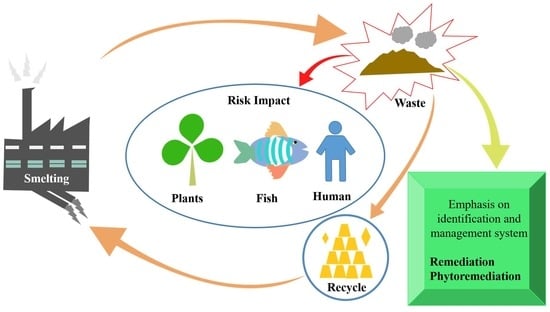
Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China
Abstract
:1. Introduction
2. Investigational Methods
2.1. Studies of Geochemical Analysis
2.2. Application of Isotopes
3. Evaluation of Heavy Metal Pollution
3.1. Mechanism of Heavy Metal Pollution
3.2. Pollution Indices
3.2.1. Geo-Accumulation Index (I-Geo)
3.2.2. Pollution Index (PI)
3.2.3. Enrichment Factor (EF)
| Index | Formula | Range of Indices | Soil Conditions | References |
|---|---|---|---|---|
| Geoaccumulation Index (I-geo) | I-geo ≤ 0 | Unpolluted | [96] | |
| 0 ≤ I-geo < 1 | Unpolluted to moderately polluted | |||
| 1 ≤ I-geo < 2 | Moderately polluted | |||
| 2 ≤ I-geo < 3 | Moderately to strongly polluted | |||
| 3 ≤ I-geo < 4 | Strongly polluted | |||
| 4 ≤ I-geo < 5 | Strongly to extremely polluted | |||
| I-geo > 5 | Extremely high polluted | |||
| Pollution Index (PI) | PI < 1 | Unpolluted, Low level of pollution | [98] | |
| 1 < PI ≤ 3 | Moderate polluted | |||
| 3 ≤ PI | Strong polluted | |||
| Enrichment Factor (EF) | EF < 2 | Deficiency to minimal enrichment | [99] | |
| EF = 2–5 | Moderate enrichment | |||
| EF = 5–20 | Significant enrichment | |||
| EF = 20–40 | Very high enrichment | |||
| EF > 40 | Extremely high enrichment | |||
| Contamination Factor (CF) | CF < 1 | Low contamination factor | [100] | |
| 1 < CF ≤ 3 | Moderately contaminated factor | |||
| 3 ≤ CF ≤ 6 | Considerably contaminated factor | |||
| 6 ≤ CF | Very high contaminated factor | |||
| Threshold Pollution Index (PIT) | PIT < 1 | Unpolluted | [101] | |
| 1 < PIT ≤ 2 | Low polluted | |||
| 2 ≤ PIT ≤ 3 | Moderate polluted | |||
| 3 ≤ PIT ≤ 5 | Strong polluted | |||
| 5 ≤ PIT | Very strong polluted |
3.2.4. Contamination Factor (CF)
3.2.5. Threshold Pollution Index (PIT)
3.3. Heavy Metal Contamination in Smelter Impacted Regions
| Province | n | SN | I-Geo | PI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Cu | Pb | Zn | Cd | Cu | Pb | Zn | NIPI | |||
| Anhui | 6 | 1 | - | 1.45 | −0.27 | −0.76 | - | 0.84 | 0.11 | 0.22 | 0.65 |
| Chongqing | 8 | 1 | 7.72 | - | 7.43 | - | 128 | 0.00 | 28.4 | - | 98.0 |
| Fujian | 25 | 1 | - | 1.30 | 4.30 | 2.67 | - | 0.84 | 4.06 | 3.30 | 3.46 |
| Gansu | 24 | 2 | 7.24 | −0.49 | 5.12 | 4.37 | 87.7 | 0.26 | 3.26 | 8.53 | 64.5 |
| Guangdong | 17 | 1 | 5.04 | - | 1.97 | - | 9.23 | 0.00 | 0.70 | - | 6.94 |
| Guangxi | 104 | 3 | 7.22 | 2.90 | 6.43 | 1.33 | 206 | 3.12 | 10.3 | 1.14 | 151 |
| Guizhou | 179 | 6 | 1.73 | 1.03 | 5.99 | 0.90 | 10.9 | 0.98 | 11.2 | 1.11 | 8.99 |
| Hebei | 9 | 1 | - | - | 3.32 | - | - | - | 1.07 | - | 1.07 |
| Henan | 131 | 3 | 4.79 | −0.01 | 2.51 | 0.09 | 10.3 | 0.29 | 0.56 | 0.38 | 7.54 |
| Hubei | 11 | 2 | 7.41 | 4.56 | 3.56 | 2.27 | 147 | 10.8 | 1.57 | 2.42 | 108 |
| Hunan | 47 | 3 | 5.89 | 2.18 | 3.41 | 2.97 | 37.3 | 1.86 | 1.57 | 4.44 | 27.6 |
| Jiangsu | 38 | 1 | - | - | 4.26 | - | - | - | 2.51 | - | 2.51 |
| Jiangxi | 12 | 1 | 3.02 | 3.46 | 1.37 | −0.32 | 4.37 | 5.05 | 0.42 | 0.33 | 4.00 |
| Liaoning | 94 | 2 | 7.39 | 4.95 | 4.53 | 4.40 | 90.33 | 9.16 | 2.47 | 8.04 | 66.8 |
| Shaanxi | 305 | 12 | 6.34 | 0.53 | 2.96 | 5.04 | 38.00 | 0.46 | 0.83 | 13.7 | 28.5 |
| Sichuan | 46 | 1 | - | - | 5.09 | - | - | - | 5.26 | - | 5.26 |
| Tibet | 17 | 1 | - | - | −0.02 | - | - | - | 0.14 | - | 0.14 |
| Yunnan | 436 | 3 | 4.54 | 1.65 | 2.89 | 3.48 | 25.3 | 2.18 | 1.51 | 6.00 | 19.0 |
| Zhejiang | 284 | 4 | 4.32 | 3.51 | 2.53 | 3.13 | 7.00 | 3.01 | 0.68 | 3.70 | 5.57 |
| China | 1793 | 49 | 5.59 | 2.08 | 3.54 | 2.27 | 61.65 | 2.59 | 4.04 | 4.10 | 32.0 |
| City | Locality | Smelting Operation | Contaminants (Maximum Concentrations in mg/kg) | Spatial Distribution | Soil Profiles | Mineralogy | Extractions | Isotopes | Bioavailability | Digestion | Measurement Method | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hunan | Zhuzhou (Hunan) | Pb/Zn smelter | Zn (3349), Pb (1197), Cu (157), As (93), Cd (41.1), Hg (2.89) | * (Topsoils) | * | HNO3-HCl-H2O2 | AAS | [33] | ||||
| Hunan | Zhuzhou (Hunan) | Pb/Zn smelter | Ag (3.71), Bi (21.4), Co (30), Cr (199) and other 6 elements | * (Topsoils) | * | HF HNO3 | ICP MS | [113] | ||||
| Hunan | Xikuangshan (Hunan) | Sb smelter | Sb (5045), As (205) | * (Topsoils) | * | * (Radish) | HCl-HNO3 | ICP-AES | [107] | |||
| Hunan | Zhuzhou Hunan | Zn/Pb Smelter | Hg (1.54) | * | * | ZAAS-HFM | [108] | |||||
| Henan | Yuguang Henan | Pb smelter | Cd (2.10), Cu (31.8), Ni (45.6), Pb (184), Zn (99.2) | * (Topsoils) | H2O2 HF HClO4 HNO3 | AAS | [53] | |||||
| Henan | Jiyuan Henan | Pb smelter | Pb (114 ± 2.17) Cd (1.19 ± 0.049) | (Wheat) | HNO3-H2O2 | AAS | [112] | |||||
| Zhejiang | Hangzhou (Zhejiang) | Cu smelter | Zn (11,840), Cu (716), Cd (8.67) | * | * | HCl/HNO3 | FAAS | [114] | ||||
| Zhejiang | Fuyang (Zhejiang) | Cu smelter (secondary) | Hg (15) | * (Topsoils) | * | HNO3-H2O2 | AFS | [109] | ||||
| Zhejiang | Zhujiawu (Zhejiang) | Cu/Zn smelter | Zn (3219), Cu (658) | * (Topsoils, transect) | * (Microbes) | HF-HClO4-HNO3 | FAAS | [115] | ||||
| Jiangxi | Guixi Jiangxi | Lengshui Pb-Zn mining & copper smelter | Mining Cd (0.84 ± 0.63) Smelting Cd (1.08 ± 0.36) | * | (Vegetables) | HNO3, HF, and HClO4 | ICP-MS | [46] | ||||
| Jiangxi | Dexing Jiangxi | Pb-Zn, Au, Cu mines | As (33.99), Cd (1.22),Cr (70.28), Cu (138.42), Mn (468.70), Ni (32.24), Pb (125.32), Zn (171.48) | * (Topsoils) | * | HNO3-HClO4-HF | ICP-AES | [28] | ||||
| Jiangxi | Guixi Jiangxi | Cu smelter | Cu (35) | * (Topsoils) | (Rice) | (HNO3), (HF), (HClO4) | ICP | [17] | ||||
| Guizhou | Magu (Guizhou) | Zn smelter | Pb (37,770), Zn (31,625), Cd (131) | * (Topsoils) | * | * | * (Pb,S) | HF-HClO4 | AAS | [116] | ||
| Hubei | Daye Hubei | Cu smelting | Cd (4.87), Cu (195.26), Pb (92.65), As (35.84) | * (Crop) | HClO4, HNO3 and HF, HClO4 and HNO3, and HNO3 and H2O2 | ICP-MS | [117] | |||||
| Guangxi | Nanning (Guangxi) | Pb/Sb smelter | Pb (992), Zn (597), Cu (39), Cd (22) (geometric means) | * (Topsoils) | * (Vegetables) | Soil HNO3/HCl = 1:3 | ICP-MS | [118] | ||||
| China | 19 major provinces | 49 Non-ferrous smelteries | Cd (19.8), Cu (265), Pb (1536), Zn (1371) | * | HCl4-H2SO4, HCl-HNO3, HCl-HClO4-HNO3, HCl-HClO4-HF-HNO3, HCl-HClO-HNO3, HF-HNO3, HCl-HF-HNO3, HClO4-HF-HNO3, HCl-HF-HNO3-H2O2, HCl-HNO3-H2O2, HClO4-HNO3, | (AAS), (ICP-AES), (ICP-MS), (ICP-OES) | [41] |
3.4. Distribution and Source of Heavy Metals
3.5. Health Risk Assessment
3.6. Metallurgical Processes
Hydrometallurgy Processes
4. Remediation Approaches
4.1. Soil Solidification/Stabilization Technology
4.2. Soil Washing/Extraction Technology
4.3. Electrokinetic Remediation Technology
4.4. Natural Attenuation
4.5. Phytoremediation
4.5.1. Phytostabilization
4.5.2. Phytostimulation
4.5.3. Phytovolatilization
4.5.4. Rhizofiltration
4.5.5. Phytoextraction
4.5.6. Phytodegradation
5. Future Scope
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spurgeon, D.J.; Lawlor, A.; Hooper, H.L.; Wadsworth, R.; Svendsen, C.; Thomas, L.D.; Ellis, J.K.; Bundy, J.G.; Keun, H.C.; Jarup, L. Outdoor and indoor cadmium distributions near an abandoned smelting works and their relations to human exposure. Environ. Pollut. 2011, 159, 3425–3432. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Duffus, J.H. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef] [Green Version]
- Kemp, D.D. The Environment Dictionary; Psychology Press: Hove, UK, 1998. [Google Scholar]
- Oves, M.; Khan, M.S.; Zaidi, A.; Ahmad, E. Soil contamination, nutritive value, and human health risk assessment of heavy metals: An overview. In Toxicity of Heavy Metals to Legumes and Bioremediation; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–27. [Google Scholar]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512, 143–153. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef]
- Wu, H.; Yang, F.; Li, H.; Li, Q.; Zhang, F.; Ba, Y.; Cui, L.; Sun, L.; Lv, T.; Wang, N.; et al. Heavy metal pollution and health risk assessment of agricultural soil near a smelter in an industrial city in China. Int. J. Environ. Health Res. 2020, 30, 174–186. [Google Scholar] [CrossRef]
- Guo, X. Cost of pollution in China: Economic estimates of physical damages. World Bank 2007, 39236, 1–151. [Google Scholar]
- Hou, D.; O’Connor, D.; Nathanail, P.; Tian, L.; Ma, Y. Integrated GIS and multivariate statistical analysis for regional scale assessment of heavy metal soil contamination: A critical review. Environ. Pollut. 2017, 231, 1188–1200. [Google Scholar] [CrossRef]
- Solgi, E.; Esmaili-Sari, A.; Riyahi-Bakhtiari, A.; Hadipour, M. Soil contamination of metals in the three industrial estates, Arak, Iran. Bull. Environ. Contam. Toxicol. 2012, 88, 634–638. [Google Scholar] [CrossRef]
- Burges, A.; Epelde, L.; Garbisu, C. Impact of repeated single-metal and multi-metal pollution events on soil quality. Chemosphere 2015, 120, 8–15. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, C.; Hong, X.; Kang, G.; Qin, M.; Yang, D.; Pang, B.; Li, Y.; He, J.; Dick, R.P. Risk assessment and source analysis of soil heavy metal pollution from lower reaches of Yellow River irrigation in China. Sci. Total Environ. 2018, 633, 1136–1147. [Google Scholar] [CrossRef]
- Giller, K.; McGrath, S. Pollution by toxic metals on agricultural soils. Nature 1988, 335, 676. [Google Scholar] [CrossRef]
- Rodrigues, S.; Cruz, N.; Coelho, C.; Henriques, B.; Carvalho, L.; Duarte, A.; Pereira, E.; Römkens, P.F. Risk assessment for Cd, Cu, Pb and Zn in urban soils: Chemical availability as the central concept. Environ. Pollut. 2013, 183, 234–242. [Google Scholar] [CrossRef]
- Zhou, J.; Liang, J.; Hu, Y.; Zhang, W.; Liu, H.; You, L.; Zhang, W.; Gao, M.; Zhou, J. Exposure risk of local residents to copper near the largest flash copper smelter in China. Sci. Total Environ. 2018, 630, 453–461. [Google Scholar] [CrossRef]
- Shi, T.; Ma, J.; Zhang, Y.; Liu, C.; Hu, Y.; Gong, Y.; Wu, X.; Ju, T.; Hou, H.; Zhao, L. Status of lead accumulation in agricultural soils across China (1979–2016). Environ. Int. 2019, 129, 35–41. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Bibi, S. Heavy Metal, Waste, COVID-19, and Rapid Industrialization in This Modern Era—Fit for Sustainable Future. Sustainability 2022, 14, 4746. [Google Scholar] [CrossRef]
- Mielke, H.W.; Reagan, P.L. Soil is an important pathway of human lead exposure. Environ. Health Perspect. 1998, 106 (Suppl. S1), 217–229. [Google Scholar]
- Wang, S.; Zhang, J. Blood lead levels in children, China. Environ. Res. 2006, 101, 412–418. [Google Scholar] [CrossRef]
- Qiu, K.; Xing, W.; Scheckel, K.; Cheng, Y.; Zhao, Z.; Ruan, X.; Li, L. Temporal and seasonal variations of As, Cd and Pb atmospheric deposition flux in the vicinity of lead smelters in Jiyuan, China. Atmos. Pollut. Res. 2016, 7, 170–179. [Google Scholar] [CrossRef]
- Stafilov, T.; Šajn, R.; Boev, B.; Cvetković, J.; Mukaetov, D.; Andreevski, M.; Lepitkova, S. Distribution of some elements in surface soil over the Kavadarci region, Republic of Macedonia. Environ. Earth Sci. 2010, 61, 1515–1530. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. Heavy Met. 2018, 10, 115–132. [Google Scholar]
- Wang, J.; Su, J.; Li, Z.; Liu, B.; Cheng, G.; Jiang, Y.; Li, Y.; Zhou, S.; Yuan, W. Source apportionment of heavy metal and their health risks in soil-dustfall-plant system nearby a typical non-ferrous metal mining area of Tongling, Eastern China. Environ. Pollut. 2019, 254, 113089. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, T.; Liu, L.; Ouyang, X. Impact of soil heavy metal pollution on food safety in China. PLoS ONE 2015, 10, e0135182. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Tao, L.; Liu, X.; Hou, J.; Wang, A.; Li, R. Heavy metal speciation and pollution of agricultural soils along Jishui River in non-ferrous metal mine area in Jiangxi Province, China. J. Geochem. Explor. 2013, 132, 156–163. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, M.; Wu, Y.; Fu, Z.; Sun, G.; Shang, L.; Li, Z.; Wang, P. Distribution patterns and sources of heavy metals in soils from an industry undeveloped city in Southern China. Ecotoxicol. Environ. Saf. 2020, 205, 111115. [Google Scholar] [CrossRef]
- Bermudez, G.M.; Jasan, R.; Plá, R.; Pignata, M.L. Heavy metals and trace elements in atmospheric fall-out: Their relationship with topsoil and wheat element composition. J. Hazard. Mater. 2012, 213, 447–456. [Google Scholar] [CrossRef]
- Luo, L.; Ma, Y.; Zhang, S.; Wei, D.; Zhu, Y.-G. An inventory of trace element inputs to agricultural soils in China. J. Environ. Manag. 2009, 90, 2524–2530. [Google Scholar] [CrossRef]
- Ran, Y.; Xing, W.; Liang, S.; Xiang, G.; Li, L. Heavy metal availability in soil near a lead smelter in the North China Plain. Asian J. Ecotoxicol. 2010, 5, 592–598. [Google Scholar]
- Li, Z.; Feng, X.; Li, G.; Bi, X.; Sun, G.; Zhu, J.; Qin, H.; Wang, J. Mercury and other metal and metalloid soil contamination near a Pb/Zn smelter in east Hunan province, China. Appl. Geochem. 2011, 26, 160–166. [Google Scholar] [CrossRef]
- Lu, H.; Li, Z.; Gasco, G.; Mendez, A.; Shen, Y.; Paz-Ferreiro, J. Use of magnetic biochars for the immobilization of heavy metals in a multi-contaminated soil. Sci. Total Environ. 2018, 622, 892–899. [Google Scholar] [CrossRef]
- Wang, S.-L.; Xu, X.-R.; Sun, Y.-X.; Liu, J.-L.; Li, H.-B. Heavy metal pollution in coastal areas of South China: A review. Mar. Pollut. Bull. 2013, 76, 7–15. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Ippolito, J.A.; Xing, W.; Qiu, K.; Yang, H. Lead smelting effects heavy metal concentrations in soils, wheat, and potentially humans. Environ. Pollut. 2020, 257, 113641. [Google Scholar] [CrossRef]
- Williams, P.; Lei, M.; Sun, G.; Huang, Q.; Lu, Y.; Deacon, C.; Meharg, A.A.; Zhu, Y.-G. Occurrence and partitioning of cadmium, arsenic and lead in mine impacted paddy rice: Hunan, China. Environ. Sci. Technol. 2009, 43, 637–642. [Google Scholar] [CrossRef]
- Xie, F.; Tan, H.; Yang, B.; He, J.L.; Chen, A.N.; Wen, X.M. The study of atmospheric transport and deposition of cadmium emitted from primitive zinc production area. Water Air Soil Pollut. 2014, 225, 2162. [Google Scholar] [CrossRef]
- Han, L.; Gao, B.; Hao, H.; Zhou, H.; Lu, J.; Sun, K. Lead contamination in sediments in the past 20 years: A challenge for China. Sci. Total Environ. 2018, 640, 746–756. [Google Scholar] [CrossRef]
- Chen, R.; De Sherbinin, A.; Ye, C.; Shi, G. China’s soil pollution: Farms on the frontline. Science 2014, 344, 691. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, Z.; Peng, C.; Liu, X.; Zhou, Z.; Xiao, X. Heavy metals in soils around non-ferrous smelteries in China: Status, health risks and control measures. Environ. Pollut. 2021, 282, 117038. [Google Scholar] [CrossRef]
- Shao, X.; Huang, B.; Zhao, Y.; Sun, W.; Gu, Z.; Qian, W. Impacts of human activities and sampling strategies on soil heavy metal distribution in a rapidly developing region of China. Ecotoxicol. Environ. Saf. 2014, 104, 1–8. [Google Scholar] [CrossRef]
- Ettler, V.; Mihaljevič, M.; Kříbek, B.; Majer, V.; Šebek, O. Tracing the spatial distribution and mobility of metal/metalloid contaminants in Oxisols in the vicinity of the Nkana copper smelter, Copperbelt province, Zambia. Geoderma 2011, 164, 73–84. [Google Scholar] [CrossRef]
- Morrison, A.L.; Gulson, B.L. Preliminary findings of chemistry and bioaccessibility in base metal smelter slags. Sci. Total Environ. 2007, 382, 30–42. [Google Scholar] [CrossRef]
- Lee, P.-K.; Kang, M.-J.; Yu, S.; Kwon, Y.K. Assessment of trace metal pollution in roof dusts and soils near a large Zn smelter. Sci. Total Environ. 2020, 713, 136536. [Google Scholar] [CrossRef]
- Du, B.; Zhou, J.; Lu, B.; Zhang, C.; Li, D.; Zhou, J.; Jiao, S.; Zhao, K.; Zhang, H. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 2020, 720, 137585. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 2016, 64, 56–74. [Google Scholar] [CrossRef] [Green Version]
- Jamal, A.; Delavar, M.A.; Naderi, A.; Nourieh, N.; Medi, B.; Mahvi, A.H. Distribution and health risk assessment of heavy metals in soil surrounding a lead and zinc smelting plant in Zanjan, Iran. Hum. Ecol. Risk Assess. Int. J. 2018, 25, 1018–1033. [Google Scholar] [CrossRef]
- Lei, K.; Giubilato, E.; Critto, A.; Pan, H.; Lin, C. Contamination and human health risk of lead in soils around lead/zinc smelting areas in China. Environ. Sci. Pollut. Res. 2016, 23, 13128–13136. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.-b.; Xin, G.; Su, Y.-b.; Gang, W. Risk assessment of heavy metals in soils and vegetables around non-ferrous metals mining and smelting sites, Baiyin, China. J. Environ. Sci. 2006, 18, 1124–1134. [Google Scholar]
- Ettler, V.; Konečný, L.; Kovářová, L.; Mihaljevič, M.; Šebek, O.; Kříbek, B.; Majer, V.; Veselovský, F.; Penížek, V.; Vaněk, A.; et al. Surprisingly contrasting metal distribution and fractionation patterns in copper smelter-affected tropical soils in forested and grassland areas (Mufulira, Zambian Copperbelt). Sci. Total Environ. 2014, 473, 117–124. [Google Scholar] [CrossRef]
- Xing, W.; Zheng, Y.; Scheckel, K.G.; Luo, Y.; Li, L. Spatial distribution of smelter emission heavy metals on farmland soil. Environ. Monit. Assess. 2019, 191, 115. [Google Scholar] [CrossRef]
- He, B.; Wang, W.; Geng, R.; Ding, Z.; Luo, D.; Qiu, J.; Zheng, G.; Fan, Q. Exploring the fate of heavy metals from mining and smelting activities in soil-crop system in Baiyin, NW China. Ecotoxicol. Environ. Saf. 2021, 207, 111234. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, S.; Qin, J.; Wu, R.; Li, H. Pollution characteristics and ecological risk assessment of heavy metals in paddy fields of Fujian province, China. Sci. Rep. 2020, 10, 12244. [Google Scholar] [CrossRef]
- Briki, M.; Zhu, Y.; Gao, Y.; Shao, M.; Ding, H.; Ji, H. Distribution and health risk assessment to heavy metals near smelting and mining areas of Hezhang, China. Environ. Monit. Assess. 2017, 189, 458. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Y.; Weiss, D.J.; Schleicher, N.J.; Wilcke, W.; Wu, L.; Guo, Q.; Chen, J.; O’Connor, D.; Hou, D. Possible application of stable isotope compositions for the identification of metal sources in soil. J. Hazard. Mater. 2021, 407, 124812. [Google Scholar] [CrossRef]
- Rabinowitz, M.B. Lead isotopes in soils near five historic American lead smelters and refineries. Sci. Total Environ. 2005, 346, 138–148. [Google Scholar] [CrossRef]
- Zhu, G.; Guo, Q.; Xiao, H.; Chen, T.; Yang, J. Multivariate statistical and lead isotopic analyses approach to identify heavy metal sources in topsoil from the industrial zone of Beijing Capital Iron and Steel Factory. Environ. Sci. Pollut. Res. 2017, 24, 14877–14888. [Google Scholar] [CrossRef]
- Pratte, S.; Bao, K.; Shen, J.; Mackenzie, L.; Klamt, A.-M.; Wang, G.; Xing, W. Recent atmospheric metal deposition in peatlands of northeast China: A review. Sci. Total Environ. 2018, 626, 1284–1294. [Google Scholar] [CrossRef]
- Kaste, J.M.; Friedland, A.J.; Stürup, S. Using stable and radioactive isotopes to trace atmospherically deposited Pb in montane forest soils. Environ. Sci. Technol. 2003, 37, 3560–3567. [Google Scholar] [CrossRef]
- Ma, Y.; Song, X. Using stable isotopes to determine seasonal variations in water uptake of summer maize under different fertilization treatments. Sci. Total Environ. 2016, 550, 471–483. [Google Scholar] [CrossRef]
- Zhou, J.; Obrist, D.; Dastoor, A.; Jiskra, M.; Ryjkov, A. Vegetation uptake of mercury and impacts on global cycling. Nat. Rev. Earth Environ. 2021, 2, 269–284. [Google Scholar] [CrossRef]
- Bigalke, M.; Weyer, S.; Kobza, J.; Wilcke, W. Stable Cu and Zn isotope ratios as tracers of sources and transport of Cu and Zn in contaminated soil. Geochim. Cosmochim. Acta 2010, 74, 6801–6813. [Google Scholar] [CrossRef]
- Ellis, A.S.; Johnson, T.M.; Bullen, T.D. Chromium isotopes and the fate of hexavalent chromium in the environment. Science 2002, 295, 2060–2062. [Google Scholar] [CrossRef] [Green Version]
- Maréchal, C.N.; Télouk, P.; Albarède, F. Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem. Geol. 1999, 156, 251–273. [Google Scholar] [CrossRef]
- Vaněk, A.; Vejvodová, K.; Mihaljevič, M.; Ettler, V.; Trubač, J.; Vaňková, M.; Teper, L.; Cabala, J.; Sutkowska, K.; Voegelin, A.; et al. Evaluation of thallium isotopic fractionation during the metallurgical processing of sulfides: An update. J. Hazard. Mater. 2022, 424, 127325. [Google Scholar] [CrossRef]
- Ventura, G.T.; Gall, L.; Siebert, C.; Prytulak, J.; Szatmari, P.; Hurlimann, M.; Halliday, A.N. The stable isotope composition of vanadium, nickel, and molybdenum in crude oils. Appl. Geochem. 2015, 59, 104–117. [Google Scholar] [CrossRef]
- Šillerová, H.; Chrastný, V.; Vítková, M.; Francová, A.; Jehlička, J.; Gutsch, M.R.; Kocourková, J.; Aspholm, P.E.; Nilsson, L.O.; Berglen, T.F.; et al. Stable isotope tracing of Ni and Cu pollution in North-East Norway: Potentials and drawbacks. Environ. Pollut. 2017, 228, 149–157. [Google Scholar] [CrossRef]
- Mulligan, C.; Yong, R.; Gibbs, B. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Liu, R.; Wang, M.; Chen, W.; Peng, C. Spatial pattern of heavy metals accumulation risk in urban soils of Beijing and its influencing factors. Environ. Pollut. 2016, 210, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Xiao, B.; Dong, H.; Wang, S. Implication of different humic acid fractions in soils under karst rocky desertification. Catena 2019, 174, 308–315. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, B.; Xiao, P. Interaction of Ca2+ and soil humic acid characterized by a joint experimental platform of potentiometric titration, UV–Visible spectroscopy, and fluorescence spectroscopy. Acta Geochim. 2021, 40, 300–311. [Google Scholar] [CrossRef]
- Chrastný, V.; Vanek, A.; Teper, L.; Cabala, J.; Procházka, J.; Pechar, L.; Drahota, P.; Penížek, V.; Komárek, M.; Novák, M. Geochemical position of Pb, Zn and Cd in soils near the Olkusz mine/smelter, South Poland: Effects of land use, type of contamination and distance from pollution source. Environ. Monit. Assess. 2012, 184, 2517–2536. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; He, Z. The impact of heavy metal contamination on soil health. Manag. Soil Health Sustain. Agric. 2018, 2, 63–95. [Google Scholar]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef]
- Song, J.; Huang, G.; Han, D.; Hou, Q.; Gan, L.; Zhang, M. A review of reactive media within permeable reactive barriers for the removal of heavy metal (loid) s in groundwater: Current status and future prospects. J. Clean. Prod. 2021, 319, 128644. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Miranda, L.S.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Adsorption-desorption behavior of heavy metals in aquatic environments: Influence of sediment, water and metal ionic properties. J. Hazard. Mater. 2022, 421, 126743. [Google Scholar] [CrossRef]
- Ji, C.; Wu, D.; Lu, J.; Shan, C.; Ren, Y.; Li, T.; Lv, L.; Pan, B.; Zhang, W. Temperature regulated adsorption and desorption of heavy metals to A-MIL-121: Mechanisms and the role of exchangeable protons. Water Res. 2021, 189, 116599. [Google Scholar] [CrossRef]
- Qu, J.; Fan, M. The current state of water quality and technology development for water pollution control in China. Crit. Rev. Environ. Sci. Technol. 2010, 40, 519–560. [Google Scholar] [CrossRef]
- Liu, R.; Guan, Y.; Chen, L.; Lian, B. Adsorption and desorption characteristics of Cd2+ and Pb2+ by micro and nano-sized biogenic CaCO3. Front. Microbiol. 2018, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Larson, C. China gets serious about its pollutant-laden soil. Am. Assoc. Adv. Sci. 2014, 343, 1415–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karami, E.; Kuhar, L.; Bona, A.; Nikoloski, A.N. A review of electrokinetic, ultrasonic and solution pulsing methods for mass transfer enhancement in in-situ processing. Miner. Eng. 2021, 170, 107029. [Google Scholar] [CrossRef]
- Varol, M. Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J. Hazard. Mater. 2011, 195, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Arthur, E.L.; Rice, P.J.; Rice, P.J.; Anderson, T.A.; Baladi, S.M.; Henderson, K.L.; Coats, J.R. Phytoremediation—an overview. Crit. Rev. Plant Sci. 2005, 24, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Morin, G.; Ostergren, J.D.; Juillot, F.; Ildefonse, P.; Calas, G.; Brown, G.E. XAFS determination of the chemical form of lead in smelter-contaminated soils and mine tailings: Importance of adsorption processes. Am. Mineral. 1999, 84, 420–434. [Google Scholar] [CrossRef]
- Popescu, I.; Nimirciag, R.; Vina, G.; Ajmone-Marsan, F. Spatial distribution of potentially toxic elements in soils polluted by mining activities. Rev. Chim. 2013, 5, 477–481. [Google Scholar]
- Popescu, I.; Stanescu, R.; Biasioli, M.; Ajmone Marsan, F.; Constantinescu, I. Assessing human risks through CSOIL exposure model for a soil contamination associated to heavy metals. Univ. Politeh. Buchar. Sci. Bull. Ser. B 2013, 75, 81–94. [Google Scholar]
- Karna, R.R.; Noerpel, M.; Betts, A.R.; Scheckel, K.G. Lead and arsenic bioaccessibility and speciation as a function of soil particle size. J. Environ. Qual. 2017, 46, 1225. [Google Scholar] [CrossRef]
- Zou, M.; Zhou, S.; Zhou, Y.; Jia, Z.; Guo, T.; Wang, J. Cadmium pollution of soil-rice ecosystems in rice cultivation dominated regions in China: A review. Environ. Pollut. 2021, 280, 116965. [Google Scholar] [CrossRef]
- Nag, R.; O’Rourke, S.M.; Cummins, E. Risk factors and assessment strategies for the evaluation of human or environmental risk from metal (loid) s–A focus on Ireland. Sci. Total Environ. 2022, 802, 149839. [Google Scholar] [CrossRef]
- Ruby, M.V.; Schoof, R.; Brattin, W.; Goldade, M.; Post, G.; Harnois, M.; Mosby, D.E.; Casteel, S.W.; Berti, W.; Carpenter, M.; et al. Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environ. Sci. Technol. 1999, 33, 3697–3705. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Farago, M.E. Lead contamination in smelting and mining environments and variations in chemical forms and bioavailability. Chem. Speciat. Bioavailab. 1995, 7, 113–123. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, J.; Wang, H.; Han, X.; Ma, J.; Ma, Y.; Luan, H. Distribution and health risk assessment of potentially toxic elements in soils around coal industrial areas: A global meta-analysis. Sci. Total Environ. 2020, 713, 135292. [Google Scholar] [CrossRef]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Yaqin, J.; Yinchang, F.; Jianhui, W.; Tan, Z.; Zhipeng, B.; Chiqing, D. Using geoaccumulation index to study source profiles of soil dust in China. J. Environ. Sci. 2008, 20, 571–578. [Google Scholar]
- Cai, L.-M.; Wang, Q.-S.; Wen, H.-H.; Luo, J.; Wang, S. Heavy metals in agricultural soils from a typical township in Guangdong Province, China: Occurrences and spatial distribution. Ecotoxicol. Environ. Saf. 2019, 168, 184–191. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Yang, Q.; Zhang, K.; Zheng, Y.; Zhou, G. Pollution characteristics of atmospheric dustfall and heavy metals in a typical inland heavy industry city in China. J. Environ. Sci. 2018, 71, 283–291. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soil and Plants, 4th ed.; Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Lu, X.; Wang, L.; Lei, K.; Huang, J.; Zhai, Y. Contamination assessment of copper, lead, zinc, manganese and nickel in street dust of Baoji, NW China. J. Hazard. Mater. 2009, 161, 1058–1062. [Google Scholar] [CrossRef]
- Šajn, R.; Aliu, M.; Stafilov, T.; Alijagić, J. Heavy metal contamination of topsoil around a lead and zinc smelter in Kosovska Mitrovica/Mitrovicë, Kosovo/Kosovë. J. Geochem. Explor. 2013, 134, 1–16. [Google Scholar] [CrossRef]
- Xu, D.-M.; Fu, R.-B.; Liu, H.-Q.; Guo, X.-P. Current knowledge from heavy metal pollution in Chinese smelter contaminated soils, health risk implications and associated remediation progress in recent decades: A critical review. J. Clean. Prod. 2021, 286, 124989. [Google Scholar] [CrossRef]
- Pérez, K.; Toro, N.; Gálvez, E.; Robles, P.; Wilson, R.; Navarra, A. Environmental, economic and technological factors affecting Chilean copper smelters—A critical review. J. Mater. Res. Technol. 2021, 15, 213–225. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Stafilov, T.; Šajn, R.; Pančevski, Z.; Boev, B.; Frontasyeva, M.V.; Strelkova, L.P. Heavy metal contamination of topsoils around a lead and zinc smelter in the Republic of Macedonia. J. Hazard. Mater. 2010, 175, 896–914. [Google Scholar] [CrossRef]
- He, M. Distribution and phytoavailability of antimony at an antimony mining and smelting area, Hunan, China. Environ. Geochem. Health 2007, 29, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, S.; Wang, L.; Liu, F.; Lin, C.-J.; Zhang, L.; Wang, F. Spatial distribution and accumulation of Hg in soil surrounding a Zn/Pb smelter. Sci. Total Environ. 2014, 496, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yao, C.; Song, J.; Li, Z.; Zhang, C.; Qian, W.; Bi, D.; Li, C.; Teng, Y.; Wu, L.; et al. Mercury contamination in vicinity of secondary copper smelters in Fuyang, Zhejiang Province, China: Levels and contamination in topsoils. Environ. Pollut. 2009, 157, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, G.; Qiu, G. A preliminary study on mercury contamination to the environment from artisanal zinc smelting using indigenous methods in Hezhang County, Guizhou, China: Part 2. Mercury contaminations to soil and crop. Sci. Total Environ. 2006, 368, 47–55. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, N.; Li, H.; Lu, Y.; Yang, Z. Potential health risk assessment for inhabitants posed by heavy metals in rice in Zijiang River basin, Hunan Province, China. Environ. Sci. Pollut. Res. 2020, 27, 24013–24024. [Google Scholar] [CrossRef]
- Xing, W.; Cao, E.; Scheckel, K.G.; Bai, X.; Li, L. Influence of phosphate amendment and zinc foliar application on heavy metal accumulation in wheat and on soil extractability impacted by a lead smelter near Jiyuan, China. Environ. Sci. Pollut. Res. 2018, 25, 31396–31406. [Google Scholar] [CrossRef]
- Li, Z.; Feng, X.; Bi, X.; Li, G.; Lin, Y.; Sun, G. Probing the distribution and contamination levels of 10 trace metal/metalloids in soils near a Pb/Zn smelter in Middle China. Environ. Sci. Pollut. Res. 2014, 21, 4149–4162. [Google Scholar] [CrossRef]
- Liu, L.; Wu, L.; Luo, Y.; Zhang, C.; Jiang, Y.; Qiu, X. The impact of a copper smelter on adjacent soil zinc and cadmium fractions and soil organic carbon. J. Soils Sediments 2010, 10, 808–817. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Wang, H.; Lin, Q.; Chen, X.; Chen, Y. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol. Environ. Saf. 2007, 67, 75–81. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Bi, X.; Wu, P.; Liu, T.; Li, F.; Liu, C. Lead, Zn, and Cd in slags, stream sediments, and soils in an abandoned Zn smelting region, southwest of China, and Pb and S isotopes as source tracers. J. Soils Sediments 2010, 10, 1527–1539. [Google Scholar] [CrossRef]
- Cai, L.-M.; Wang, Q.-S.; Luo, J.; Chen, L.-G.; Zhu, R.-L.; Wang, S.; Tang, C.-H. Heavy metal contamination and health risk assessment for children near a large Cu-smelter in central China. Sci. Total Environ. 2019, 650, 725–733. [Google Scholar] [CrossRef]
- Cui, Y.-J.; Zhu, Y.-G.; Zhai, R.-H.; Chen, D.-Y.; Huang, Y.-Z.; Qiu, Y.; Liang, J.-Z. Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environ. Int. 2004, 30, 785–791. [Google Scholar] [CrossRef]
- Girault, F.; Perrier, F.; Poitou, C.; Isambert, A.; Théveniaut, H.; Laperche, V.; Clozel-Leloup, B.; Douay, F. Effective radium concentration in topsoils contaminated by lead and zinc smelters. Sci. Total Environ. 2016, 566, 865–876. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Wan, Y.; Wu, R.; Feng, D.; Li, K. Source identification and comprehensive apportionment of the accumulation of soil heavy metals by integrating pollution landscapes, pathways, and receptors. Sci. Total Environ. 2021, 786, 147436. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, Z.; Wang, Y.; Qiu, K.; Fu, Y.; Zhao, X.; Li, L. Soil heavy metal pollution in the farmland near a lead-smelter in Henan Province. Chin. J. Soil Sci. 2014, 45, 1505–1510. [Google Scholar]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—A case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Y.; Ma, J.; Hu, Y.; Su, B.; Fang, G.; Wang, L.; Xiang, B. A review of heavy metal pollution levels and health risk assessment of urban soils in Chinese cities. Environ. Sci. Pollut. Res. 2018, 25, 1055–1069. [Google Scholar] [CrossRef]
- Jiang, J.; Khan, A.U.; Shi, B.; Tang, S.; Khan, J. Application of positive matrix factorization to identify potential sources of water quality deterioration of Huaihe River, China. Appl. Water Sci. 2019, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Fei, J.-C.; Min, X.-B.; Wang, Z.-X.; Pang, Z.-h.; Liang, Y.-J.; Ke, Y. Health and ecological risk assessment of heavy metals pollution in an antimony mining region: A case study from South China. Environ. Sci. Pollut. Res. 2017, 24, 27573–27586. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Yutong, Z.; Shenggao, L. Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicol. Environ. Saf. 2015, 120, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, J.; Liu, X.; Liu, X.; Li, X.; Ren, Y.; Wang, J.; Dong, L. Partitioning and geochemical fractions of heavy metals from geogenic and anthropogenic sources in various soil particle size fractions. Geoderma 2018, 312, 104–113. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Y.; Li, H.; Tu, Y.; Liu, B.; Yang, Z. Assessment of heavy metal contamination, distribution and source identification in the sediments from the Zijiang River, China. Sci. Total Environ. 2018, 645, 235–243. [Google Scholar] [CrossRef]
- Quan, Z.; Huang, W.; Liao, Y.; Liu, W.; Xu, H.; Yan, N.; Qu, Z. Study on the regenerable sulfur-resistant sorbent for mercury removal from nonferrous metal smelting flue gas. Fuel 2019, 241, 451–458. [Google Scholar] [CrossRef]
- Cheng, H.; Li, M.; Zhao, C.; Li, K.; Peng, M.; Qin, A.; Cheng, X. Overview of trace metals in the urban soil of 31 metropolises in China. J. Geochem. Explor. 2014, 139, 31–52. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Cheng, H.; Li, Q.; Lin, C. Anthropogenic atmospheric emissions of cadmium in China. Atmos. Environ. 2013, 79, 155–160. [Google Scholar] [CrossRef]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef]
- Guo, B.; Su, Y.; Pei, L.; Wang, X.; Wei, X.; Zhang, B.; Zhang, D.; Wang, X. Contamination, Distribution and Health Risk Assessment of Risk Elements in Topsoil for Amusement Parks in Xi’an, China. Pol. J. Environ. Stud. 2020, 30, 601–617. [Google Scholar] [CrossRef]
- Jo, I.; Koh, M. Chemical changes in agricultural soils of Korea: Data review and suggested countermeasures. Environ. Geochem. Health 2004, 26, 105–117. [Google Scholar] [CrossRef]
- Sow, A.Y.; Ismail, A.; Zulkifli, S.Z.; Amal, M.N.; Hambali, K.A. Survey on heavy metals contamination and health risk assessment in commercially valuable Asian swamp eel, Monopterus albus from Kelantan, Malaysia. Sci. Rep. 2019, 9, 6391. [Google Scholar] [CrossRef]
- Li, M.-M.; Gao, Z.-Y.; Dong, C.; Wu, M.-Q.; Yan, J.; Cao, J.; Ma, W.-J.; Wang, J.; Gong, Y.-L.; Xu, J.; et al. Contemporary blood lead levels of children aged 0–84 months in China: A national cross-sectional study. Environ. Int. 2020, 134, 105288. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Dong, K.F.; Wu, Y.; Yang, X.; Yang, J.; Tan, H.; Niu, X.; Zhao, X.; Xiao, G.; et al. Regional characteristics of children’s blood lead levels in China: A systematic synthesis of national and subnational population data. Sci. Total Environ. 2021, 769, 144649. [Google Scholar] [CrossRef]
- Ji, A.; Wang, F.; Luo, W.; Yang, R.; Chen, J.; Cai, T. Lead poisoning in China: A nightmare from industrialisation. Lancet 2011, 377, 1474–1476. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Cai, L.-M.; Wang, Q.-S.; Hu, G.-C.; Chen, L.-G. A comprehensive exploration of risk assessment and source quantification of potentially toxic elements in road dust: A case study from a large Cu smelter in central China. Catena 2021, 196, 104930. [Google Scholar] [CrossRef]
- Kandra, P.; Pavuluri, H.; Grandhi, S.K.; Kandula, V.N.; Challa, L. Current Trends and Future Perspectives of Biobased Methods for Recovery of Metals from WEEE for a Sustainable Environment. In Environmental Management of Waste Electrical and Electronic Equipment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 125–151. [Google Scholar]
- Gunarathne, V.; Rajapaksha, A.U.; Vithanage, M.; Alessi, D.S.; Selvasembian, R.; Naushad, M.; You, S.; Oleszczuk, P.; Ok, Y.S. Hydrometallurgical processes for heavy metals recovery from industrial sludges. Crit. Rev. Environ. Sci. Technol. 2020, 52, 1022–1062. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Fernández, Á.M.; Torres, V.M. Hydrometallurgical processes for the recovery of metals from steel industry by-products: A critical review. J. Sustain. Metall. 2020, 6, 505–540. [Google Scholar] [CrossRef]
- Jadhav, U.; Hocheng, H. Hydrometallurgical recovery of metals from large printed circuit board pieces. Sci. Rep. 2015, 5, 14574. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Yang, C.; Chiang, J.F.; Li, J. Innovating e-waste management: From macroscopic to microscopic scales. Sci. Total Environ. 2017, 575, 1–5. [Google Scholar] [CrossRef]
- Gupta, C.K. Chemical Metallurgy: Principles and Practice; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Vestola, E.A.; Kuusenaho, M.K.; Närhi, H.M.; Tuovinen, O.H.; Puhakka, J.A.; Plumb, J.J.; Kaksonen, A. Acid bioleaching of solid waste materials from copper, steel and recycling industries. Hydrometallurgy 2010, 103, 74–79. [Google Scholar] [CrossRef]
- Yong, Y.S.; Lim, Y.A.; Ilankoon, I. An analysis of electronic waste management strategies and recycling operations in Malaysia: Challenges and future prospects. J. Clean. Prod. 2019, 224, 151–166. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, L.-J.; Lin, C.-B.; Xie, X.-X.; Yong, X.-Y.; Wu, X.-Y.; Zhou, J.; Jia, H.-H.; Wei, P. Extracting heavy metals from electroplating sludge by acid and bioelectrical leaching using Acidithiobacillus ferrooxidans. Hydrometallurgy 2020, 191, 105225. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Nancharaiah, Y.V. Biotechnology in the management and resource recovery from metal bearing solid wastes: Recent advances. J. Environ. Manag. 2018, 211, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, F.; Ma, Y.; Cai, T.; Li, H.; Huang, Z.; Yuan, G. Multiple heavy metals extraction and recovery from hazardous electroplating sludge waste via ultrasonically enhanced two-stage acid leaching. J. Hazard. Mater. 2010, 178, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Kumar, V.; Singh, R. Review of hydrometallurgical recovery of zinc from industrial wastes. Resour. Conserv. Recycl. 2001, 33, 1–22. [Google Scholar] [CrossRef]
- Hannula, P.-M.; Khalid, M.K.; Janas, D.; Yliniemi, K.; Lundström, M. Energy efficient copper electrowinning and direct deposition on carbon nanotube film from industrial wastewaters. J. Clean. Prod. 2019, 207, 1033–1039. [Google Scholar] [CrossRef]
- Trellu, C.; Mousset, E.; Pechaud, Y.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. J. Hazard. Mater. 2016, 306, 149–174. [Google Scholar] [CrossRef]
- Jensen, J.K.; Holm, P.E.; Nejrup, J.; Larsen, M.B.; Borggaard, O.K. The potential of willow for remediation of heavy metal polluted calcareous urban soils. Environ. Pollut. 2009, 157, 931–937. [Google Scholar] [CrossRef]
- He, C.; Zhao, Y.; Wang, F.; Oh, K.; Zhao, Z.; Wu, C.; Zhang, X.; Chen, X.; Liu, X. Phytoremediation of soil heavy metals (Cd and Zn) by castor seedlings: Tolerance, accumulation and subcellular distribution. Chemosphere 2020, 252, 126471. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Xiao, X.-Y.; Guo, Z.-H. Identification of indicators of giant reed (Arundo donax L.) ecotypes for phytoremediation of metal-contaminated soil in a non-ferrous mining and smelting area in southern China. Ecol. Indic. 2019, 101, 249–260. [Google Scholar] [CrossRef]
- Fu, P.; Yang, H.; Zhang, G.; Fu, P.; Li, Z. In-situ immobilization of Cd-contaminated soils using ferronickel slag as potential soil amendment. Bull. Environ. Contam. Toxicol. 2019, 103, 756–762. [Google Scholar] [CrossRef]
- Hou, D.; Al-Tabbaa, A. Sustainability: A new imperative in contaminated land remediation. Environ. Sci. Policy 2014, 39, 25–34. [Google Scholar] [CrossRef]
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Chang, T.; Yen, J. On-site mercury-contaminated soils remediation by using thermal desorption technology. J. Hazard. Mater. 2006, 128, 208–217. [Google Scholar] [CrossRef]
- Tran, H.T.; Lin, C.; Hoang, H.G.; Bui, X.T.; Vu, C.T. Soil washing for the remediation of dioxin-contaminated soil: A Review. J. Hazard. Mater. 2021, 421, 12676. [Google Scholar] [CrossRef]
- Yan, D.; Guo, Z.; Xiao, X.; Peng, C.; He, Y.; Yang, A.; Wang, X.; Hu, Y.; Li, Z. Cleanup of arsenic, cadmium, and lead in the soil from a smelting site using N, N-bis (carboxymethyl)-L-glutamic acid combined with ascorbic acid: A lab-scale experiment. J. Environ. Manag. 2021, 296, 113174. [Google Scholar] [CrossRef]
- Mousset, E.; Oturan, M.A.; Van Hullebusch, E.D.; Guibaud, G.; Esposito, G. Soil washing/flushing treatments of organic pollutants enhanced by cyclodextrins and integrated treatments: State of the art. Crit. Rev. Environ. Sci. Technol. 2014, 44, 705–795. [Google Scholar] [CrossRef]
- Vu, C.T.; Tran, H.T.; Kaewlaoyoong, A.; Huang, W.-Y.; Lin, C. Efficacy of indigenously prepared sugarcane and pineapple wine solvents for washing highly dioxin-contaminated field soils. Appl. Sci. 2019, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Makino, T.; Kamiya, T.; Takano, H.; Itou, T.; Sekiya, N.; Sasaki, K.; Maejima, Y.; Sugahara, K. Remediation of cadmium-contaminated paddy soils by washing with calcium chloride: Verification of on-site washing. Environ. Pollut. 2007, 147, 112–119. [Google Scholar] [CrossRef]
- Kim, S.-O.; Jeong, J.Y.; Lee, W.-C.; Yun, S.-T.; Jo, H.Y. Electrokinetic remediation of heavy metal-contaminated soils: Performance comparison between one-and two-dimensional electrode configurations. J. Soils Sediments 2021, 21, 2755–2769. [Google Scholar] [CrossRef]
- Tang, X.; Li, Q.; Wu, M.; Lin, L.; Scholz, M. Review of remediation practices regarding cadmium-enriched farmland soil with particular reference to China. J. Environ. Manag. 2016, 181, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.T.; Hsu, C.-N. Electrokinetic remediation of cadmium-contaminated clay. J. Environ. Eng. 2005, 131, 298–304. [Google Scholar] [CrossRef]
- Lu, P.; Feng, Q.; Meng, Q.; Yuan, T. Electrokinetic remediation of chromium-and cadmium-contaminated soil from abandoned industrial site. Sep. Purif. Technol. 2012, 98, 216–220. [Google Scholar] [CrossRef]
- Alvarez, G.B.; Bento, N.J.S.; Neves, T.A.; Dos Santos, F.S.; Silva, G.C.; de Sousa, P.A.P. Numerical study of the influence of electrode arrangements in electrokinetic remediation technique. Environ. Sci. Pollut. Res. 2017, 24, 26424–26435. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Husain, T. Evaluation of a petroleum hydrocarbon contaminated site for natural attenuation using ‘RBMNA’methodology. Environ. Model. Softw. 2003, 18, 179–194. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Murtaza, G.; Kunhikrishnan, A.; Seshadri, B.; Shahid, M.; Ali, S.; Bolan, N.S.; Ok, Y.S.; et al. Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit. Rev. Environ. Sci. Technol. 2016, 46, 467–499. [Google Scholar] [CrossRef]
- Wahla, I.H.; Kirkham, M. Heavy metal displacement in salt-water-irrigated soil during phytoremediation. Environ. Pollut. 2008, 155, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Gul, I.; Manzoor, M.; Hashim, N.; Shah, G.M.; Waani, S.P.T.; Shahid, M.; Antoniadis, V.; Rinklebe, J.; Arshad, M. Challenges in microbially and chelate-assisted phytoextraction of cadmium and lead—A review. Environ. Pollut. 2021, 287, 117667. [Google Scholar] [CrossRef]
- Kos, B.; Leštan, D. Chelator induced phytoextraction and in situ soil washing of Cu. Environ. Pollut. 2004, 132, 333–339. [Google Scholar] [CrossRef]
- Kumar, V.; Shahi, S.; Singh, S. Bioremediation: An Eco-Sustainable Approach for Restoration of Contaminated Sites. In Microbial Bioprospecting for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2018; pp. 115–136. [Google Scholar]
- Erakhrumen, A.A.; Agbontalor, A. Phytoremediation: An environmentally sound technology for pollution prevention, control and remediation in developing countries. Educ. Res. Rev. 2007, 2, 151–156. [Google Scholar]
- Prasad, M. Phytoremediation of metal-polluted ecosystems: Hype for commercialization. Russ. J. Plant Physiol. 2003, 50, 686–701. [Google Scholar] [CrossRef]
- Tomé, F.V.; Rodríguez, P.B.; Lozano, J. Elimination of natural uranium and 226Ra from contaminated waters by rhizofiltration using Helianthus annuus L. Sci. Total Environ. 2008, 393, 351–357. [Google Scholar] [CrossRef]
- Kristanti, R.A.; Ngu, W.J.; Yuniarto, A.; Hadibarata, T. Rhizofiltration for Removal of Inorganic and Organic Pollutants in Groundwater: A Review. Biointerafce Res. Appl. Chem 2021, 4, 12326–12347. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231. https://doi.org/10.3390/toxics10050231
Adnan M, Xiao B, Xiao P, Zhao P, Li R, Bibi S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics. 2022; 10(5):231. https://doi.org/10.3390/toxics10050231
Chicago/Turabian StyleAdnan, Muhammad, Baohua Xiao, Peiwen Xiao, Peng Zhao, Ruolan Li, and Shaheen Bibi. 2022. "Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China" Toxics 10, no. 5: 231. https://doi.org/10.3390/toxics10050231