffmann-Eubanks, PharmD, MBA
REFERENCES
1. Shah Y, Patel D, Khan N. Iron deficiency anemia in IBD: an overlooked comorbidity. Expert Rev Gastroenterol Hepatol. 2021:1-11. doi: 10.1080/17474124.2021.1900730
2. Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematol. 2019;142(1):30-36. doi: 10.1159/000496728
3. Calvo P, Pablo L. Managing IBD outside the gut: ocular manifestations. Dig Dis. 2013;31(2):229-232. doi: 10.1159/000353375
4. Kumar A, Brookes MJ. Iron therapy in inflammatory bowel disease. Nutrients. 2020;12(11):3478. doi: 10.3390/nu12113478
5. Patel D, Yang YX, Trivedi C, et al. Incidence, duration, and management of anemia: a nationwide comparison between IBD and non-IBD populations. Inflamm Bowel Dis. 2020;26(6):934-940. doi: 10.1093/ibd/izz206
6. Høivik ML, Reinisch W, Cvancarova M, Moum B; IBSEN study group. Anaemia in inflammatory bowel disease: a population-based 10-year follow-up. Aliment Pharmacol Ther. 2014;39(1):69-76. doi: 10.1111/apt.12541
7. Khan N, Patel D, Shah Y,Yang YX. Factors predicting testing and treatment of iron deficiency in a nationwide cohort of anemic UC patients. Inflamm Bowel Dis. 2016;22(12):2894-2901. doi: 10.1097/mib.0000000000000947
8. Lopez A, Cacoub P, Macdougall IC,Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387(10021):907-916. doi: 10.1016/s0140-6736(15)60865-0
9. Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823(9):1434-1443. doi: 10.1016/j.bbamcr.2012.01.014
10. Nielsen OH, Soendergaard C, Vikner ME, Weiss G. Rational management of iron-deficiency anaemia in inflammatory bowel disease. Nutrients. 2018;10(1):82. doi: 10.3390/nu10010082
11. Nemeth E, Tuttle MS, Powelson J,et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090-2093. doi: 10.1126/science.1104742
12. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessement of severity. Vitamin and Mineral Nutrition Information System; 2011. Accessed May 10, 2021. www.who.int/vmnis/indicators/haemoglobin.pdf
13. Stein J, Dignass AU. Management of iron deficiency anemia in inflammatory bowel disease - a practical approach. Ann Gastroenterol. 2013;26(2):104-113.
14. Weiss G. Anemia of chronic disorders: new diagnostic tools and new treatment strategies. Semin Hematol. 2015;52(4):313-320. doi: 10.1053/j.seminhematol.2015.07.004
15. Bhandari S, Pereira DIA, Chappell HF, Drakesmith H. Intravenous irons: from basic science to clinical practice. Pharmaceuticals (Basel). 2018;11(3):82. doi: 10.3390/ph11030082
16. Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn’s disease. Aliment Pharmacol Ther. 2006;24(11-12):1507-1523. doi: 10.1111/j.1365-2036.2006.03146.x
17. Jimenez K, Gasche C, Auerbach M. On both sides of the ocean. Blood Transfus. 2016;14(2):197-198. doi: 10.2450/2016.0304-15
18. Gasche C, Ahmad T, Tulassay Z,et al; AEGIS Study Group. Ferric maltol is effective in correcting iron deficiency anemia in patients with inflammatory bowel disease: results from a phase-3 clinical trial program. Inflamm Bowel Dis. 2015;21(3):579-588. doi: 10.1097/mib.0000000000000314
19. Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13(12):1545-1553. doi: 10.1002/ibd.20285
20. Rizvi S, Schoen RE. Supplementation with oral vs. intravenous iron for anemia with IBD or gastrointestinal bleeding: is oral iron getting a bad rap? Am J Gastroenterol. 2011;106(11):1872-1879. doi: 10.1038/ajg.2011.232
21. Niepel D, Klag T, Malek NP,Wehkamp J. Practical guidance for the management of iron deficiency in patients with inflammatory bowel disease. Therap Adv Gastroenterol. 2018;11:1756284818769074. doi: 10.1177/1756284818769074
22. Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. doi: 10.1182/blood-2018-05-815944
23. Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6(3):62-72. doi: 10.4291/wjgp.v6.i3.62
24. Gómez-Ramírez S, Brilli E, Tarantino G,Muñoz M. Sucrosomial iron: a new generation iron for improving oral supplementation. Pharmaceuticals (Basel). 2018;11(4):97. doi: 10.3390/ph11040097
25. Dignass AU, Gasche C, Bettenworth D, et al; European Crohn’s and Colitis Organisation. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9(3):211-222. doi: 10.1093/ecco-jcc/jju009
26. D’Amico F, Peyrin-Biroulet L, Danese S. Oral iron for IBD patients: lessons learned at time of COVID-19 pandemic. J Clin Med. 2020;9(5). doi: 10.3390/jcm9051536
27. Gisbert JP, Bermejo F, Pajares R,et al. Oral and intravenous iron treatment in inflammatory bowel disease: hematological response and quality of life improvement. Inflamm Bowel Dis. 2009;15(10):1485-1491. doi: 10.1002/ibd.20925
28. Stoffel NU, Cercamondi CI, Brittenham G,et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4(11):e524-e533. doi: 10.1016/s2352-3026(17)30182-5
29. Khoury A, Pagan KA, Farland MZ. Ferric maltol: a new oral iron formulation for the treatment of iron deficiency in adults. Ann Pharmacother. 2021;55(2):222-229. doi: 10.1177/1060028020941014
30. Feraccru. Product information. European Medicines Agency; 2021. Accessed April 18, 2021. www.ema.europa.eu/en/medicines/human/EPAR/feraccru#product-information-section
31. Howaldt S, Jacob I, Sampson M,Akriche F. P567. Impact of oral ferric maltol and IV iron on health-related quality of life in patients with iron deficiency anaemia and inflammatory bowel disease, and relationship with haemoglobin and serum iron. J Crohns Colitis. 2020;14(suppl 1):S478-S479. doi: 10.1093/ecco-jcc/jjz203.695
32. A Study Comparing Two Ferric Carboxymaltose Formulations in Patients With Iron Deficiency Anemia. National Institutes of Health.Updated May 30, 2019. Accessed June 14, 2021. clinicaltrials.gov/ct2/show/NCT03399084?cond=Ferric+Carboxymaltose&draw=2&rank=2
33. Abbati G, Incerti F, Boarini C,et al. Safety and efficacy of sucrosomial iron in inflammatory bowel disease patients with iron deficiency anemia. Intern Emerg Med. 2019;14(3):423-431. doi: 10.1007/s11739-018-1993-9
34. Riccio E, Sabbatini M, Capuano I,Pellegrino AM, Petruzzelli LA, Pisani A.. Oral Sucrosomial iron versus intravenous iron for recovering iron deficiency anaemia in ND-CKD patients: a cost-minimization analysis. BMC Nephrol. 2020;21(1):57. doi: 10.1186/s12882-020-01716-w
35. Lee T, Clavel T, Smirnov K,et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2017;66(5):863-871. doi: 10.1136/gutjnl-2015-309940
36. Feraheme. Prescribing information. AMAG Pharmaceuticals; 2020. Accessed June 16, 2021. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=32b0e320-a739-11dc-a704-0002a5d5c51b&type=display
37. Venofer. Prescribing information. American Regent; 2020. Accessed June 16, 2021. www.venofer.com/pdfs/venofer-prescribing-information.pdf
38. Injectafer. Prescribing information. American Regent; 2021. Accessed June 16, 2021. https://injectafer.com/prescribing-information-portlet/getDocument?product=IF&inline=true
39. Monoferric. Prescribing information. Pharmacosmos; 2020. Accessed June 16, 2021. www.monoferric.com/?gclid=CjwKCAjwwqaGBhBKEiwAMk-FtCzPbktLcRumS3EXJpV5e4hk_su6c3RLJ6O42hOntOrL1ezJ-kqPtxoCQfYQAvD_BwE
40. INFeD. Prescribing information. Watson Pharmaceuticals; 2021. Accessed June 16, 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abacb7fa-2fc2-471e-9200-944eeac8ca2a
41. Bonovas S, Fiorino G, Allocca M,et al. Intravenous versus oral iron for the treatment of anemia in inflammatory bowel disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95(2):e2308. doi: 10.1097/md.0000000000002308
42. Stein J, Haas JS, Ong SH,et al. Oral versus intravenous iron therapy in patients with inflammatory bowel disease and iron deficiency with and without anemia in Germany - a real-world evidence analysis. Clinicoecon Outcomes Res. 2018;10:93-103. doi: 10.2147/ceor.S150900
43. Schröder O, Mickisch O, Seidler U,et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease—a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100(11):2503-2509. doi: 10.1111/j.1572-0241.2005.00250.x
44. Gasche C, Lomer MC, Cavill I,Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53(8):1190-1197. doi: 10.1136/gut.2003.035758
45. Muñoz M, Gómez-Ramírez S, García-Erce JA. Intravenous iron in inflammatory bowel disease. World J Gastroenterol. 2009;15(37):4666-4674. doi: 10.3748/wjg.15.4666
46. Nielsen OH, Ainsworth M, Coskun M,Weiss G. Management of iron-deficiency anemia in inflammatory bowel disease: a systematic review. Medicine (Baltimore). 2015;94(23):e963. doi: 10.1097/md.0000000000000963
47. Evstatiev R, Marteau P, Iqbal T,et al; FERGI Study Group. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141(3):846-853.e841-e842. doi: 10.1053/j.gastro.2011.06.005
48. Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (Ferinject) randomized controlled trial. Am J Gastroenterol. 2008;103(5):1182-1192. doi: 10.1111/j.1572-0241.2007.01744.x
49. Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28(8):1793-1803. doi: 10.1002/jbmr.1923
50. Shimizu Y, Tada Y, Yamauchi M,et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone. 2009;45(4):814-816. doi: 10.1016/j.bone.2009.06.017
51. Khalil A, Goodhand JR, Wahed M, Yeung J, Ali FR, Rampton DS. Efficacy and tolerability of intravenous iron dextran and oral iron in inflammatory bowel disease: a case-matched study in clinical practice. Eur J Gastroenterol Hepatol. 2011;23(11):1029-1035. doi: 10.1097/MEG.0b013e32834a58d1
52. Koutroubakis IE, Oustamanolakis P, Karakoidas C, Mantzaris GJ, Kouroumalis EA. Safety and efficacy of total-dose infusion of low molecular weight iron dextran for iron deficiency anemia in patients with inflammatory bowel disease. Dig Dis Sci. 2010;55(8):2327-2331. doi:10.1007/s10620-009-1022-y
53. Silverstein SB, Rodgers GM. Parenteral iron therapy options. Am J Hematol. 2004;76(1):74-78. doi: 10.1002/ajh.20056
54. Provenzano R, Schiller B, Rao M,Coyne D, Brenner L, Pereira BJ. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(2):386-393 doi: 10.2215/cjn.02840608
55. Goldberg ND. Iron deficiency anemia in patients with inflammatory bowel disease. Clin Exp Gastroenterol. 2013;6:61-70. doi: 10.2147/ceg.S43493
56. Singh H, Reed J, Noble S,Cangiano JL, Van Wyck DB; United States Iron Sucrose (Venofer) Clinical Trials Group. Effect of intravenous iron sucrose in peritoneal dialysis patients who receive erythropoiesis-stimulating agents for anemia: a randomized, controlled trial. Clin J Am Soc Nephrol. 2006;1(3):475-482. doi: 10.2215/cjn.01541005
57. Auerbach M, Henry D, Derman RJ,Achebe MM, Thomsen LL, Glaspy J. A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial. Am J Hematol. 2019;94(9):1007-1014. doi: 10.1002/ajh.25564
58. Bhandari S, Kalra PA, Berkowitz M,Belo D, Thomsen LL, Wolf M. Safety and efficacy of iron isomaltoside 1000/ferric derisomaltose versus iron sucrose in patients with chronic kidney disease: the FERWON-NEPHRO randomized, open-label, comparative trial. Nephrol Dial Transplant. 2021;36(1):111-120. doi: 10.1093/ndt/gfaa011
59. Reinisch W, Staun M, Tandon RK, et al. A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). Am J Gastroenterol. 2013;108(12):1877-1888. doi: 10.1038/ajg.2013.335
60. Patel D, Trivedi C, Khan N. Management of anemia in patients with inflammatory bowel disease (IBD). Curr Treat Options Gastroenterol. 2018;16(1):112-128. doi: 10.1007/s11938-018-0174-2
61. Aksan A, Isik H, Radeke HH,Dignass A, Stein J. Systematic review with network meta-analysis: comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45(10):1303-1318. doi: 10.1111/apt.14043
62. Vikrant S, Parashar A. The safety and efficacy of high dose ferric carboxymaltose in patients with chronic kidney disease: a single center study. Indian J Nephrol. 2015;25(4):213-221. doi: 10.4103/0971-4065.144421
63. Miller HJ, Hu J, Valentine JK,Gable PS. Efficacy and tolerability of intravenous ferric gluconate in the treatment of iron deficiency anemia in patients without kidney disease. Arch Intern Med. 2007;167(12):1327-1328. doi: 10.1001/archinte.167.12.1327
64. Ford DC, Dahl NV, Strauss WE,et al. Ferumoxytol versus placebo in iron deficiency anemia: Efficacy, safety, and quality of life in patients with gastrointestinal disorders. Clin Exp Gastroenterol. 2016;9:151-162. doi: 10.2147/ceg.S101473
65. Evstatiev R, Alexeeva O, Bokemeyer B, et al; FERGI Study Group. Ferric carboxymaltose prevents recurrence of anemia in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11(3):269-277. doi: 10.1016/j.cgh.2012.10.013
66. Reinisch W, Altorjay I, Zsigmond F,et al. A 1-year trial of repeated high-dose intravenous iron isomaltoside 1000 to maintain stable hemoglobin levels in inflammatory bowel disease. Scand J Gastroenterol. 2015;50(10):1226-1233. doi: 10.3109/00365521.2015.1031168
67. Tsiolakidou G, Koutroubakis IE. Stimulating erythropoiesis in inflammatory bowel disease associated anemia. World J Gastroenterol. 2007;13(36):4798-4806. doi: 10.3748/wjg.v13.i36.4798
