Acetylation of Scaled-Down Chitin Nanofiber Films to Improve Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of a Film of Partially Deacetylated Chitin Nanofiber (PDA-ChNF)
2.3. Preparation of Scaled-Down Chitin Nanofiber (SD-ChNF) Dispersion and Film
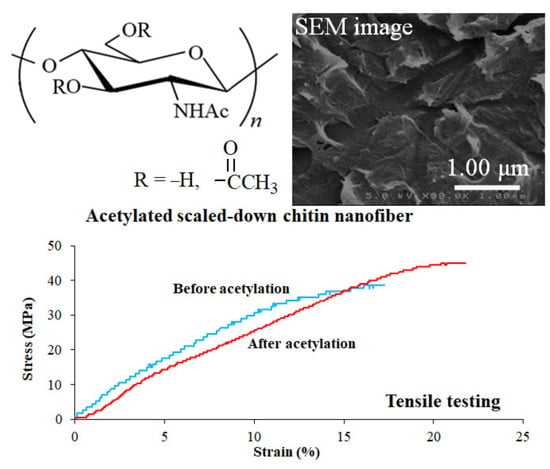
2.4. Acetylation of SD-ChNF Film
2.5. Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schuerch, C. Polysaccharides. In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Mark, H.F., Bilkales, N., Overberger, C.G., Eds.; John Wiley & Sons: New York, NY, USA, 1986; Volume 13, pp. 87–162. [Google Scholar]
- Kasapis, S.; Norton, I.T.; Ubbink, J.B. Modern Biopolymer Science: Bridging the Divide between Fundamental Treatise and Industrial Application; Academic Press: San Diego, CA, USA, 2009. [Google Scholar]
- Song, E.H.; Shang, J.; Ratner, D.M. 9.08—Polysaccharides. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 137–155. [Google Scholar]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Kadokawa, J.; Takegawa, A.; Mine, S.; Prasad, K. Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydr. Polym. 2011, 84, 1408–1412. [Google Scholar] [CrossRef]
- Zeng, J.B.; He, Y.S.; Li, S.L.; Wang, Y.Z. Chitin whiskers: An overview. Biomacromolecules 2012, 13, 1–11. [Google Scholar]
- Ifuku, S. Preparation of chitin nanofibers from crab shell and their applications. Kobunshi Ronbunshu 2012, 69, 460–467. [Google Scholar]
- Tajiri, R.; Setoguchi, T.; Wakizono, S.; Yamamoto, K.; Kadokawa, J. Preparation of self-assembled chitin nanofibers by regeneration from ion gels using calcium halide dihydrate/methanol solutions. J. Biobased Mater. Bioenergy 2013, 7, 655–659. [Google Scholar] [CrossRef]
- Kadokawa, J. Ionic liquid as useful media for dissolution, derivatization, and nanomaterial processing of chitin. Green Sustain. Chem. 2013, 3, 19–25. [Google Scholar]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Mattioli-Belmonte, M. Emerging biomedical applications of nano-chitins and nano-chitosans obtained via advanced eco-friendly technologies from marine resources. Mar. Drugs 2014, 12, 5468–5502. [Google Scholar] [CrossRef] [Green Version]
- Kadokawa, J. Fabrication of nanostructured and microstructured chitin materials through gelation with suitable dispersion media. RSC Adv. 2015, 5, 12736–12746. [Google Scholar] [CrossRef]
- You, J.; Li, M.; Ding, B.; Wu, X.; Li, C. Crab chitin-based 2D soft nanomaterials for fully biobased electric devices. Adv. Mater. 2017, 29, 1606895. [Google Scholar]
- Anraku, M.; Tabuchi, R.; Ifuku, S.; Nagae, T.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Miyamura, S.; Hirayama, F.; et al. An oral absorbent, surface-deacetylated chitin nano-fiber ameliorates renal injury and oxidative stress in 5/6 nephrectomized rats. Carbohydr. Polym. 2017, 161, 21–25. [Google Scholar] [CrossRef]
- Koizumi, R.; Azuma, K.; Izawa, H.; Morimoto, M.; Ochi, K.; Tsuka, T.; Imagawa, T.; Osaki, T.; Ito, N.; Okamoto, Y.; et al. Oral administration of surface-deacetylated chitin nanofibers and chitosan inhibit 5-fluorouracil-induced intestinal mucositis in mice. Int. J. Mol. Sci. 2017, 18, 279. [Google Scholar] [CrossRef] [Green Version]
- Satam, C.C.; Irvin, C.W.; Lang, A.W.; Jallorina, J.C.R.; Shofner, M.L.; Reynolds, J.R.; Meredith, J.C. Spray-coated multilayer cellulose nanocrystal—Chitin nanofiber films for barrier applications. ACS Sustain. Chem. Eng. 2018, 6, 10637–10644. [Google Scholar] [CrossRef]
- Mushi, N.E.; Nishino, T.; Berglund, L.A.; Zhou, Q. Strong and tough chitin film from α-chitin nanofibers prepared by high pressure homogenization and chitosan addition. ACS Sustain. Chem. Eng. 2019, 7, 1692–1697. [Google Scholar] [CrossRef]
- Naghdi, T.; Golmohammadi, H.; Yousefi, H.; Hosseinifard, M.; Kostiv, U.; Horák, D.; Merkoçi, A. Chitin nanofiber paper toward optical (bio)sensing applications. ACS Appl. Mater. Interfaces 2020, 12, 15538–15552. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Water purification: Nanocellulose-enabled membranes for water purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 2070009. [Google Scholar] [CrossRef]
- Prasad, K.; Murakami, M.; Kaneko, Y.; Takada, A.; Nakamura, Y.; Kadokawa, J. Weak gel of chitin with ionic liquid, 1-allyl-3-methylimidazolium bromide. Int. J. Biol. Macromol. 2009, 45, 221–225. [Google Scholar] [CrossRef]
- Kadokawa, J.; Kawano, A.; Yamamoto, K. Fabrication of semi-crystalline film by hexanoylation on self-assembled chitin nanofibers. ChemistrySelect 2019, 4, 797–801. [Google Scholar]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef]
- Ifuku, S.; Shervani, Z.; Saimoto, H. Preparation, modification and application of chitin nanofibers. In Nanofibers: Synthesis, Properties, and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 167–181. [Google Scholar]
- Ifuku, S. Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules 2014, 19, 18367–18380. [Google Scholar]
- Ifuku, S. Chitin nanofibers: Preparations, modifications, and applications. In Handbook of Polymer Nanocomposites. Processing, Performance and Application: Volume C: Polymer Nanocomposites of Cellulose Nanoparticles; Springer: Berlin, Germany, 2015; pp. 165–178. [Google Scholar]
- Kadokawa, J. Surface derivatization and grafting on self-assembled chitin nanofibers for modification, functionalization, and application. In Surface Treatment Methods of Natural Fibres and Their Effects on Biocomposites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 187–202. [Google Scholar]
- Ifuku, S.; Morooka, S.; Morimoto, M.; Saimoto, H. Acetylation of chitin nanofibers and their transparent nanocomposite films. Biomacromolecules 2010, 11, 1326–1330. [Google Scholar] [CrossRef]
- Kurita, K.; Ishii, S.; Tomita, K.; Nishimura, S.I.; Shimoda, K. Reactivity characteristics of squid β-chitin as compared with those of shrimp chitin: High potentials of squid chitin as a starting material for facile chemical modifications. J. Polym. Sci. Polym. Chem. 1994, 32, 1027–1032. [Google Scholar] [CrossRef]
- Hashiguchi, T.; Yamamoto, K.; Kadokawa, J. Fabrication of highly flexible nanochitin film and its composite film with anionic polysaccharide. Carbohydr. Polym. 2021, 270, 118369. [Google Scholar]
- Zhao, D.B.; Fei, Z.F.; Geldbach, T.J.; Scopelliti, R.; Laurenczy, G.; Dyson, P.J. Allyl-functionalised ionic liquids: Synthesis, characterisation, and reactivity. Helv. Chim. Acta 2005, 88, 665–675. [Google Scholar] [CrossRef]
- Mine, S.; Izawa, H.; Kaneko, Y.; Kadokawa, J. Acetylation of a-chitin in ionic liquids. Carbohydr. Res. 2009, 344, 2263–2265. [Google Scholar]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Ando, T.; Kataoka, S. Acylation of chitin with acid anhydrides in trichloroacetic acid system. Kobunshi Ronbunshu 1980, 37, 1–7. [Google Scholar] [CrossRef]




| Entry | Equiv. of Pyridine (b) | Temp. (°C) | DA (c) (KBr Pellet Method) | DA (c) (ATR Method) | CI (d) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|---|
| SD-ChNF film | - | - | - | - | 95.7 | 38.6 | 17.2 |
| 1 | 0.6 | 50 | 0.81 | 1.82 | 94.7 | 28.5 | 21.4 |
| 2 | 10 | 50 | 0.96 | 1.83 | 93.8 | 45.0 | 21.8 |
| 3 | 10 | 80 | 1.18 | 1.84 | 93.6 | 44.1 | 24.9 |
| 4 | 100 | 80 | 1.22 | 1.86 | 92.7 | 14.0 | 15.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadokawa, J.-i.; Iiyama, C.; Nakashima, A. Acetylation of Scaled-Down Chitin Nanofiber Films to Improve Mechanical Properties. Surfaces 2023, 6, 249-256. https://doi.org/10.3390/surfaces6030017
Kadokawa J-i, Iiyama C, Nakashima A. Acetylation of Scaled-Down Chitin Nanofiber Films to Improve Mechanical Properties. Surfaces. 2023; 6(3):249-256. https://doi.org/10.3390/surfaces6030017
Chicago/Turabian StyleKadokawa, Jun-ichi, Chiharu Iiyama, and Aoi Nakashima. 2023. "Acetylation of Scaled-Down Chitin Nanofiber Films to Improve Mechanical Properties" Surfaces 6, no. 3: 249-256. https://doi.org/10.3390/surfaces6030017