Baculovirus Display of Peptides and Proteins for Medical Applications
Abstract
:1. Introduction
1.1. Characteristics of the Baculovirus Expression Vector System
1.2. Baculovirus Display Strategies
1.3. GP64 Glycoprotein
1.4. Surface Display by Fusion to Complete GP64
1.5. Using Peptide Insertions on GP64
1.6. Capsid Display by Fusion to VP39
1.7. Baculovirus Display with Complete Proteins
1.8. Advantages and Applications of Baculoviral Surface-Display Technology
1.9. Baculovirus as Antigen Carriers
1.10. Development of Vaccines Based on Baculovirus Display
1.11. Display of Bioactives Peptides
2. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jehle, J.A.; Blissard, G.W.; Bonning, B.C.; Cory, J.S.; Herniou, E.A.; Rohrmann, G.F.; Theilmann, D.A.; Thiem, S.M.; Vlak, J.M. On the classification and nomenclature of baculoviruses: A proposal for revision. Arch. Virol. 2006, 151, 1257–1266. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Z. Advances in Molecular Biology of Baculoviruses. Curr. Issues Mol. Biol. 2020, 34, 183–214. [Google Scholar] [CrossRef]
- Cox, M.M.J. Innovations in the Insect Cell Expression System for Industrial Recombinant Vaccine Antigen Production. Vaccines 2021, 9, 1504. [Google Scholar] [CrossRef]
- Koonin, E.V.; Krupovic, M.; Agol, V.I. The Baltimore Classification of Viruses 50 Years Later: How Does It Stand in the Light of Virus Evolution?. Microbiol. Mol. Biol. Rev. 2021, 85, e0005321. [Google Scholar] [CrossRef]
- Luz-Madrigal, A.; Asanov, A.; Camacho-Zarco, A.R.; Sampieri, A.; Vaca, L. A Cholesterol Recognition Amino Acid Consensus Domain in GP64 Fusion Protein Facilitates Anchoring of Baculovirus to Mammalian Cells. J. Virol. 2013, 87, 11894–11907. [Google Scholar] [CrossRef]
- Monsma, S.A.; Oomens, A.G.; Blissard, G.W. The GP64 envelope fusion protein is an essential baculovirus protein required for cell-to-cell transmission of infection. J. Virol. 1996, 70, 4607–4616. [Google Scholar] [CrossRef]
- Kukkonen, S.P.; Airenne, K.J.; Marjomäki, V.; Laitinen, O.H.; Lehtolainen, P.; Kankaanpää, P.; Mähönen, A.J.; Räty, J.K.; Nordlund, H.R.; Oker-Blom, C.; et al. Baculovirus capsid display: A novel tool for transduction imaging. Mol. Ther. 2003, 8, 853–862. [Google Scholar] [CrossRef]
- Wang, R.; Deng, F.; Hou, D.; Zhao, Y.; Guo, L.; Wang, H.; Hu, Z. Proteomics of the Autographa californica Nucleopolyhedrovirus Budded Virions. J. Virol. 2010, 84, 7233–7242. [Google Scholar] [CrossRef]
- Zhao, S.; He, G.; Yang, Y.; Liang, C. Nucleocapsid Assembly of Baculoviruses. Viruses 2019, 11, 595. [Google Scholar] [CrossRef]
- Döhner, K.; Ramos-Nascimento, A.; Bialy, D.; Anderson, F.; Hickford-Martinez, A.; Rother, F.; Koithan, T.; Rudolph, K.; Buch, A.; Prank, U.; et al. Importin α1 is required for nuclear import of herpes simplex virus proteins and capsid assembly in fibroblasts and neurons. PLoS Pathog. 2018, 14, e1006823. [Google Scholar] [CrossRef]
- Rohrmann, G.F. The Baculovirus Replication Cycle Effects on Cells and Insects. In Baculovirus Molecular Biology; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2019; pp. 57–82. [Google Scholar]
- Saxena, A.; Byram, P.K.; Singh, S.K.; Chakraborty, J.; Murhammer, D.; Giri, L. A structured review of baculovirus infection process: Integration of mathematical models and biomolecular information on cell–virus interaction. J. Gen. Virol. 2018, 99, 1151–1171. [Google Scholar] [CrossRef]
- Smith, G.E.; Summers, M.D.; Fraser, M.J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol. Cell. Biol. 1983, 3, 2156. [Google Scholar] [CrossRef]
- Felberbaum, R.S. The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol. J. 2015, 10, 702–714. [Google Scholar] [CrossRef]
- Hüser, A.; Hofmann, C. Baculovirus vectors: Novel mammalian cell gene-delivery vehicles and their applications. Am. J. Pharm. 2003, 3, 53–63. [Google Scholar] [CrossRef]
- van Oers, M.M. Opportunities and challenges for the baculovirus expression system. J. Invertebr. Pathol. 2011, 107, S3–S15. [Google Scholar] [CrossRef]
- Hitchman, R.B.; Possee, R.D.; King, L.A. Protein Expression in Insect Cells. In Comprehensive Biotechnology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 1, pp. 323–340. ISBN 9780080885049. [Google Scholar]
- Merrington, C.L.; Bailey, M.J.; Possee, R.D. Manipulation of baculovirus vectors. Mol. Biotechnol. 1997, 8, 283–297. [Google Scholar] [CrossRef]
- Possee, R.D.; King, L.A. Baculovirus Transfer Vectors. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2016; Volume 1350, pp. 51–71. [Google Scholar]
- Grose, C.; Putman, Z.; Esposito, D. A review of alternative promoters for optimal recombinant protein expression in baculovirus-infected insect cells. Protein Expr. Purif. 2021, 186, 105924. [Google Scholar] [CrossRef]
- Saarenpää, T.; Jaakola, V.P.; Goldman, A. Baculovirus-Mediated Expression of GPCRs in Insect Cells. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 556, pp. 185–218. [Google Scholar]
- Kitts, P.A.; Ayres, M.D.; Possee, R.D. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucleic Acids Res. 1990, 18, 5667–5672. [Google Scholar] [CrossRef]
- Kitts, P.A.; Possee, R.D. A method for producing recombinant baculovirus expression vectors at high frequency. Biotechniques 1993, 14, 810–817. [Google Scholar]
- Hong, M.; Li, T.; Xue, W.; Zhang, S.; Cui, L.; Wang, H.; Zhang, Y.; Zhou, L.; Gu, Y.; Xia, N.; et al. Genetic engineering of baculovirus-insect cell system to improve protein production. Front. Bioeng. Biotechnol. 2022, 10, 1–15. [Google Scholar] [CrossRef]
- Luckow, V.A.; Lee, S.C.; Barry, G.F.; O Olins, P. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 1993, 67, 4566–4579. [Google Scholar] [CrossRef] [Green Version]
- Hitchman, R.B.; Locanto, E.; Possee, R.D.; King, L.A. Optimizing the baculovirus expression vector system. Methods 2011, 55, 52–57. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Yao, K.; Wu, T.-Y. Baculovirus as an expression and/or delivery vehicle for vaccine antigens. Expert Rev. Vaccines 2008, 7, 363–371. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Wei, S.-C.; Lo, H.-R.; Chao, Y.-C. Baculovirus as Versatile Vectors for Protein Display and Biotechnological Applications. Curr. Issues Mol. Biol. 2020, 34, 231–256. [Google Scholar] [CrossRef]
- Trianti, I.; Akeprathumchai, S.; Mekvichitsaeng, P.; Poomputsa, K. Baculovirus Surface Display Using Infuenza Neuraminidase (NA) Transmembrane Anchor. J. Trop. Life Sci. 2012, 6, 205–209. [Google Scholar] [CrossRef]
- Blissard, G.W.; Theilmann, D.A. Baculovirus Entry and Egress from Insect Cells. Annu. Rev. Virol. 2018, 5, 113–139. [Google Scholar] [CrossRef]
- Pidre, M.L.; Ferrelli, M.L.; Haase, S.; Romanowski, V. Baculovirus Display: A Novel Tool for Vaccination. In Current Issues in Molecular Virology—Viral Genetics and Biotechnological Applications; InTech: Vienna, Austria, 2013; pp. 137–164. [Google Scholar]
- Grabherr, R.; Ernst, W. Baculovirus for eukaryotic protein display. Curr. Gene Ther. 2010, 10, 195–200. [Google Scholar] [CrossRef]
- Rahman, M.; Gopinathan, K.P. Bombyx mori nucleopolyhedrovirus-based surface display system for recombinant proteins. J. Gen. Virol. 2003, 84, 2023–2031. [Google Scholar] [CrossRef]
- Tami, C.; Farber, M.; Palma, E.L.; Taboga, O. Presentation of antigenic sites from foot-and-mouth disease virus on the surface of baculovirus and in the membrane of infected cells. Arch. Virol. 2000, 145, 1815–1828. [Google Scholar] [CrossRef]
- Peralta, A.; Molinari, P.; Conte-Grand, D.; Calamante, G.; Taboga, O. A chimeric baculovirus displaying bovine herpesvirus-1 (BHV-1) glycoprotein D on its surface and their immunological properties. Appl. Microbiol. Biotechnol. 2007, 75, 407–414. [Google Scholar] [CrossRef]
- Meng, T.; Kolpe, A.B.; Kiener, T.K.; Chow, V.T.K.; Kwang, J. Display of VP1 on the Surface of Baculovirus and Its Immunogenicity against Heterologous Human Enterovirus 71 Strains in Mice. PLoS ONE 2011, 6, e21757. [Google Scholar] [CrossRef] [Green Version]
- Ernst, W.J.; Spenger, A.; Toellner, L.; Katinger, H.; Grabherr, R.M. Expanding Baculovirus Surface Display. Modification of the Native Coat Protein Gp64 of Autographa Californica NPV. Eur. J. Biochem. 2000, 267, 4033–4039. [Google Scholar] [CrossRef] [PubMed]
- Spenger, A.; Grabherr, R.; Töllner, L.; Katinger, H.; Ernst, W. Altering the surface properties of baculovirus Autographa californica NPV by insertional mutagenesis of the envelope protein gpJBIC. Eur. J. Biochem. 2002, 269, 4458–4467. [Google Scholar] [CrossRef]
- Tung, M.-C.; Lu, H.-Y.; Chang, Y.-K.; Huang, W.-R.; Liao, T.-L.; Wu, H.-Y.; Chang, C.-D.; Fan, H.-C.; Nielsen, B.L.; Liu, H.-J. Baculovirus surface display of the HA protein of H5N2 avian influenza virus and its immunogenicity against a lethal challenge with H5N1 virus in chickens. Veter. Microbiol. 2020, 243, 108640. [Google Scholar] [CrossRef]
- Tami, C.; Peralta, A.; Barbieri, R.; Berinstein, A.; Carrillo, E.; Taboga, O. Immunological properties of FMDV-gP64 fusion proteins expressed on SF9 cell and baculovirus surfaces. Vaccine 2004, 23, 840–845. [Google Scholar] [CrossRef]
- Räty, J.K.; Airenne, K.J.; Marttila, A.T.; Marjomäki, V.; Hytönen, V.P.; Lehtolainen, P.; Laitinen, O.H.; Mähönen, A.J.; Kulomaa, M.S.; Ylä-Herttuala, S. Enhanced Gene Delivery by Avidin-Displaying Baculovirus. Mol. Ther. 2004, 9, 282–291. [Google Scholar] [CrossRef]
- Kiener, T.K.; Premanand, B.; Kwang, J. Immune responses to baculovirus-displayed enterovirus 71 VP1 antigen. Expert Rev. Vaccines 2013, 12, 357–364. [Google Scholar] [CrossRef]
- Xue, W.; Li, T.; Zhang, S.; Wang, Y.; Hong, M.; Cui, L.; Wang, H.; Zhang, Y.; Chen, T.; Zhu, R.; et al. Baculovirus Display of Varicella–Zoster Virus Glycoprotein E Induces Robust Humoral and Cellular Immune Responses in Mice. Viruses 2022, 14, 1785. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Ren, F.; Zou, S.; Feng, M.; Xu, L.; Yao, L.; Sun, J. Construction of a highly efficient display system for baculovirus and its application on multigene co-display. Mol. Genet. Genom. 2018, 293, 1265–1277. [Google Scholar] [CrossRef]
- Oomens, A.; Blissard, G. Requirement for GP64 to Drive Efficient Budding ofAutographa californicaMulticapsid Nucleopolyhedrovirus. Virology 1999, 254, 297–314. [Google Scholar] [CrossRef]
- Zhou, J.; Blissard, G.W. Display of Heterologous Proteins on gp64 null Baculovirus Virions and Enhanced Budding Mediated by a Vesicular Stomatitis Virus G-Stem Construct. J. Virol. 2008, 82, 1368–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, W.; Schinko, T.; Spenger, A.; Oker-Blom, C.; Grabherr, R. Improving baculovirus transduction of mammalian cells by surface display of a RGD-motif. J. Biotechnol. 2006, 126, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, J.P.; Mäkelä, A.R.; Kakkonen, E.; Turkki, P.; Kukkonen, S.; Peränen, J.; Ylä-Herttuala, S.; Airenne, K.J.; Oker-Blom, C.; Vihinen-Ranta, M.; et al. Clathrin-Independent Entry of Baculovirus Triggers Uptake of E. coli in Non-Phagocytic Human Cells. PLoS ONE 2009, 4, e5093. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Z.; Wu, C.P.; Chao, Y.-C.; Liu, C.Y.-Y. Membrane penetrating peptides greatly enhance baculovirus transduction efficiency into mammalian cells. Biochem. Biophys. Res. Commun. 2011, 405, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, Y.; Chen, J. Baculoviral capsid display of His-tagged ZnO inorganic binding peptide. Cytotechnology 2010, 62, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Molinari, P.; Crespo, M.I.; Gravisaco, M.J.; Taboga, O.; Morón, G. Baculovirus Capsid Display Potentiates OVA Cytotoxic and Innate Immune Responses. PLoS ONE 2011, 6, e24108. [Google Scholar] [CrossRef]
- Tavarone, E.; Molina, G.N.; Amalfi, S.; Peralta, A.; Molinari, P.; Taboga, O. The localization of a heterologous displayed antigen in the baculovirus-budded virion determines the type and strength of induced adaptive immune response. Appl. Microbiol. Biotechnol. 2017, 101, 4175–4184. [Google Scholar] [CrossRef]
- Musthaq, S.S.; Madhan, S.; Hameed, A.S.; Kwang, J. Localization of VP28 on the baculovirus envelope and its immunogenicity against white spot syndrome virus in Penaeus monodon. Virology 2009, 391, 315–324. [Google Scholar] [CrossRef]
- Wu, Q.; Fang, L.; Wu, X.; Li, B.; Luo, R.; Yu, Z.; Jin, M.; Chen, H.; Xiao, S. A pseudotype baculovirus-mediated vaccine confers protective immunity against lethal challenge with H5N1 avian influenza virus in mice and chickens. Mol. Immunol. 2009, 46, 2210–2217. [Google Scholar] [CrossRef]
- Yoon, K.-W.; Chu, K.-B.; Kang, H.-J.; Kim, M.-J.; Eom, G.-D.; Lee, S.-H.; Moon, E.-K.; Quan, F.-S. Mucosal Administration of Recombinant Baculovirus Displaying Toxoplasma gondii ROP4 Confers Protection Against T. gondii Challenge Infection in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Hu, Y.C.; Luo, Y.L.; Ji, W.T.; Chulu, J.L.; Chang, P.C.; Shieh, H.; Wang, C.Y.; Liu, H.J. Dual expression of the HA protein of H5N2 avian influenza virus in a baculovirus system. J. Virol. Methods 2006, 135, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-G.; Chung, Y.-C.; Lai, Y.-K.; Lai, C.-W.; Liu, H.-J.; Hu, Y.-C. Avian Influenza Virus Hemagglutinin Display on Baculovirus Envelope: Cytoplasmic Domain Affects Virus Properties and Vaccine Potential. Mol. Ther. 2007, 15, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Münch, R.C.; Janicki, H.; Völker, I.; Rasbach, A.; Hallek, M.; Büning, H.; Buchholz, C.J. Displaying High-affinity Ligands on Adeno-associated Viral Vectors Enables Tumor Cell-specific and Safe Gene Transfer. Mol. Ther. 2013, 21, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.M.; Chen, M.Y.; Lam, M.T.; Ykema, M.R.; Suh, J. Display of Self-Peptide on Adeno-Associated Virus Capsid Decreases Phagocytic Uptake in Vitro. ACS Synth. Biol. 2020, 9, 2246–2251. [Google Scholar] [CrossRef]
- Fan, H.; Pan, Y.; Fang, L.; Wang, D.; Wang, S.; Jiang, Y.; Chen, H.; Xiao, S. Construction and immunogenicity of recombinant pseudotype baculovirus expressing the capsid protein of porcine circovirus type 2 in mice. J. Virol. Methods 2008, 150, 21–26. [Google Scholar] [CrossRef]
- Meysami, P.; Rezaei, F.; Marashi, S.M.; Amiri, M.M.; Bakker, E.; Mokhtari-Azad, T. Antitumor effects of a recombinant baculovirus displaying anti-HER2 scFv expressing Apoptin in HER2 positive SK-BR-3 breast cancer cells. Future Virol. 2019, 14, 139–152. [Google Scholar] [CrossRef]
- Martyn, J.C.; Cardin, A.J.; Wines, B.D.; Cendron, A.; Li, S.; MacKenzie, J.; Powell, M.; Gowans, E.J. Surface display of IgG Fc on baculovirus vectors enhances binding to antigen-presenting cells and cell lines expressing Fc receptors. Arch. Virol. 2009, 154, 1129–1138. [Google Scholar] [CrossRef]
- Ono, C.; Okamoto, T.; Abe, T.; Matsuura, Y. Baculovirus as a Tool for Gene Delivery and Gene Therapy. Viruses 2018, 10, 510. [Google Scholar] [CrossRef]
- Targovnik, A.M.; Simonin, J.A.; Mc Callum, G.J.; Smith, I.; Warlet, F.U.C.; Nugnes, M.V.; Miranda, M.V.; Belaich, M.N. Solutions against emerging infectious and noninfectious human diseases through the application of baculovirus technologies. Appl. Microbiol. Biotechnol. 2021, 105, 8195–8226. [Google Scholar] [CrossRef]
- Krammer, F.; Schinko, T.; Palmberger, D.; Tauer, C.; Messner, P.; Grabherr, R. Trichoplusia ni cells (High FiveTM) are highly efficient for the production of influenza A virus-like particles: A comparison of two insect cell lines as production platforms for influenza vaccines. Mol. Biotechnol. 2010, 45, 226–234. [Google Scholar] [CrossRef]
- Possee, R.D.; Chambers, A.C.; Graves, L.P.; Aksular, M.; King, L.A. Recent Developments in the Use of Baculovirus Expression Vectors. Curr. Issues Mol. Biol. 2020, 34, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kaname, Y.; Wen, X.; Tani, H.; Moriishi, K.; Uematsu, S.; Takeuchi, O.; Ishii, K.J.; Kawai, T.; Akira, S.; et al. Baculovirus Induces Type I Interferon Production through Toll-Like Receptor-Dependent and -Independent Pathways in a Cell-Type-Specific Manner. J. Virol. 2009, 83, 7629–7640. [Google Scholar] [CrossRef]
- Premanand, B.; Prabakaran, M.; Kiener, T.K.; Kwang, J. Recombinant Baculovirus Associated with Bilosomes as an Oral Vaccine Candidate against HEV71 Infection in Mice. PLoS ONE 2013, 8, e55536. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Liu, H.-J.; Tsai, C.-P.; Chung, C.-Y.; Shih, Y.-S.; Chang, P.-C.; Chiu, Y.-T.; Hu, Y.-C. Baculovirus as an avian influenza vaccine vector: Differential immune responses elicited by different vector forms. Vaccine 2010, 28, 7644–7651. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Tani, H.; Limn, C.K.; Matsunaga, T.M.; Moriishi, K.; Matsuura, Y. Ligand-Directed Gene Targeting to Mammalian Cells by Pseudotype Baculoviruses. J. Virol. 2005, 79, 3639–3652. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, M.; Madhan, S.; Prabhu, N.; Geng, G.Y.; New, R.; Kwang, J. Reverse micelle-encapsulated recombinant baculovirus as an oral vaccine against H5N1 infection in mice. Antivir. Res. 2010, 86, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, M.; Madhan, S.; Prabhu, N.; Qiang, J.; Kwang, J. Gastrointestinal Delivery of Baculovirus Displaying Influenza Virus Hemagglutinin Protects Mice against Heterologous H5N1 Infection. J. Virol. 2010, 84, 3201–3209. [Google Scholar] [CrossRef]
- Basak, S.; Chu, K.-B.; Kang, H.-J.; Kim, M.-J.; Lee, S.-H.; Yoon, K.-W.; Jin, H.; Suh, J.W.; Moon, E.-K.; Quan, F.-S. Orally administered recombinant baculovirus vaccine elicits partial protection against avian influenza virus infection in mice. Microb. Pathog. 2020, 149, 104495. [Google Scholar] [CrossRef]
- Tang, X.-C.; Lu, H.-R.; Ross, T.M. Hemagglutinin Displayed Baculovirus Protects Against Highly Pathogenic Influenza. Vaccine 2010, 28, 6821–6831. [Google Scholar] [CrossRef]
- Kumar, S.R.; Khader, S.M.S.; Kiener, T.K.; Szyporta, M.; Kwang, J. Intranasal Immunization of Baculovirus Displayed Hemagglutinin Confers Complete Protection against Mouse Adapted Highly Pathogenic H7N7 Reassortant Influenza Virus. PLoS ONE 2013, 8, e63856. [Google Scholar] [CrossRef]
- Musthaq, S.K.S.; Kumar, S.R.; Szyporta, M.; Kwang, J. Immunization with baculovirus displayed H6 hemagglutinin vaccine protects mice against lethal H6 influenza virus challenge. Antivir. Res. 2014, 109, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Kolpe, A.B.; Kiener, T.K.; Grotenbreg, G.M.; Kwang, J. Display of enterovirus 71 VP1 on baculovirus as a type II transmembrane protein elicits protective B and T cell responses in immunized mice. Virus Res. 2012, 168, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Wang, J.Y.; Xu, X.G.; Tong, D.W.; Lu, H.Y.; Chen, Y.H.; Chiou, M.T.; Chang, C.D.; Liu, H.J. Localization of the VP2 Protein of Canine Parvovirus Type 2 on the Baculovirus Envelop and Its Immunogenicity in a Mouse Model. Open J. Veter. Med. 2012, 02, 178–185. [Google Scholar] [CrossRef]
- Musthaq, S.S.; Kwang, J. Oral Vaccination of Baculovirus-Expressed VP28 Displays Enhanced Protection against White Spot Syndrome Virus in Penaeus monodon. PLoS ONE 2011, 6, e26428. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, S.; Nan, H.; Zhao, K.; Xu, X.; Wang, G.; Ji, H.; Chen, H. Immersion immunization with recombinant baculoviruses displaying cyprinid herpesvirus 2 membrane proteins induced protective immunity in gibel carp. Fish Shellfish Immunol. 2019, 93, 879–887. [Google Scholar] [CrossRef]
- Wu, Q.; Yu, F.; Xu, J.; Li, Y.; Chen, H.; Xiao, S.; Fu, Z.F.; Fang, L. Rabies-virus-glycoprotein-pseudotyped recombinant baculovirus vaccine confers complete protection against lethal rabies virus challenge in a mouse model. Veter. Microbiol. 2014, 171, 93–101. [Google Scholar] [CrossRef]
- Ye, Y.; Cheng, X.; Zhang, J.; Tong, T.; Lin, W.; Liao, M.; Fan, H. Induction of robust immunity response in mice by dual-expression-system-based recombinant baculovirus expressing the capsid protein of porcine circovirus type. Virol. J. 2013, 10, 316. [Google Scholar] [CrossRef]
- Tao, Y.; Li, G.; Zheng, W.; Shu, J.; Chen, J.; Yang, F.; Wu, Y.; He, Y. Development of a Combined Genetic Engineering Vaccine for Porcine Circovirus Type 2 and Mycoplasma Hyopneumoniae by a Baculovirus Expression System. Int. J. Mol. Sci. 2019, 20, 4425. [Google Scholar] [CrossRef]
- Xu, X.-G.; Wang, Z.-S.; Zhang, Q.; Li, Z.-C.; Ding, L.; Li, W.; Wu, H.-Y.; Chang, C.-D.; Lee, L.-H.; Tong, D.-W.; et al. Baculovirus as a PRRSV and PCV2 bivalent vaccine vector: Baculovirus virions displaying simultaneously GP5 glycoprotein of PRRSV and capsid protein of PCVJ. Virol. Methods 2012, 179, 359–366. [Google Scholar] [CrossRef]
- Karuppannan, A.K.; Qiang, J.; Chang, C.; Kwang, J. A novel baculovirus vector shows efficient gene delivery of modified porcine reproductive and respiratory syndrome virus antigens and elicits specific immune response. Vaccine 2013, 31, 5471–5478. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Hsu, W.-T.; Chao, Y.-C.; Chang, H.-W. Display of Porcine Epidemic Diarrhea Virus Spike Protein on Baculovirus to Improve Immunogenicity and Protective Efficacy. Viruses 2018, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-G.; Wang, Z.-S.; Zhang, Q.; Li, Z.-C.; Zhao, H.-N.; Li, W.; Tong, D.-W.; Liu, H.-J. Baculovirus surface display of E envelope glycoprotein of Japanese encephalitis virus and its immunogenicity of the displayed proteins in mouse and swine models. Vaccine 2011, 29, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-G.; Chiou, M.-T.; Zhang, Y.-M.; Tong, D.-W.; Hu, J.-H.; Zhang, M.-T.; Liu, H.-J. Baculovirus surface display of Erns envelope glycoprotein of classical swine fever virus. J. Virol. Methods 2008, 153, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-G.; Liu, H.-J. Baculovirus surface display of E2 envelope glycoprotein of classical swine fever virus and immunogenicity of the displayed proteins in a mouse model. Vaccine 2008, 26, 5455–5460. [Google Scholar] [CrossRef]
- Xu, X.-G.; Tong, D.-W.; Chiou, M.-T.; Hsieh, Y.-C.; Shih, W.-L.; Chang, C.-D.; Liao, M.-H.; Zhang, Y.-M.; Liu, H.-J. Baculovirus surface display of NS3 nonstructural protein of classical swine fever virus. J. Virol. Methods 2009, 159, 259–264. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.-W.; Tong, T.-Z.; Ye, Y.; Liao, M.; Fan, H.-Y. BacMam virus-based surface display of the infectious bronchitis virus (IBV) S1 glycoprotein confers strong protection against virulent IBV challenge in chickens. Vaccine 2014, 32, 664–670. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Qu, X.; Deng, H.; Ding, M.; Lau, T.L.; Yu, A.C.-H.; Chen, J. Baculovirus Surface Display of SARS Coronavirus (SARS-CoV) Spike Protein and Immunogenicity of the Displayed Protein in Mice Models. DNA Cell Biol. 2006, 25, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Mlambo, G.; Kumar, N.; Yoshida, S. Functional immunogenicity of baculovirus expressing Pfs25, a human malaria transmission-blocking vaccine candidate antigen. Vaccine 2010, 28, 7025–7029. [Google Scholar] [CrossRef]
- Blagborough, A.M.; Yoshida, S.; Sattabongkot, J.; Tsuboi, T.; Sinden, R.E. Intranasal and intramuscular immunization with Baculovirus Dual Expression System-based Pvs25 vaccine substantially blocks Plasmodium vivax transmission. Vaccine 2010, 28, 6014–6020. [Google Scholar] [CrossRef]
- Yoshida, S.; Araki, H.; Yokomine, T. Baculovirus-Based Nasal Drop Vaccine Confers Complete Protection against Malaria by Natural Boosting of Vaccine-Induced Antibodies in Mice. Infect. Immun. 2010, 78, 595–602. [Google Scholar] [CrossRef]
- Yoshida, S.; Nagumo, H.; Yokomine, T.; Araki, H.; Suzuki, A.; Matsuoka, H. Plasmodium berghei Circumvents Immune Responses Induced by Merozoite Surface Protein 1- and Apical Membrane Antigen 1-Based Vaccines. PLoS ONE 2010, 5, e13727. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kondoh, D.; Arai, E.; Matsuoka, H.; Seki, C.; Tanaka, T.; Okada, M.; Ishii, A. Baculovirus virions displaying Plasmodium berghei circumsporozoite protein protect mice against malaria sporozoite infection. Virology 2003, 316, 161–170. [Google Scholar] [CrossRef]
- Yoshida, S.; Kawasaki, M.; Hariguchi, N.; Hirota, K.; Matsumoto, M. A Baculovirus Dual Expression System-Based Malaria Vaccine Induces Strong Protection against Plasmodium berghei Sporozoite Challenge in Mice. Infect. Immun. 2009, 77, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Iyori, M.; Blagborough, A.M.; Salman, A.M.; Dulal, P.; Sala, K.A.; Yamamoto, D.S.; Khan, S.M.; Janse, C.J.; Biswas, S.; et al. Adenovirus-prime and baculovirus-boost heterologous immunization achieves sterile protection against malaria sporozoite challenge in a murine model. Sci. Rep. 2018, 8, 3896. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Iyori, M.; Blagborough, A.M.; Fukumoto, S.; Funatsu, T.; Sinden, R.E.; Yoshida, S. Baculovirus-Vectored Multistage Plasmodium vivax Vaccine Induces Both Protective and Transmission-Blocking Immunities against Transgenic Rodent Malaria Parasites. Infect. Immun. 2014, 82, 4348–4357. [Google Scholar] [CrossRef]
- Strauss, R.; Hüser, A.; Ni, S.; Tuve, S.; Kiviat, N.; Sow, P.S.; Hofmann, C.; Lieber, A. Baculovirus-based Vaccination Vectors Allow for Efficient Induction of Immune Responses Against Plasmodium falciparum Circumsporozoite Protein. Mol. Ther. 2007, 15, 193–202. [Google Scholar] [CrossRef]
- Iyori, M.; Yamamoto, D.S.; Sakaguchi, M.; Mizutani, M.; Ogata, S.; Nishiura, H.; Tamura, T.; Matsuoka, H.; Yoshida, S. DAF-shielded baculovirus-vectored vaccine enhances protection against malaria sporozoite challenge in mice. Malar. J. 2017, 16, 390. [Google Scholar] [CrossRef]
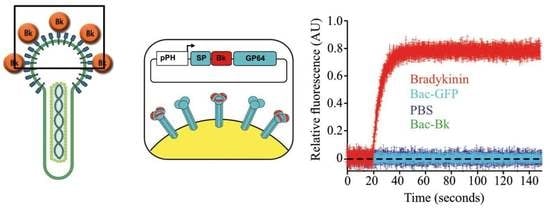
- Hall, J.M. Bradykinin receptors. Gen. Pharmacol. Vasc. Syst. 1997, 28, 1–6. [Google Scholar] [CrossRef]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef]
- Reyes-Cruz, G.; Vazquez-Prado, J.; Müller-Esterl, W.; Vaca, L. Regulation of the human bradykinin B2 receptor expressed in sf21 insect cells: A possible role for tyrosine kinases. J. Cell. Biochem. 2000, 76, 658–673. [Google Scholar] [CrossRef]
- Wirth, K.; Hock, F.; Albus, U.; Linz, W.; Alpermann, H.; Anagnostopoulos, H.; Henke, S.; Breipohl, G.; König, W.; Knolle, J.; et al. Hoe 140 a New Potent and Long Acting Bradykinin-Antagonist: In Vivo Studies. Br. J. Pharmacol. 1991, 102, 774–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokudome, T.; Otani, K. Molecular Mechanism of Blood Pressure Regulation through the Atrial Natriuretic Peptide. Biology 2022, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Diep, P.-T.; Carter, S.; Carbone, M.G. Oxytocin: An Old Hormone, a Novel Psychotropic Drug and Its Possible Use in Treating Psychiatric Disorders. Curr. Med. Chem. 2022, 29, 5615–5687. [Google Scholar] [CrossRef] [PubMed]
- Fathabadipour, S.; Mohammadi, Z.; Roshani, F.; Goharbakhsh, N.; Alizadeh, H.; Palizgar, F.; Cumming, P.; Michel, T.M.; Vafaee, M.S. The neural effects of oxytocin administration in autism spectrum disorders studied by fMRI: A systematic review. J. Psychiatr. Res. 2022, 154, 80–90. [Google Scholar] [CrossRef]
- Szafoni, S.; Piegza, M. Progress in Personalized Psychiatric Therapy with the Example of Using Intranasal Oxytocin in PTSD Treatment. J. Pers. Med. 2022, 12, 1067. [Google Scholar] [CrossRef]
- Lustig, R.H.; Fennoy, I. The History of Obesity Research. Horm. Res. Paediatr. 2022, 95, 638–648. [Google Scholar] [CrossRef]


| Platform Design | Displayed Antigen | Disease | Model/Dose | Adjuvant | Route of Administration | Immune Response | Reference |
|---|---|---|---|---|---|---|---|
| Virus vaccines | |||||||
| Display HA protein cloned under ie1 promoter from WSSV | HA | Influenza H5N1 | Mouse/3 doses (1 × 108 pfu) inactivated or live recombinant baculovirus | With or without rCTB Reverse micelle (liposomes of phosphatidylcholine) based carried vehicle | Oral | Specific serum IgG and mucosal IgA antibodies. High HI titers. Cross neutralization. Protection (100% for live baculovirus) in challenge study | [71,72] |
| Display HA protein cloned under ie1 promoter from WSSV | HA | Influenza H5N1 | Mouse/2 doses (5.6 × 106 pfu) | With or without rCTB | Oral | Production IgG, IgG1, IgG2a and IgA systemic; IgG and IgA at mucosal sites. Induces germinal center B cell response. Significant neutralizing antibody titers. Partial protection against lethal challenge. | [73] |
| Display combinations of full-length or ED of HA fused to SP or TM or CTD domains of GP64 derived from AcMNPV, all under Pph | HA | Influenza H1N1 and H5N1 | Mouse/2 doses (1 × 107 or 1 × 108 or 2 × 107 or 4 × 106 pfu) | Baculovirus act as adjuvant | i.m. | Elicits HI activity. High levels of IFN-γ secreting and HA-specific CD8+ T cells. Protection around 60–100% in challenge study | [74] |
| Display HA cloned between SP and CTD of GP64 under Pp10 | ED and TM domains of HA | Influenza H5N2 | Mouse/3 doses of 200 μL (1 × 108–1 × 1010 pfu) | Baculovirus act as adjuvant | i.m., s.c., and i.n. | High specific IgG2a and IgG1 neutralizing antibodies. Induces high HI titers. Induces IgA antibodies. Elicits cellular immune response (Th1 and Th2). | [69] |
| Display HA protein under ie1 promoter from WSSV | HA | Influenza H7N7 or H6N1 | Mouse/H7N7: 2 doses (28 HA units). H6N1: 2 doses (1 × 109 pfu/mL) | i.n.: baculovirus act as adjuvant s.c.: Montanide ISA 201 VG | i.n. and s.c. | High levels of systemic IgG and mucosal IgA. Enhances neutralizing activity. High levels of IFN-γ and IL-4. Protection (100%) in challenge study. | [75,76] |
| Display VP1 protein fused between C-terminus of GP64 from AcMNPV and the N-terminus of TM region from H3N2 under ie1 or Pph promoters | VP1 from HEV71 | HFMD | Mouse/2–3 doses (1 × 107 − 1 × 108 pfu) | Bilosomes (lipid-based vesicles incorporating bile salts) or Freund’s adjuvant | Oral and s.c. | Specific systemic IgG and mucosal IgA immune responses. High neutralizing antibody titers. | [36,68] |
| Display VP1 protein cloned into the C-terminus of NA from influenza A under ie1 promoter | VP1 from HEV71 | HFMD | Mouse/3 doses (1 × 108 pfu) | Freund’s adjuvant or Montanide adjuvant | s.c. | Specific IgG antibodies. High neutralizing antibody titers. Produces significant levels of IFN-γ. Protection (100%) against lethal challenge. | [77] |
| Display VP2 protein fused to TM and CTD of GP64GP64 protein under Pp10 | VP2 protein from CPV-2 | Parvoviruses | Mouse/2 doses (3 × 108 pfu) | Baculovirus act as adjuvant | i.p. | Elicits immune response. High levels of virus neutralization. | [78] |
| Display VP28 protein fused to SP and C-terminus TM of GP64 protein under ie1 promoter | VP28 | WSSV | Shrimp/ Injection: 2 doses (50 μL 1 × 108 pfu/mL) Oral: pellet feed continuously for 7 days (3 mL 1 × 108 pfu/mL). Immersion: seawater 2 L (3 mL with 1 × 108 pfu/mL) | Injection, oral and immersion | Injection: 73–86% protection post challenge Oral: 76–82% protection post challenge Immersion: 68–75% protection post challenge | [53,79] | |
| Display nine truncated CyHV-2 membrane glycoproteins, fused between SP and TM of GP64 under chicken β-actin promoter. | Membrane glycoproteins ORF25, ORF25C, ORF25D, ORF30, ORF124, ORF131, ORF136, ORF142A and ORF146 | Herpesviral haematopoie-tic necrosis disease | Carp/30 mL of 6 × 105 TCID50/mL were diluted into 5 L freshwater | immersion | Expression of IL-11, INF-α, INF-γ and a complement component gene C3 significant up-regulated. Protection greater than 70% in challenge study. | [80] | |
| Display RVG protein cloned one copy under Pph and another copy under PCMV. | RVG | Rabies | Mouse/2 doses (1 × 108 IFU) | Baculovirus act as adjuvant | i.m. | High levels of virus-neutralizing antibodies. Elicits cellular immune response Protection (100%) against rabies viral challenge. | [81] |
| Display ORF2 protein cloned between SP and TM domains of GP64 under Pph and PCMV promoters and, VSV-G under Pp10. | Cap or ORF2 from PCV2 | PMWS | Mouse/2 doses (1 × 109 pfu/mL) | Baculovirus act as adjuvant | i.m. | IgG antibodies. High specific neutralizing antibody titers. Production of IFN-γ. | [82] |
| Display P97R1P46P24 protein under Pph promoter; Cap protein cloned under Pph promoter. Genes were cloned between SP and TM of GP64. | P97R1, P46 and P24 from Mhp; Cap from PCV2 | MPS and PMWS | Mouse and swine/3 doses for mice (1 × 108 pfu/mL) 2 doses for piglets (1 × 109 pfu/mL) | Baculovirus act as adjuvant | s.c. | High levels of P97R1P46P24-Cap specific IgG. Induction of cellular immune response. | [83] |
| Display GP5 protein under Pp10 and Cap protein under Pph. Both proteins cloned between TM and CTD domains of GP64. | GP5 from PRRSV and Cap from PCV2 | PRRS and PMWS | Mouse and swine/2 doses for mice and piglets (1 × 109 pfu) | Baculovirus act as adjuvant | i.m. | High GP5 and Cap antibody titers. High virus neutralization titers. Induces specific cell-mediated immune response. | [84] |
| Display ORF2a, ORF3, ORF4 and ORF5 proteins between C-terminus SP of GP64 and N-terminus TM HA from H3N2 that continuous of CTD of GP64, under ie1 promoter | ORF2a (36-207aa), ORF3 (27-265 aa), ORF4 (23-157aa) and ORF5 (33-200aa or without 64-90/108-129 regions) | PRRS | Mouse/2 doses of 100 μL (1 × 108 pfu) | Baculovirus act as adjuvant | s.c. | Production of high specific and neutralizing antibodies. | [85] |
| Display S protein or S1 subunit cloned between SP and TM of GP64 protein, under Pp10. | S and S1 from PEDV | PED | Mouse and swine/ mice: 2 doses of 200 μL (1 × 109 TCID50/mL) Piglets: 2 doses of 2 mL (1 × 109 TCID50/mL) | Baculovirus act as adjuvant | i.m. | Systemic S-specific IgG. S-specific neutralizing antibodies. Decrease severity of illness in challenge study. | [86] |
| Display E glycoprotein linked to SP and TM domains of GP64 under Pp10 | E from JEV | JE | Mouse and swine/2 doses for mice and piglets (1 × 109 pfu) | Baculovirus act as adjuvant | i.p. and i.m. | Produces E-specific antibodies. Induces neutralizing antibody response and protective immunity toward a lethal challenge. Induces cell-mediated immune response. | [87] |
| Display E2 glycoprotein fused to TM and CTD domains of GP64 under Pp10. | E2 from CSFV | CSF | Mouse/3 doses (1.5 × 108 pfu) | Freund’s adjuvant | i.p. | High E2 antibody titers. Significant level of neutralizing antibodies. | [88,89] |
| Display NS3 protein fused to TM and CTD domains of GP64 under Pp10. | NS3 from CSFV | CSF | Mouse/3 doses (1.5 × 108 pfu) | Freund’s adjuvant | i.p. | High specific NS3 antibody titers. | [90] |
| Display E protein fused to SP and TM domains of GP64 | E | ZIKV | Mouse/3 doses (15 μg) | Freund’s adjuvant | i.p. | High levels of systemic IgG. High neutralizing antibody titers. High levels of IFN-γ and IL-4. Protection greater than 80% in challenge study. | [39] |
| Display S1 peptide between SP and TM of GP64 under Pph and PCMV promoters and, VSV-G under Pp10. | ED of S1 glycoprotein from IBV | IB | Chicken/2 doses of 200 μL (2 × 108 pfu) | Baculovirus act as adjuvant | i.m. | Strong humoral and cell-mediated immune responses. Induces cytotoxic T lymphocyte responses. Protection greater than 80% in challenge study. | [91] |
| Display D glycoprotein fused to GP64 | ED of gD from BHV-1 | BHV-1 | Mouse/3 doses of 200 μL (5 × 108 pfu) | Freund’s adjuvant | i.p. | High specific IgG and neutralizing antibodies. | [35] |
| Display S protein fused between SP of GP64 and TM domain of VSV-G under Pph | ED of S protein from SARS-CoV | SARS | Mouse/2 doses (~5 × 108 pfu/mL) | Baculovirus act as adjuvant | s.c. | Production of high specific and neutralizing S protein antibodies. | [92] |
| Display A or P1 fused to GP64 under Pph | Site A (138-156aa) of VP1 from FMDV | HFMD | Mouse/2 doses (1 × 109 pfu) | Freund’s adjuvant | i.p. | High specific antibodies and seroneutralizing titers. Shows protection in challenge study. | [40] |
| Parasite vaccines | |||||||
| Display Pfs25 cloned between SP and TM of GP64 protein, under Pph. | Pfs25 (23-195aa) from Plasmodium falciparum | Malaria | Mouse/3 doses (1 × 108 pfu) | Baculovirus act as adjuvant | i.n. and i.m. | High levels of Pfs25-specific antibodies. In passive immunization shows transmission-blocking effect (>90% reduction in infection intensity). In active immunization shows transmission blocking (83% i.n. and ~95% i.m.) | [93] |
| Display Pvs25 cloned between SP and TM of GP64 protein, under PCMV and Pph | Pvs25 (23-195aa) from Plasmodium vivax | Malaria | Mouse/4 doses (5 × 107 pfu) | Baculovirus act as adjuvant | i.n. and i.m. | High Pvs25-specific antibody titers. Mixed Th1/Th2 response (IgG1, IgG2a and IgG2b). Transmission-blocking effect (94%) Active immunization shows transmission-blocking (92.1% i.n. and 83.8% i.m.) | [94] |
| Display PyMSP119 protein cloned between SP and TM of GP64 protein under Pph. | PyMSP119 from Plasmodium yoelii | Malaria | Mouse/3 doses (5 × 107 pfu) | Baculovirus act as adjuvant | Oral, i.n. and i.m. | High titers of PyMSP119 -specific antibodies (i.n. and i.m.). Mixed Th1/Th2 response (IgG1, IgG2a and IgG2b). Partial protection against lethal challenge. | [95] |
| Display MSP119 or AMA1 cloned between SP and TM of GP64 protein under Pph. | MSP119 from Pb, Pf and Py. AMA1 (domain I, II and III o only domain III) from Py. | Malaria | Mouse/3 doses (5 × 107 pfu) | Baculovirus act as adjuvant | i.m. and i.n. | High level PfMSP119-specifc antibody titers. High titers of PyAMA1-specifc antibodies. Partial protection in challenge study. | [96] |
| Display CSP protein cloned between SP and TM of GP64 protein | CSP from Pb | Malaria | Mouse/2 or 3 doses (1 × 108 pfu) | Baculovirus act as adjuvant | i.m. | High levels and IFN-γ. Protection (60%) in challenge study. | [97] |
| Display CSP protein cloned between SP and TM of GP64 under PCMV and Pph promoters | CSP(21-305) from Pb | Malaria | Mouse/3 doses (1 × 108 pfu) | Baculovirus act as adjuvant | i.m. | High PbCSP-specific antibody titers. Mixed Th1/Th2 response (IgG1 and IgG2a). Specific CD8+ T-cell response. Partial parasitemia protection (~70%) in challenge study. | [98] |
| Display CSP protein under Pph and another copy under PCMV | CSP from Pf | Malaria | Mouse/2 doses Prime: ChAd63-PfCSP (5 × 107 pfu) Boost: emBDES-PfCSP/IL12 (2 × 108 pfu) | Baculovirus act as adjuvant | i.m. | Induces high anti-PfCSP IgG titers. High levels and IFN-γ. Increases memory CD8+ T-cell numbers. Protection in challenge study. | [99] |
| Display CSP and Pvs25 proteins cloned between SP of GP64 and TM of VSV-G under PCMV and Pph | CSP and Pvs25 from Pv | Malaria | Mouse/3 doses (1 × 108 pfu) | Baculovirus act as adjuvant | i.m. | High CSP and vs25-specific antibody titers. Transmission-blocking effect (82%). Transmission-blocking in vivo (84%). Partial protection in challenge study. | [100] |
| Display CSP protein cloned between SP and TM of GP64 under Pph | CSP from Pf | Malaria | Mouse/2 doses (1 × 108 pfu) | Baculovirus act as adjuvant | i.m. | High anti-CSP antibody titers. Mixed Th1/Th2 response (IgG1 and IgG2a). High levels and IFN-γ. Induces specific CD4+ and CD8+ T-cell responses. | [101] |
| Display CSP proteins cloned in C-terminus of SP of GP64 and N-terminus of TM GP64 or TM VSV-G under PCMV and Pph promoters | CSP from Pf | Malaria | Mouse/4 doses (1 × 108 pfu) | Baculovirus act as adjuvant | i.m. | High antibody titers. Induces IgG1, IgG2a and IgG2b (Th1/Th2). Partial protection in challenge study. | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Hernández, A.P.; Martínez-Flores, D.; Cruz-Reséndiz, A.; Padilla-Flores, T.; González-Flores, R.; Estrada, K.; Sampieri, A.; Camacho-Zarco, A.R.; Vaca, L. Baculovirus Display of Peptides and Proteins for Medical Applications. Viruses 2023, 15, 411. https://doi.org/10.3390/v15020411
Rodríguez-Hernández AP, Martínez-Flores D, Cruz-Reséndiz A, Padilla-Flores T, González-Flores R, Estrada K, Sampieri A, Camacho-Zarco AR, Vaca L. Baculovirus Display of Peptides and Proteins for Medical Applications. Viruses. 2023; 15(2):411. https://doi.org/10.3390/v15020411
Chicago/Turabian StyleRodríguez-Hernández, Aaron Pavel, Daniel Martínez-Flores, Adolfo Cruz-Reséndiz, Teresa Padilla-Flores, Rodrigo González-Flores, Kenia Estrada, Alicia Sampieri, Aldo Román Camacho-Zarco, and Luis Vaca. 2023. "Baculovirus Display of Peptides and Proteins for Medical Applications" Viruses 15, no. 2: 411. https://doi.org/10.3390/v15020411