Impact of Carbon Impurities on Air Stability of MOCVD 2D-MoS2
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of As-Deposited 2D-MoS2 Layers
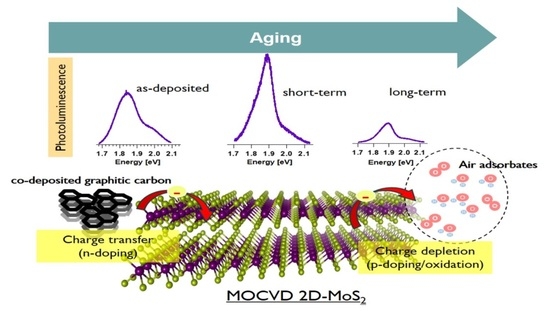
3.2. Aging of MOCVD 2D-MoS2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-Layer MoS2 Transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.R.; Song, W.; Han, J.K.; Lee, Y.B.; Kim, S.J.; Myung, S.; Lee, S.S.; An, K.S.; Choi, C.J.; Lim, J. Wafer-Scale, Homogeneous MoS2 Layers on Plastic Substrates for Flexible Visible-Light Photodetectors. Adv. Mater. 2016, 28, 5025–5030. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Meng, P.; Chen, J.; Liu, H.; Bian, R.; Zhu, C.; Liu, F.; Liu, Z. 2D Material Based Synaptic Devices for Neuromorphic Computing. Adv. Funct. Mater. 2020, 31, 2005443. [Google Scholar] [CrossRef]
- Andrzejewski, D.; Oliver, R.; Beckmann, Y.; Grundmann, A.; Heuken, M.; Kalisch, H.; Vescan, A.; Kümmell, T.; Bacher, G. Flexible Large-Area Light-Emitting Devices Based on WS2 Monolayers. Adv. Opt. Mater. 2020, 8, 2000694. [Google Scholar] [CrossRef]
- Jiao, K.; Duan, C.; Wu, X.; Chen, J.; Wang, Y.; Chen, Y. The Role of MoS2 as an Interfacial Layer in Graphene/Silicon Solar Cells. Phys. Chem. Chem. Phys. 2015, 17, 8182–8186. [Google Scholar] [CrossRef]
- Rai, A.; Movva, H.C.P.; Roy, A.; Taneja, D.; Chowdhury, S.; Banerjee, S.K. Progress in Contact, Doping and Mobility Engineering of MoS2: An Atomically Thin 2D Semiconductor. Crystals 2018, 8, 316. [Google Scholar] [CrossRef]
- Schranghamer, T.F.; Sharma, M.; Singh, R.; Das, S. Review and Comparison of Layer Transfer Methods for Two-Dimensional Materials for Emerging Applications. Chem. Soc. Rev. 2021, 50, 11032–11054. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Movva, H.C.P.; Satpati, B.; Kim, K.; Dey, R.; Rai, A.; Pramanik, T.; Guchhait, S.; Tutuc, E.; Banerjee, S.K. Structural and Electrical Properties of MoTe2 and MoSe2 Grown by Molecular Beam Epitaxy. ACS Appl. Mater. Interfaces 2016, 8, 7396–7402. [Google Scholar] [CrossRef]
- Mortelmans, W.; El Kazzi, S.; Groven, B.; Nalin Mehta, A.; Balaji, Y.; De Gendt, S.; Heyns, M.; Merckling, C. Epitaxial Registry and Crystallinity of MoS2 via Molecular Beam and Metalorganic Vapor Phase van Der Waals Epitaxy. Appl. Phys. Lett. 2020, 117, 033101. [Google Scholar] [CrossRef]
- Timpel, M.; Ligorio, G.; Ghiami, A.; Gavioli, L.; Cavaliere, E.; Chiappini, A.; Rossi, F.; Pasquali, L.; Gärisch, F.; List-Kratochvil, E.J.W.; et al. 2D-MoS2 Goes 3D: Transferring Optoelectronic Properties of 2D MoS2 to a Large-Area Thin Film. npj 2D Mater. Appl. 2021, 5, 64. [Google Scholar] [CrossRef]
- Ghiami, A.; Timpel, M.; Chiappini, A.; Nardi, M.V.; Verucchi, R. Synthesis of MoS2 Thin Film by Ionized Jet Deposition: Role of Substrate and Working Parameters. Surfaces 2020, 3, 683–693. [Google Scholar] [CrossRef]
- Serna, M.I.; Yoo, S.H.; Moreno, S.; Xi, Y.; Oviedo, J.P.; Choi, H.; Alshareef, H.N.; Kim, M.J.; Minary-Jolandan, M.; Quevedo-Lopez, M.A. Large-Area Deposition of MoS2 by Pulsed Laser Deposition with in Situ Thickness Control. ACS Nano 2016, 10, 6054–6061. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, C.; Shaw, J.C.; Cheng, R.; Chen, Y.; Huang, X.; Liu, Y.; Weiss, N.O.; Lin, Z.; Huang, Y.; et al. Large Area Growth and Electrical Properties of P-Type WSe2 Atomic Layers. Nano Lett. 2015, 15, 709–713. [Google Scholar] [CrossRef]
- Huang, J.; Yang, L.; Liu, D.; Chen, J.; Fu, Q.; Xiong, Y.; Lin, F.; Xiang, B. Large-Area Synthesis of Monolayer WSe2 on a SiO2/Si Substrate and Its Device Applications. Nanoscale 2015, 7, 4193–4198. [Google Scholar] [CrossRef]
- Kim, H.; Ovchinnikov, D.; Deiana, D.; Unuchek, D.; Kis, A. Suppressing Nucleation in Metal–Organic Chemical Vapor Deposition of MoS2 Monolayers by Alkali Metal Halides. Nano Lett. 2017, 17, 5056–5063. [Google Scholar] [CrossRef]
- Kim, I.S.; Sangwan, V.K.; Jariwala, D.; Wood, J.D.; Park, S.; Chen, K.S.; Shi, F.; Ruiz-Zepeda, F.; Ponce, A.; Jose-Yacaman, M.; et al. Influence of Stoichiometry on the Optical and Electrical Properties of Chemical Vapor Deposition Derived MoS2. ACS Nano 2014, 8, 10551–10558. [Google Scholar] [CrossRef]
- Chubarov, M.; Choudhury, T.H.; Hickey, D.R.; Bachu, S.; Zhang, T.; Sebastian, A.; Bansal, A.; Zhu, H.; Trainor, N.; Das, S.; et al. Wafer-Scale Epitaxial Growth of Unidirectional WS2 Monolayers on Sapphire. ACS Nano 2021, 15, 2532–2541. [Google Scholar] [CrossRef]
- Shi, Y.; Groven, B.; Serron, J.; Wu, X.; Nalin Mehta, A.; Minj, A.; Sergeant, S.; Han, H.; Asselberghs, I.; Lin, D.; et al. Engineering Wafer-Scale Epitaxial Two-Dimensional Materials through Sapphire Template Screening for Advanced High-Performance Nanoelectronics. ACS Nano 2021, 15, 9482–9494. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Patsha, A.; Mohapatra, P.K.; Kazes, M.; Ranganathan, K.; Houben, L.; Oron, D.; Ismach, A. Growth-Etch Metal–Organic Chemical Vapor Deposition Approach of WS 2 Atomic Layers. ACS Nano 2021, 15, 526–538. [Google Scholar] [CrossRef]
- Grundmann, A.; McAleese, C.; Conran, B.R.; Pakes, A.; Andrzejewski, D.; Kümmell, T.; Bacher, G.; Khin Teo, K.B.; Heuken, M.; Kalisch, H.; et al. MOVPE of Large-Scale MoS2/WS2, WS2/MoS2, WS2/Graphene and MoS2/Graphene 2D-2D Heterostructures for Optoelectronic Applications. MRS Adv. 2020, 5, 1625–1633. [Google Scholar] [CrossRef]
- Schaefer, C.M.; Caicedo Roque, J.M.; Sauthier, G.; Bousquet, J.; Hébert, C.; Sperling, J.R.; Pérez-Tomás, A.; Santiso, J.; del Corro, E.; Garrido, J.A. Carbon Incorporation in MOCVD of MoS2 Thin Films Grown from an Organosulfide Precursor. Chem. Mater. 2021, 33, 4474–4487. [Google Scholar] [CrossRef]
- Tuxen, A.; Gøbel, H.; Hinnemann, B.; Li, Z.; Knudsen, K.G.; Topsøe, H.; Lauritsen, J.V.; Besenbacher, F. An Atomic-Scale Investigation of Carbon in MoS2 Hydrotreating Catalysts Sulfided by Organosulfur Compounds. J. Catal. 2011, 281, 345–351. [Google Scholar] [CrossRef]
- Marx, M.; Grundmann, A.; Lin, Y.-R.; Andrzejewski, D.; Kümmell, T.; Bacher, G.; Heuken, M.; Kalisch, H.; Vescan, A. Metalorganic Vapor-Phase Epitaxy Growth Parameters for Two-Dimensional MoS2. J. Electron. Mater. 2018, 47, 910–916. [Google Scholar] [CrossRef]
- Liang, T.; Habib, M.R.; Xiao, H.; Xie, S.; Kong, Y.; Yu, C.; Iwai, H.; Fujita, D.; Hanagata, N.; Chen, H.; et al. Intrinsically Substitutional Carbon Doping in CVD-Grown Monolayer MoS2 and the Band Structure Modulation. ACS Appl. Electron. Mater. 2020, 2, 1055–1064. [Google Scholar] [CrossRef]
- Choudhury, T.H.; Simchi, H.; Boichot, R.; Chubarov, M.; Mohney, S.E.; Redwing, J.M. Chalcogen Precursor Effect on Cold-Wall Gas-Source Chemical Vapor Deposition Growth of WS2. Cryst. Growth Des. 2018, 18, 4357–4364. [Google Scholar] [CrossRef]
- Kang, K.; Xie, S.; Huang, L.; Han, Y.; Huang, P.Y.; Mak, K.F.; Kim, C.-J.; Muller, D.; Park, J. High-Mobility Three-Atom-Thick Semiconducting Films with Wafer-Scale Homogeneity. Nature 2015, 520, 656–660. [Google Scholar] [CrossRef]
- Eichfeld, S.M.; Hossain, L.; Lin, Y.C.; Piasecki, A.F.; Kupp, B.; Birdwell, A.G.; Burke, R.A.; Lu, N.; Peng, X.; Li, J.; et al. Highly Scalable, Atomically Thin WSe2 grown via Metal-Organic Chemical Vapor Deposition. ACS Nano 2015, 9, 2080–2087. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman Spectroscopy as a Versatile Tool for Studying the Properties of Graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Hu, Z.; Probert, M.; Li, K.; Lv, D.; Yang, X.; Gu, L.; Mao, N.; Feng, Q.; Xie, L.; et al. Exploring Atomic Defects in Molybdenum Disulphide Monolayers. Nat. Commun. 2015, 6, 6293. [Google Scholar] [CrossRef]
- Carozo, V.; Wang, Y.; Fujisawa, K.; Carvalho, B.R.; McCreary, A.; Feng, S.; Lin, Z.; Zhou, C.; Perea-López, N.; Elías, A.L.; et al. Optical Identification of Sulfur Vacancies: Bound Excitons at the Edges of Monolayer Tungsten Disulfide. Sci. Adv. 2017, 3, e1602813. [Google Scholar] [CrossRef]
- Wu, Z.; Ni, Z. Spectroscopic Investigation of Defects in Two-Dimensional Materials. Nanophotonics 2017, 6, 1219–1237. [Google Scholar] [CrossRef]
- Gao, J.; Li, B.; Tan, J.; Chow, P.; Lu, T.M.; Koratkar, N. Aging of Transition Metal Dichalcogenide Monolayers. ACS Nano 2016, 10, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Pető, J.; Ollár, T.; Vancsó, P.; Popov, Z.I.; Magda, G.Z.; Dobrik, G.; Hwang, C.; Sorokin, P.B.; Tapasztó, L. Spontaneous Doping of the Basal Plane of MoS2 Single Layers through Oxygen Substitution under Ambient Conditions. Nat. Chem. 2018, 10, 1246–1251. [Google Scholar] [CrossRef]
- Yao, K.; Banerjee, D.; Femi-Oyetoro, J.D.; Hathaway, E.; Jiang, Y.; Squires, B.; Jones, D.C.; Neogi, A.; Cui, J.; Philipose, U.; et al. Growth of Monolayer MoS2 on Hydrophobic Substrates as a Novel and Feasible Method to Prevent the Ambient Degradation of Monolayer MoS2. MRS Adv. 2020, 5, 2707–2715. [Google Scholar] [CrossRef]
- Kotsakidis, J.C.; Zhang, Q.; Vazquez De Parga, A.L.; Currie, M.; Helmerson, K.; Gaskill, D.K.; Fuhrer, M.S. Oxidation of Monolayer WS2 in Ambient Is a Photoinduced Process. Nano Lett. 2019, 19, 5205–5215. [Google Scholar] [CrossRef]
- Grundmann, A.; Andrzejewski, D.; Kümmell, T.; Bacher, G.; Heuken, M.; Kalisch, H.; Vescan, A. H2S-Free Metal-Organic Vapor Phase Epitaxy of Coalesced 2D WS2 Layers on Sapphire—ERRATUM. MRS Adv. 2019, 4, e1. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of Disorder on Raman Scattering of Single-Layer MoS2. Phys. Rev. B 2015, 91, 195411. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H. Two-Dimensional MoS2: Properties, Preparation, and Applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef]
- Ghiami, A.; Timpel, M.; Nardi, M.V.; Chiappini, A.; Nozar, P.; Quaranta, A.; Verucchi, R. Unravelling Work Function Contributions and Their Engineering in 2H-MoS2 Single Crystal Discovered by Molecular Probe Interaction. J. Phys. Chem. C 2020, 124, 6732–6740. [Google Scholar] [CrossRef]
- Ganta, D.; Sinha, S.; Haasch, R.T. 2-D Material Molybdenum Disulfide Analyzed by XPS. Surf. Sci. Spectra 2014, 21, 19–27. [Google Scholar] [CrossRef]
- Baer, D.R.; Artyushkova, K.; Cohen, H.; Easton, C.D.; Engelhard, M.; Gengenbach, T.R.; Greczynski, G.; Mack, P.; Morgan, D.J.; Roberts, A. XPS Guide: Charge Neutralization and Binding Energy Referencing for Insulating Samples. J. Vac. Sci. Technol. A 2020, 38, 031204. [Google Scholar] [CrossRef]
- Bertolazzi, S.; Bonacchi, S.; Nan, G.; Pershin, A.; Beljonne, D.; Samorì, P. Engineering Chemically Active Defects in Monolayer MoS2 Transistors via Ion-Beam Irradiation and Their Healing via Vapor Deposition of Alkanethiols. Adv. Mater. 2017, 29, 1606760. [Google Scholar] [CrossRef]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase Engineering of Transition Metal Dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef]
- Islam, M.A.; Church, J.; Han, C.; Chung, H.-S.; Ji, E.; Kim, J.H.; Choudhary, N.; Lee, G.-H.; Lee, W.H.; Jung, Y. Noble Metal-Coated MoS2 Nanofilms with Vertically-Aligned 2D Layers for Visible Light-Driven Photocatalytic Degradation of Emerging Water Contaminants. Sci. Rep. 2017, 7, 14944. [Google Scholar] [CrossRef] [PubMed]
- Najmaei, S.; Yuan, J.; Zhang, J.; Ajayan, P.; Lou, J. Synthesis and Defect Investigation of Two-Dimensional Molybdenum Disulfide Atomic Layers. Acc. Chem. Res. 2015, 48, 31–40. [Google Scholar] [CrossRef]
- Romanov, R.I.; Slavich, A.S.; Kozodaev, M.G.; Myakota, D.I.; Lebedinskii, Y.Y.; Novikov, S.M.; Markeev, A.M. Band Alignment in As-Transferred and Annealed Graphene/MoS2 Heterostructures. Phys. Status Solidi-Rapid Res. Lett. 2020, 14, 1900406. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.; Wang, Z.; Xie, S.; Zhang, Y.; Shen, Y.; Yu, M.; Deng, B.; Huang, Q.; Fan, C.; et al. Radiation Induced Reduction: An Effective and Clean Route to Synthesize Functionalized Graphene. J. Mater. Chem. 2012, 22, 7775–7781. [Google Scholar] [CrossRef]
- Fujimoto, A.; Yamada, Y.; Koinuma, M.; Sato, S. Origins of Sp3C Peaks in C1s X-Ray Photoelectron Spectra of Carbon Materials. Anal. Chem. 2016, 88, 6110–6114. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Giannakopoulos, A.; Nardi, M.V.; Ligorio, G.; Oehzelt, M.; Chen, L.; Pasquali, L.; Timpel, M.; Giglia, A.; Nannarone, S.; et al. Tuning the Work Function of Graphene-on-Quartz with a High Weight Molecular Acceptor. J. Phys. Chem. C 2014, 118, 4784–4790. [Google Scholar] [CrossRef]
- Vasquez, R.P. Highly Oriented Pyrolytic Graphite by XPS. Surf. Sci. Spectra 1992, 1, 238–241. [Google Scholar] [CrossRef]
- Blyth, R.I.R.; Buqa, H.; Netzer, F.P.; Ramsey, M.G.; Besenhard, J.O.; Golob, P.; Winter, M. XPS Studies of Graphite Electrode Materials for Lithium Ion Batteries. Appl. Surf. Sci. 2000, 167, 99–106. [Google Scholar] [CrossRef]
- Blanton, T.N.; Majumdar, D. Characterization of X-Ray Irradiated Graphene Oxide Coatings Using X-Ray Diffraction, X-Ray Photoelectron Spectroscopy, and Atomic Force Microscopy. Powder Diffr. 2013, 28, 68–71. [Google Scholar] [CrossRef]
- Fan, Z.J.; Kai, W.; Yan, J.; Wei, T.; Zhi, L.J.; Feng, J.; Ren, Y.M.; Song, L.P.; Wei, F. Facile Synthesis of Graphene Nanosheets via Fe Reduction of Exfoliated Graphite Oxide. ACS Nano 2011, 5, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, B.; Li, Y.; Luo, X.; Li, G.; Chen, Y.; Zhang, C.; He, J. Tunable Ultrafast Nonlinear Optical Properties of Graphene/MoS2 van Der Waals Heterostructures and Their Application in Solid-State Bulk Lasers. ACS Nano 2018, 12, 11376–11385. [Google Scholar] [CrossRef]
- Li, Z.; Ye, R.; Feng, R.; Kang, Y.; Zhu, X.; Tour, J.M.; Fang, Z. Graphene Quantum Dots Doping of MoS2 Monolayers. Adv. Mater. 2015, 27, 5235–5240. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 2–5. [Google Scholar] [CrossRef]
- Mouri, S.; Miyauchi, Y.; Matsuda, K. Tunable Photoluminescence of Monolayer MoS2 via Chemical Doping. Nano Lett. 2013, 13, 5944–5948. [Google Scholar] [CrossRef]
- Sim, D.M.; Kim, M.; Yim, S.; Choi, M.J.; Choi, J.; Yoo, S.; Jung, Y.S. Controlled Doping of Vacancy-Containing Few-Layer MoS2 via Highly Stable Thiol-Based Molecular Chemisorption. ACS Nano 2015, 9, 12115–12123. [Google Scholar] [CrossRef]
- Panasci, S.E.; Schilirò, E.; Greco, G.; Cannas, M.; Gelardi, F.M.; Agnello, S.; Roccaforte, F.; Giannazzo, F. Strain, Doping, and Electronic Transport of Large Area Monolayer MoS2 Exfoliated on Gold and Transferred to an Insulating Substrate. ACS Appl. Mater. Interfaces 2021, 13, 31248–31259. [Google Scholar] [CrossRef]
- Chae, D.; Cain, J.D.; Hanson, E.D.; Murthy, A.; Dravid, V.P. Substrate-Induced Strain and Charge Doping in CVD-Grown Monolayer MoS2 Substrate-Induced Strain and Charge Doping in CVD-Grown Monolayer MoS2. Appl. Phys. Lett. 2017, 111, 143106. [Google Scholar] [CrossRef]
- Pierucci, D.; Henck, H.; Naylor, C.H.; Sediri, H.; Lhuillier, E.; Balan, A.; Rault, J.E.; Dappe, Y.J.; Bertran, F.; Le Fèvre, P.; et al. Large Area Molybdenum Disulphide- Epitaxial Graphene Vertical Van Der Waals Heterostructures. Sci. Rep. 2016, 6, 26656. [Google Scholar] [CrossRef]
- Shim, G.W.; Yoo, K.; Seo, S.B.; Shin, J.; Jung, D.Y.; Kang, I.S.; Ahn, C.W.; Cho, B.J.; Choi, S.Y. Large-Area Single-Layer MoSe2 and Its van Der Waals Heterostructures. ACS Nano 2014, 8, 6655–6662. [Google Scholar] [CrossRef] [PubMed]
- Bhanu, U.; Islam, M.R.; Tetard, L.; Khondaker, S.I. Photoluminescence Quenching in Gold-MoS2 Hybrid Nanoflakes. Sci. Rep. 2014, 4, 5575. [Google Scholar] [CrossRef]
- Kang, K.; Godin, K.; Kim, Y.D.; Fu, S.; Cha, W.; Hone, J.; Yang, E.H. Graphene-Assisted Antioxidation of Tungsten Disulfide Monolayers: Substrate and Electric-Field Effect. Adv. Mater. 2017, 29, 1603898. [Google Scholar] [CrossRef]
- Fabbri, F.; Dinelli, F.; Forti, S.; Sementa, L.; Pace, S.; Piccinini, G.; Fortunelli, A.; Coletti, C.; Pingue, P. Edge Defects Promoted Oxidation of Monolayer WS2 Synthesized on Epitaxial Graphene. J. Phys. Chem. C 2020, 124, 9035–9044. [Google Scholar] [CrossRef]
- Jia, L.; Liu, B.; Zhao, Y.; Chen, W.; Mou, D.; Fu, J.; Wang, Y.; Xin, W.; Zhao, L. Structure Design of MoS2@Mo2C on Nitrogen-Doped Carbon for Enhanced Alkaline Hydrogen Evolution Reaction. J. Mater. Sci. 2020, 55, 16197–16210. [Google Scholar] [CrossRef]
- Jeon, J.; Park, Y.; Choi, S.; Lee, J.; Lim, S.S.; Lee, B.H.; Song, Y.J.; Cho, J.H.; Jang, Y.H.; Lee, S. Epitaxial Synthesis of Molybdenum Carbide and Formation of a Mo2C/MoS2 Hybrid Structure via Chemical Conversion of Molybdenum Disulfide. ACS Nano 2018, 12, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Pan, Y.; Luo, K.; Yu, J.; Zhang, Y.; Jia, K.; Yin, H.; Zhu, H.; Tian, H.; et al. The Evolution of MoS2 Properties under Oxygen Plasma Treatment and Its Application in MoS2 Based Devices. J. Mater. Sci. Mater. Electron. 2019, 30, 18185–18190. [Google Scholar] [CrossRef]
- Coy Diaz, H.; Addou, R.; Batzill, M. Interface Properties of CVD Grown Graphene Transferred onto MoS2. Nanoscale 2014, 6, 1071–1078. [Google Scholar] [CrossRef]
- Nan, H.; Wang, Z.; Wang, W.; Liang, Z.; Lu, Y.; Chen, Q.; He, D.; Tan, P.; Miao, F.; Wang, X.; et al. Strong Photoluminescence Enhancement of MoS2 through Defect Engineering and Oxygen Bonding. ACS Nano 2014, 8, 5738–5745. [Google Scholar] [CrossRef] [PubMed]
- Tongay, S.; Zhou, J.; Ataca, C.; Liu, J.; Kang, J.S.; Matthews, T.S.; You, L.; Li, J.; Grossman, J.C.; Wu, J. Broad-Range Modulation of Light Emission in Two-Dimensional Semiconductors by Molecular Physisorption Gating. Nano Lett. 2013, 13, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Deng, J.; Guan, J.; Yang, Q.; Yu, Z.; Lu, Y.; Xu, Z.; Yao, Z.; Zheng, J. Photoluminescence of Monolayer MoS2 modulated by Water/O2/Laser Irradiation. Phys. Chem. Chem. Phys. 2021, 23, 24579–24588. [Google Scholar] [CrossRef] [PubMed]
- Cun, H.; Macha, M.; Kim, H.; Liu, K.; Zhan, Y.; LaGrange, T.; Kis, A.; Radenovic, A. Wafer-scale MOCVD growth of monolayer MoS2 on sapphire and SiO2. Nano Res. 2019, 12, 2646–2652. [Google Scholar] [CrossRef]
- Gredelj, S.; Gerson, A.R.; Kumar, S.; Cavallaro, G.P. Characterization of aluminium surfaces with and without plasma nitriding by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2001, 174, 240–250. [Google Scholar] [CrossRef]
- Xie, K.; Yuan, K.; Li, X.; Lu, W.; Shen, C.; Liang, C.; Vajtai, R.; Ajayan, P.; Wei, B. Superior Potassium Ion Storage via Vertical MoS2 “Nano-Rose” with Expanded Interlayers on Graphene. Small 2017, 13, 1701471. [Google Scholar] [CrossRef]





| Mo 3d5/2 (eV) | S 2p3/2 (eV) | Carbon (Atom. %) | PL A Intensity * (cts) | |
|---|---|---|---|---|
| SC | 229.6 | 162.5 | - | |
| 2DTBS | 228.6 | 161.5 | 5 | 57.52 |
| 2N2 | 228.6 | 161.4 | 3 | 87.56 |
| BN2 | 228.7 | 161.5 | 4 | 66.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiami, A.; Grundmann, A.; Tang, S.; Fiadziushkin, H.; Wang, Z.; Aussen, S.; Hoffmann-Eifert, S.; Heuken, M.; Kalisch, H.; Vescan, A. Impact of Carbon Impurities on Air Stability of MOCVD 2D-MoS2. Surfaces 2023, 6, 351-363. https://doi.org/10.3390/surfaces6040025
Ghiami A, Grundmann A, Tang S, Fiadziushkin H, Wang Z, Aussen S, Hoffmann-Eifert S, Heuken M, Kalisch H, Vescan A. Impact of Carbon Impurities on Air Stability of MOCVD 2D-MoS2. Surfaces. 2023; 6(4):351-363. https://doi.org/10.3390/surfaces6040025
Chicago/Turabian StyleGhiami, Amir, Annika Grundmann, Songyao Tang, Hleb Fiadziushkin, Zhaodong Wang, Stephan Aussen, Susanne Hoffmann-Eifert, Michael Heuken, Holger Kalisch, and Andrei Vescan. 2023. "Impact of Carbon Impurities on Air Stability of MOCVD 2D-MoS2" Surfaces 6, no. 4: 351-363. https://doi.org/10.3390/surfaces6040025