JOURNAL OF CLINICAL AND MEDICAL RESEARCH
Invasive Candidiasis with Cavitary Lung Lesion in a Post-Covid-19 Diabetic Patient- A Case Report
| ReceivedJun 5, 2021 | RevisedJun 30, 2021 | AcceptedJul 5, 2021 | PublishedAug 5, 2021 |
Harveen Kaur1*, Dilbag Singh1, N.C Kajal2 and Rupali3
1Junior resident, Department of Pulmonary Medicine, Government Medical College, Amritsar, Punjab, India
2Professor, Department of Pulmonary Medicine, Government Medical College, Amritsar, Punjab, India
3Senior resident, ENT Department, Government Medical College, Amritsar, Punjab, India
*Corresponding Author: Harveen Kaur, Junior resident, Department of Pulmonary Medicine, Government Medical College, Amritsar, Punjab, India.
Accepted Date: 07-05-2021; Published Date: 08-05-2021
Copyright© 2021 by Kaur H, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
There have been reported several complications after corona virus disease-2019 (COVID-19). Superinfections, especially secondary fungal diseases are now on rise in post-COVID-19 patients. Candida usually reflects airway colonization and true Candida pneumonia is rare, but can occur after hematologic dissemination from other body sites, such as the skin, gastrointestinal and genitourinary tract. Diabetes mellitus (DM) is an independent risk factor for both severe COVID-19 and increased susceptibility to fungal infections. We describe a case of invasive candidiasis in a 72-year-old post-COVID-19 diabetic male, who presented with cough, fever and cavitary lesion in lung seen on contrast-enhanced computed tomography (CECT) Chest. The patient’s sputum and blood cultures were positive for Candida.
Keywords
Invasive candidiasis, Cavitary lesion, COVID-19, Diabetes mellitus (DM), CECT chest.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with many opportunistic bacterial and fungal infections. The main fungal pathogens reported for co-infection in people with COVID-19 are both Aspergillus and Candida [1]. Although in the background of COVID-19 pandemic, Mucormycosis is seen more often in immunocompromised individuals and Aspergillus fumigatus is an important reason of fungal super-infections among critically-ill patients, but the incidence of candidiasis in such patients is yet to be evaluated. Amongst the Candida genus: Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis, and Candida krusei, are the most prevalent species that inhabit various mucosal surfaces, such as the skin and the respiratory, gastrointestinal, and urinary tracts [2,3]. These commensals within the human host are equipped with virulence attributes, enabling them to invade when opportunities arise and cause various infections in humans, especially when the immune system is impaired [3]. The established clinical entities of candidiasis include: superficial infections, such as skin disorders; mucosal infections, including oropharyngeal or vulvovaginitis candidiasis; and invasive candidiasis.
The hematogenous spread of Candida, rather than oropharyngeal secretion aspiration is responsible for lung infection [4]. The various predisposing factors include: immunosuppression, neutropenia, sepsis, prolonged antibiotic use, total parenteral nutrition [5].
Individuals with diabetes mellitus (DM), have several alterations in cell-mediated immunity, such as chemotaxis, phagocytosis and cytokine secretion, along with reduced natural killer cell activity, which affects the host response and paves the way for secondary fungal infections.
The various radiological presentations of pulmonary candidiasis can vary from pneumonia, nodules, ground-glass opacity (GGO), micro-abscesses, miliary patterns, bronchial wall thickening and a rare occurrence of cavitary lesions [6].
High mortality is reported in adult patients of invasive candidiasis, approximately 15%-25% infected individuals [7]. Thus, it is important to have a high index of suspicion for fungal co-infection in post-COVID-19 patients with comorbidities, who present with worsening symptoms.
All studies of fungal infections reported in COVID-19 patients usually occur mostly 14 days after appearance of COVID-19 symptoms. In the current case, the patient was previously diagnosed with COVID-19, one and a half month back; after a few weeks from his recovery, he developed breathlessness, cough and fever again.
Case Report
A 72-year-old diabetic male, post-COVID-19 infection, presented with chief complaints of fever, cough and breathlessness. Cough was associated with small amount of expectoration, progressively worsening for 15 days. He was a known case of type 2 DM from past 20 years, with poor glycaemic control. He had no previous history of tuberculosis.
Four weeks before this complaint, he had cured of COVID-19.
On physical examination, he was alert, pale, febrile 101 F and oxygen saturation 94% on room air. On auscultation, basilar crackles on left side present with normal heart sounds.
Initial laboratory evaluation revealed – Haemoglobin 9.1 g/dl; TLC 14,000; HbA1c 10.4; renal and liver function tests were within normal limits. Mantoux test was negative. Sputum for AFB was negative and sputum for CBNAAT did not detect Mycobacterium tuberculosis. Sputum and blood cultures tested positive for Candida.
CECT Chest showed areas of consolidation with cavitation and surrounding coarse ground glass opacities (GGO’s) along with inter & intra lobular septal thickening in left upper lobe and pleural thickening in apical region, and focal invasion of the left subclavian artery. Bronchoscopy with bronchoalveolar lavage (BAL) subsequently showed white exudate and examination of tissue culture obtained during transbronchial biopsy revealed Candida. The patient was started on antifungal medications. Subsequently, repeat blood cultures tested negative for fungus.
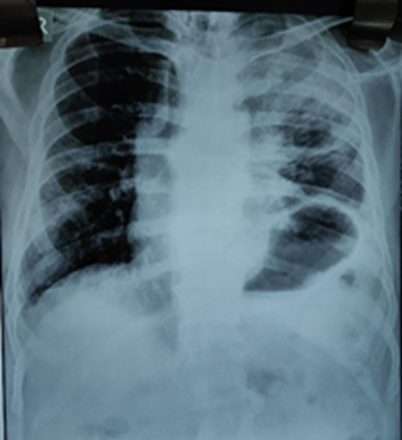
Figure 1: Chest X-ray showed areas of consolidation with cavitation in left upper lobe along with elevated left dome of diaphragm.

Figure 2: CECT Chest showing consolidation with cavitation and surrounding coarse GGO’s along with inter & intra lobular septal thickening in left upper lobe.

Figure 3: Budding yeast cells seen on Gram staining of bronchoalveolar lavage.
Discussion
Recently, the pandemic of COVID-19 has paved way for super-infections in individuals with immune alterations. In majority of the viral respiratory diseases, such as influenza, SARS, MERS, and others, secondary infections are a well-described occurrence. But in COVID-19 pneumonia, super-infections and co-infections are under exploration. Presence of comorbidities, including DM further predispose an individual to secondary fungal infections. The rates of COVID-19 associated candidiasis (CAC) vary by country and region; the reported rates from Spain [8], India [9], Iran [10], Italy [11], UK [12] and China [13] being 0.7%,2.5%,5%,8%,12.6%, and 23.5%, respectively.
The wide usage of antibiotics, steroids, along with insult by SARS CoV-2 infection, causes commensal Candida to invade internal organs. When Candida enters the blood and spreads to other body sites, there occurs Invasive candidiasis. The various predisposing factors include immunosuppression, surgical procedures, renal failure, prolonged placement of central venous catheter, malignancy, prolonged antibiotic usage, late sepsis [5]. The sepsis syndrome associated with severe COVID-19 damages the intestinal mucosal barrier and enables the translocation of these commensals, leading to fungemia [14,15]. Candida-related immune dysfunction adds on to the increased susceptibility to other respiratory pathogens.
Invasion of the pulmonary parenchyma by Candida is rare, due to which its presence in respiratory specimens is usually regarded as contamination. Kassner et al. described three histologic forms of pulmonary candidiasis: embolic, disseminated and bronchopulmonary [16].
As determined by el-Ebiary, the incidence of Candida-pneumonia is 8%, and pattern of colonization is uniform throughout the lung usually [17]. The most prevalent yeast species as per the recent studies in critically ill COVID-19 patients, is Candida albicans (44.1%); followed by C. auris (23.2%); C.glabrata, C. parapsilosis, C.tropicalis, and S. cerevisiae (4.6% each); and C.krusei and Rhodotorula spp. (2.3% each) [9,12,18,19].
To reliably establish the diagnosis of bronchopulmonary and disseminated Candida infection, bronchoalveolar lavage (BAL), cultures with cytologic and morphologic analyses, and histopathology (the gold standard) should be performed. The diagnosis of invasive candidiasis is challenging and requires invasive procedure for deep-seated candidiasis, and the use of non-fungal-specific media to culture clinical samples [20]. In order to improve the sensitivity of the techniques, combining multiple techniques is recommended.
Cavitary pneumonia presentation of pulmonary candidiasis is rare but was seen in the present case. We diagnosed this case as invasive candidiasis by the patient’s positive blood cultures, chest CT and BAL findings.
Invasive yeast infections are associated with a higher mortality in COVID-19 cases not receiving antifungal treatment, so prompt diagnosis and treatment is of importance to achieve clinical success. The treatment of invasive candidiasis in patients with COVID-19 is similar to that of non-COVID-19 patients. The treatment of choice for invasive Candida infections are Echinocandins [12].
The fungal diseases add insult to the injury in a significant proportion of post-COVID-19 patients with immune alterations and are associated with high mortality, thereby require early diagnosis and timely initiation of appropriate antifungal treatment.
Conclusion
The presence of DM in post-COVID-19 patients increases the risk of contracting secondary fungal infections. The colonization of respiratory tract by Candida often leads to poor outcomes clinically, along with development of complications. In invasive candidiasis, delay in therapy initiation adds on to the increased mortality. Emphasis should be given for early and comprehensive diagnosis of invasive candidiasis in immunosuppressed patients, for timely initiation of antifungal therapy appropriate for infection clearance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Ethical approval
Not applicable.
Declaration of patient consent
All appropriate consent forms obtained from the patient.
References
1. Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;31:1-8. PubMed | CrossRef
2. Hallen-Adams HE, Suhr MJ. Fungi in the healthy human gastrointestinal tract. Virulence. 2017;8(3):352-8. PubMed | CrossRef
3. Rolling T, Hohl TM, Zhai B. Minority report: The intestinal mycobiota in systemic infections. Curr Opin Microbiol. 2020;56:1-6. PubMed | CrossRef
4. Azoulay E, Timsit JF, Tafflet M, de Lassence A, Darmon M, Zahar JR, et al. Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest. 2006;129(1):110-7. PubMed | CrossRef
5. Taschdjian CL, Kozinn PJ, Toni EF. Opportunistic yeast infections, with special reference to candidiasis. Ann N Y Acad Sci. 1970;174(2):606-22. PubMed | CrossRef
6. Franquet T, Müller NL, Lee KS, Oikonomou A, Flint JD. Pulmonary candidiasis after hematopoietic stem cell transplantation: thin-section CT findings. Radiology. 2005;236(1):332-7. PubMed | CrossRef
7. Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41(9):1232-9. PubMed | CrossRef
8. Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83-8. PubMed | CrossRef
9. Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerging Infect Dis. 2020;26(11):2694. PubMed | CrossRef
10. Salehi M, Ahmadikia K, Mahmoudi S, Kalantari S, Jamalimoghadamsiahkali S, Izadi A, et al. Oropharyngeal candidiasis in hospitalised COVID‐19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses. 2020;63(8):771-8. PubMed | CrossRef
11. Antinori S, Bonazzetti C, Gubertini G, Capetti A, Pagani C, Morena V, et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia?. Autoimmun Rev. 2020;19(7):102564. PubMed | CrossRef
12. White L, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020. PubMed | CrossRef
13. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-13. PubMed | CrossRef
14. Hoenigl M, Lin J, Finkelman M, Zhang Y, Karris MY, Letendre SL, et al. Glucan rich nutrition does not increase gut translocation of beta‐glucan. Mycoses. 2021;64(1):24-9. PubMed | CrossRef
15. Leelahavanichkul A, Worasilchai N, Wannalerdsakun S, Jutivorakool K, Somparn P, Issara-Amphorn J, et al. Gastrointestinal leakage detected by serum (1→3)-β-D-glucan in mouse models and a pilot study in patients with sepsis. Shock: Injury, Inflammation, and Sepsis: Lab Clin Approaches. 2016;46(5):506-18. PubMed | CrossRef
16. Kassner EG, Kauffman SL, Yoon JJ, Semiglia M, Kozinn PJ, Goldberg PL. Pulmonary candidiasis in infants: clinical, radiologic, and pathologic features. AJR Am J Roentgenol. 1981;137(4):707-16. PubMed | CrossRef
17. El-Ebiary M, Torres A, Fabregas N, de la BELLACASA JP, Gonzalez J, Ramirez J, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients: an immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156(2):583-90. PubMed | CrossRef
18. Ventoulis I, Sarmourli T, Amoiridou P, Mantzana P, Exindari M, Gioula G, et al. Bloodstream infection by Saccharomyces cerevisiae in two COVID-19 patients after receiving supplementation of Saccharomyces in the ICU. J Fungi. 2020;6(3):98. PubMed | CrossRef
19. Posteraro B, Torelli R, Vella A, Leone PM, De Angelis G, De Carolis E, et al. Pan-echinocandin-resistant Candida glabrata bloodstream infection complicating COVID-19: A fatal case report. J Fungi. 2020;6(3):163. PubMed | CrossRef
20. Arastehfar A, Wickes BL, Ilkit M, Pincus DH, Daneshnia F, Pan W, et al. Identification of mycoses in developing countries. J Fungi. 2019 Dec;5(4):90. PubMed | CrossRef
